Abstract.
One of the current strategies to prevent malaria in pregnancy is intermittent preventive treatment with sulfadoxine-pyrimethamine (IPTp-SP). However, in order for pregnant women to receive an adequate number of SP doses, they should attend a health facility on a regular basis. In addition, SP resistance may decrease IPTp-SP efficacy. New or additional interventions for preventing malaria during pregnancy are therefore warranted. Because it is known that community health workers (CHWs) can diagnose and treat malaria in children, in this study screening and treatment of malaria in pregnancy by CHWs was evaluated as an addition to the regular IPTp-SP program. CHWs used rapid diagnostic tests (RDTs) for screening and artemether–lumefantrine was given in case of a positive RDT. Overall, CHWs were able to conduct RDTs with a sensitivity of 81.5% (95% confidence interval [CI] 67.9–90.2) and high specificity of 92.1% (95% CI 89.9–93.9) compared with microscopy. After a positive RDT, 79.1% of women received artemether–lumefantrine. When treatment was not given, this was largely due to the woman being already under treatment. Almost all treated women finished the full course of artemether–lumefantrine (96.4%). In conclusion, CHWs are capable of performing RDTs with high specificity and acceptable sensitivity, the latter being dependent on the limit of detection of RDTs. Furthermore, CHWs showed excellent adherence to test results and treatment guidelines, suggesting they can be deployed for screen and treat approaches of malaria in pregnancy.
BACKGROUND
Malaria in pregnancy can cause several adverse outcomes such as maternal anemia, stillbirths, miscarriage, and low birth weight (reviewed by Desai and others).1 The current key strategy for prevention of malaria in pregnancy is intermittent preventive treatment with sulfadoxine–pyrimethamine (IPTp-SP). IPTp-SP consists in the administration of SP during the second and third trimester when the women attend the antenatal care clinic (ANC),2 and has proven effective in preventing placental malaria and low birth weight.3 The World Health Organization (WHO) recommends that SP is administered at each antenatal care visit in the second and third trimester, provided it is at least a month apart from the previous dose.2 However, IPTp-SP coverage of at least two doses is low in many sub-Sahara African countries (on average 21.5%).4 Reasons for low coverage are a lack of coordination and leadership, financial constraints, unmotivated and unsupported health staff, perceived risk of the medication, logistic challenges, and ANC attendance among others.5–7 Adolescents and primigravidae, who have among pregnant women the highest risk of malaria, are even less likely to receive sufficient doses of IPTp-SP, mainly due to low ANC attendance.6 Besides the unsatisfactory uptake of IPTp-SP, SP resistance is widespread and rising in most sub-Saharan African countries.8–10 In east Africa, where resistance is the highest of sub-Saharan Africa,11 the efficacy of IPTp-SP seems already compromised.12–15 Therefore, it is important to explore alternative or additional preventive measures.
Community case management of malaria (CCMm) aims at reducing malaria morbidity in children, by improving the access to diagnosis and treatment of malaria. CCMm relies on members of community, often named community health workers (CHW), who have been trained in diagnosing malaria with rapid diagnostic tests (RDTs) and administering an antimalarial treatment to positive tested children.16 The strategy is effective as CHWs are capable of performing RDTs with a fair sensitivity (generally more than 85% compared with microscopy).17 Because of these positive results, an intervention based on this strategy was considered for pregnant women. In this intervention, CHWs were mobilized to screen pregnant women for malaria with RDTs at monthly intervals, between antenatal care visits.18 Besides increasing the chance of detecting and treating malaria infections, this strategy also increases the total number of health-care contacts during pregnancy, something that is strongly encouraged by the WHO.19 The performance of RDTs used by CHWs on pregnant women in rural Burkina Faso is reported here, including the adherence of CHWs and pregnant women to the test results and to treatment guidelines.
METHODS
This study was nested in a cluster-randomized controlled trial (COSMIC; Trial Registration: Current Controlled Trials ISRCTN37259296 and clinicaltrials.gov NCT01941564) carried out in Benin, The Gambia, and Burkina Faso, as described previously.18 The aim of the main trial was to assess the efficacy of community screening and treatment of malaria during pregnancy on placental malaria. Here, the quality of RDT use by CHWs in pregnant women and adherence to treatment guidelines is described for the Burkina Faso study site.
Study procedures.
The intervention of community screening and treatment of malaria during pregnancy was conducted in 15 villages in the Nanoro health-center catchment area, about 85 km northwest of Ouagadougou. Malaria is endemic in this region, but has a seasonal pattern with peak transmission occurring toward the end of the rainy season (that lasts from June until October). CHWs were instructed to pay monthly visits to pregnant women in their second and third trimester up to delivery. In each village there was a single CHW participating in the study, except for one village in which the initial CHW got a new job outside the study area and was therefore replaced. At each home visit, CHWs performed a RDT (SD Bioline Ag-Pf, Standard Diagnostics, Gyeonggi-do, Republic of Korea), irrespective of symptoms, and if positive administered artemether–lumefantrine (COARTEM®, Novartis, Basel, Switzerland). Treatment was not given if a woman reported to have received a course of artemether–lumefantrine in the past 3 weeks. Seriously ill women were referred to the health center. RDT results and any given treatment were recorded on a case record form by the CHW (Supplemental Material 1). In addition, the CHW collected a blood slide and blood spots on filter paper. All treated women were visited after 3 days by the CHW, to assess treatment adherence by administering a questionnaire and by checking the empty blisters. The number of tablets remaining and reasons for nonadherence were recorded in another CRF (Supplemental Material 2). Besides the home visits, pregnant women were encouraged by the CHWs to visit the ANC. At each ANC visit the woman received standard care, including IPTp-SP. Furthermore, a blood slide and filter paper were collected and clinical data and any given treatment was recorded on a CRF (Supplemental Materials 3 and 4).
Study population and sample size.
All women resident in the study area and without a known sensitivity to sulphonamides were eligible for inclusion in the COSMIC study. Pregnant women enrolled in intervention villages (N = 900) in Burkina Faso were included in the analyses of the current study.18
Community health workers.
CHWs included in the study were already involved in community sensitization and organization of vaccination and malnutrition campaigns. Each of the CHWs was linked to a health facility. CHWs followed a program before the beginning of the trial in which they were trained in malaria symptoms and recognition of danger signs, the use of RDTs and the need and purpose of screening pregnant women for malaria. They were also explained the benefits of IPTp–SP and advised to promote ANC visits and SP uptake among the pregnant women. CHWs were supervised by field workers.
Rapid diagnostic tests.
RDTs used by the CHWs targeted the Plasmodium falciparum histidine-rich protein 2 (HRP2) antigen. A central stock of RDTs was kept at the Unité de Recherche Clinique de Nanoro (URCN) and the CHWs were supplied with small stocks at a regular basis. In case of an invalid test result, CHWs were instructed to repeat the RDT.
Microscopy.
Filter papers and blood slides collected at home visits in intervention villages were transferred to the laboratory (URCN) on the same day. Blood slides were stained with Giemsa 3% for 45–60 minutes. Slides were read by two independent expert microscopists blinded to the RDT results. The number of parasites were counted against 200 leukocytes, or against 500 leukocytes if the count was < 10 parasites/200 leukocytes. Slides were considered negative if no parasites were seen after examination of 100 high power fields. Any discrepancies between the two readings were resolved by consulting a third independent blinded reader.
Real-time Polymerase chain reaction.
Filter papers were air dried, sealed in bags with silica, and stored at room temperature until shipment to the Netherlands (Academic Medical Center, Amsterdam). For each selected filter paper, a blood spot was punched out using Acu-Punch skin biopsy punchers (acuderm® inc, FL) and transferred to a 5-mL polystyrene tube with lysis buffer (bioMérieux, Marcy-l’Étoile, France). The tubes were placed on a roller bank for 30 minutes. After lysis, the fluid was transferred to easyMag vessels and Magnetic Bead Silica were added. DNA was extracted using the NucliSENS easyMag DBS 1.0 protocol (bioMérieux, Marcy-l’Étoile, France). Positive and negative controls were included (blood spots from ethylenediaminetetraacetic acid (EDTA) blood spiked with 3D7 or FCR3 culture and blood spots of uninfected EDTA blood of the Dutch blood bank). Samples were stored at −20°C.
Real-time polymerase chain reaction (PCR) for detection of P. falciparum DNA was performed as previously described with minor modifications.20,21 In each reaction 2.5 μL of DNA, 5 mM MgCl2, 2.5 μL of 10 × PCR Buffer, 0.125 μL of HotStarTaq DNA Polymerase, 0.25 mM of each dNTP, 0.4 μM of each primer, and 0.1 μM of FAM-labeled probe (‘5-aacaattggagggcaagg-3′) were used. PCR Mix reagents were ordered from Qiagen (Hilden, Germany) and all primers from Biolegio (Nijmegen, the Netherlands). In each plate, a dilution series of P. falciparum FCR3 culture was included (104 parasites/μL–1 parasites/μL) as well as positive and negative DNA extraction controls and Milli-Q water. Reactions were run on Bio-Rad CFX real-time PCR machine (Hercules, CA) with the following settings: initial denaturation 95°C for 10 minutes, 40 cycles of 95°C for 60 seconds, and 60°C for 20 seconds. Results were analyzed using Bio-Rad CFX manager software (version 3.1).
Statistical analyses.
All analyses were done using Stata 14.0 (College Station, TX). Microscopy was used as the reference test for RDT performance. A subanalysis at first home visit was done with real-time PCR as the reference test, because it has been shown that submicroscopic infections are also clinically important as they can result in maternal anemia and preterm or low birth weight babies.22 Sensitivity (proportion of correctly identified positive samples), specificity (proportion of correctly identified negative samples), positive predictive value (proportion of diseased after a positive RDT result; positive predictive value [PPV]), negative predictive values (proportion of nondiseased after a negative RDT result; negative predictive value NPV), and prevalence (proportion of positive test results of all tests performed) were calculated by using logistic regression with robust standard errors to take clustering of tests within CHWs into account. For sensitivity and specificity analysis stratified by CHW, logistic regression with robust standard errors was used to account for repeated measurements of participants. However, for CHWs with 100% RDT sensitivity exact binomial confidence intervals were calculated since no between-woman variation was observed. For comparisons of parasite density, Mann–Whitney U tests were performed for skewed data distributions.
Ethics.
Informed consent was obtained for each participating community prior to the start of the trial. During the study, informed consent was obtained for each participating woman. The study protocol was reviewed and ethically approved by the Institutional Ethics Committee of Center Muraz in Burkina Faso on 19 September 2013 (ref A20-2013/CE-CM).
RESULTS
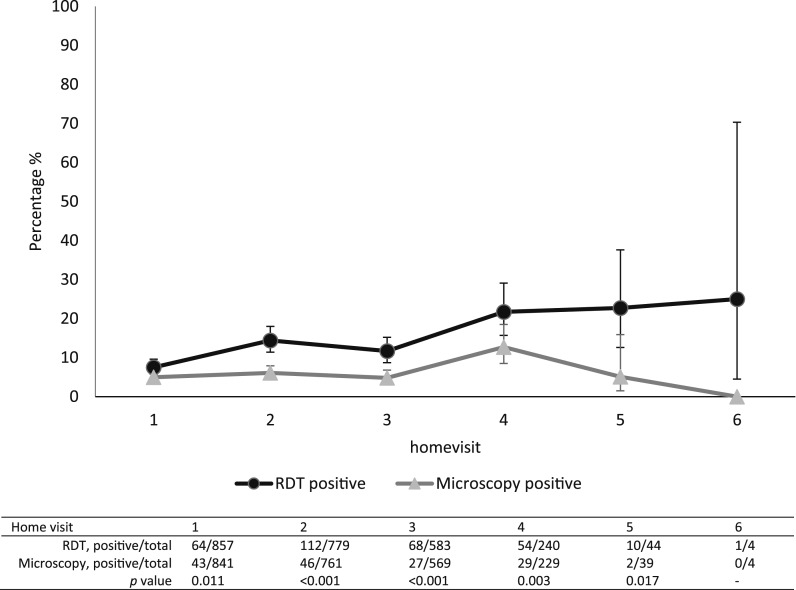
Pregnant women were enrolled over a period of 18 months; including follow-up the study lasted approximately 2 years in Burkina Faso (March 2014–January 2016). A total of 900 women were recruited in 15 villages allocated to the intervention arm; of 861 women at least one home visit was recorded (Figure 1). The mean age of the women was 26 years old (standard deviation 6.3), with 10.5% of the women aged 18 or below; 19.2% (165/861) of women were primigravidae and 14.9% (128/861) secundigravidae. The modal number of visits per pregnancy was three (40.0%) though some women had up to six visits (Table 1). In total, 2,516 home visits were done. There were 2,507 recorded RDT results, with 307 (12.2%) positive tests; 242 women tested positive at least once (up to a maximum of four times) (Figure 1). Of all microscopy slides, 147 of 2443 (6.0%) were positive. Over subsequent home visits, the proportion of positive RDTs was consistently higher than that of positive microscopy (P values 0.011; < 0.001; < 0.001; 0.003; and 0.017 for home visits 1–5, respectively) (Figure 2).
Figure 1.
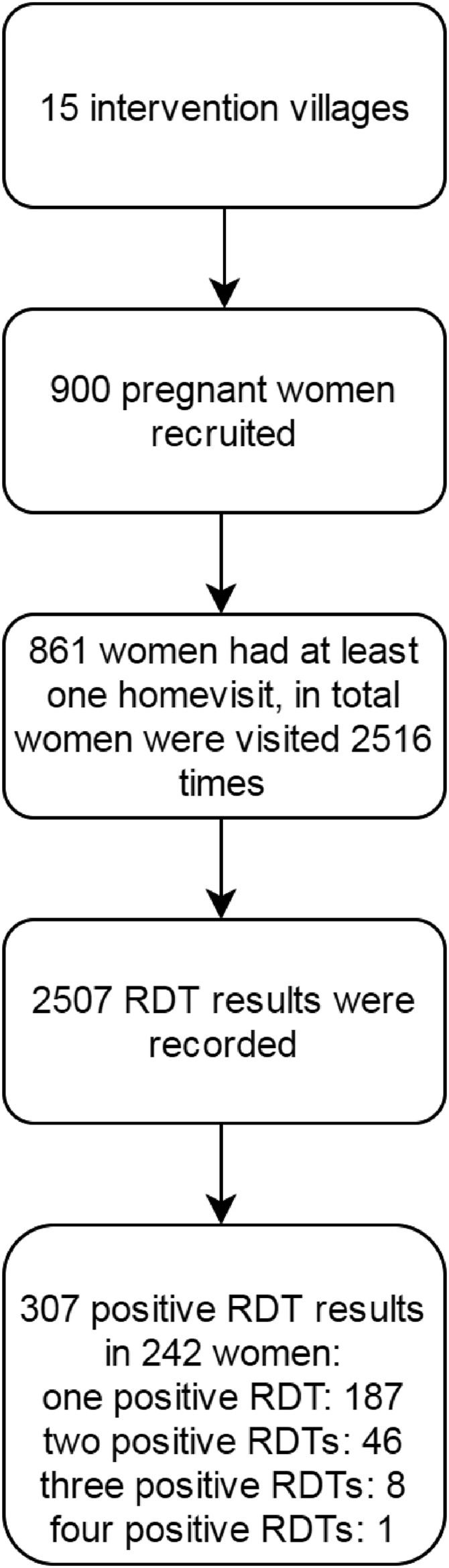
Flow chart of rapid diagnostic tests (RDTs) performed during home visits in intervention arm (COSMIC).
Table 1.
Characteristics of pregnant women with at least one home visit
| Participant characteristics (N = 861) | |
|---|---|
| Age, mean ± SD (median, IQR) | 26 ± 6.3 (26, 21–30) |
| Gravidity | |
| Primigravidae, % (no.) | 19.2 (165) |
| Secundigravidae, % (no.) | 14.9 (128) |
| Multigravidae, % (no.) | 66.0 (568) |
| No. of home visits per woman, % (no.) | |
| 1 | 9.3 (80) |
| 2 | 22.8 (196) |
| 3 | 40.0 (344) |
| 4 | 22.9 (197) |
| 5 | 4.6 (40) |
| 6 | 0.5 (4) |
IQR = interquartile range; SD = standard deviation.
Figure 2.
Plasmodium falciparum positive tests (rapid diagnostic test and microscopy) stratified by home visit (% and 95% confidence intervals).
RDT versus microscopy.
Using microscopy as the reference test, RDT sensitivity was 81.5% (95% confidence interval [CI] 67.9–90.2) and specificity 92.1% (95% CI 89.9–93.9); PPV was 39.8% (95% CI 33.0–47.0) and NPV was 98.7% (95% CI 97.6–99.3) (Table 2). When stratified by home visit (Table 3), sensitivity ranged between 74.1% and 87.0% without a clear trend over successive home visits (P = 0.115). However, specificity decreased over successive home visits (point estimates decreased from 96.4 to 87.4%, P < 0.001). Consequently, the PPV differed significantly between home visits (P < 0.001); a decrease in PPV was seen over successive home visits, except at home visit 4 due to higher malaria prevalence by microscopy. The NPV remained high over successive home visits without significant differences (P = 0.086).
Table 2.
RDT performance compared with microscopy; sensitivity, specificity, PPV and NPV
| RDT vs. microscopy (N = 2,434) | Microscopy positive | Microscopy negative | Total |
|---|---|---|---|
| RDT positive | 119 | 180 | 299 |
| RDT negative | 27 | 2,108 | 2,135 |
| Total | 146 | 2,288 | |
| Sensitivity % (95% CI) | 81.5 (67.9–90.2) | ||
| Specificity % (95% CI) | 92.1 (89.9–93.9) | ||
| Positive predictive value % (95% CI) | 39.8 (33.0–47.0) | ||
| Negative predictive value % (95% CI) | 98.7 (97.6–99.3) |
CI = confidence interval; NPV = positive predictive value; PPV = positive predictive value; RDT = rapid diagnostic test.
Table 3.
RDT sensitivity and specificity compared with microscopy and real-time PCR, stratified for home visits 1–4
| Home visit 1 | Home visit 2 | Home visit 3 | Home visit 4 | P value | |
|---|---|---|---|---|---|
| RDT vs. microscopy, n | 837 | 759 | 567 | 228 | |
| Sensitivity (95% CI) | 78.6 (62.6–88.9) | 87.0 (71.9–94.6) | 74.1 (48.1–89.8) | 82.8 (55.3–94.9) | 0.115 |
| Specificity (95% CI) | 96.4 (94.4–97.7) | 90.3 (86.9–92.9) | 91.1 (87.8–93.6) | 87.4 (80.6–92.1) | 0.000 |
| Positive predictive value % (95% CI) | 53.2 (39.2–66.7) | 36.7 (28.0–46.3) | 29.4 (20.4–40.3) | 49.0 (34.2–64.0) | 0.000 |
| Negative predictive value % (95% CI) | 98.8 (97.7–99.4) | 99.0 (97.9–99.6) | 98.6 (96.5–99.5) | 97.2 (91.3–99.1) | 0.086 |
| RDT vs. qPCR, n | 621 | NA | NA | NA | |
| Sensitivity (95% CI) | 75.7 (66.2–83.2) | NA | NA | NA | NA |
| Specificity (95% CI) | 96.6 (94.2–98.0) | NA | NA | NA | NA |
| Positive predictive value % (95% CI) | 58.3 (42.3–72.8) | NA | NA | NA | NA |
| Negative predictive value % (95% CI) | 98.4 (97.5–99.0) | NA | NA | NA | NA |
CI = confidence interval; qPCR = quantitative polymerase chain reaction. Home visits 5 and 6 not presented because of small sample sizes (N = 39 and N = 4, respectively).
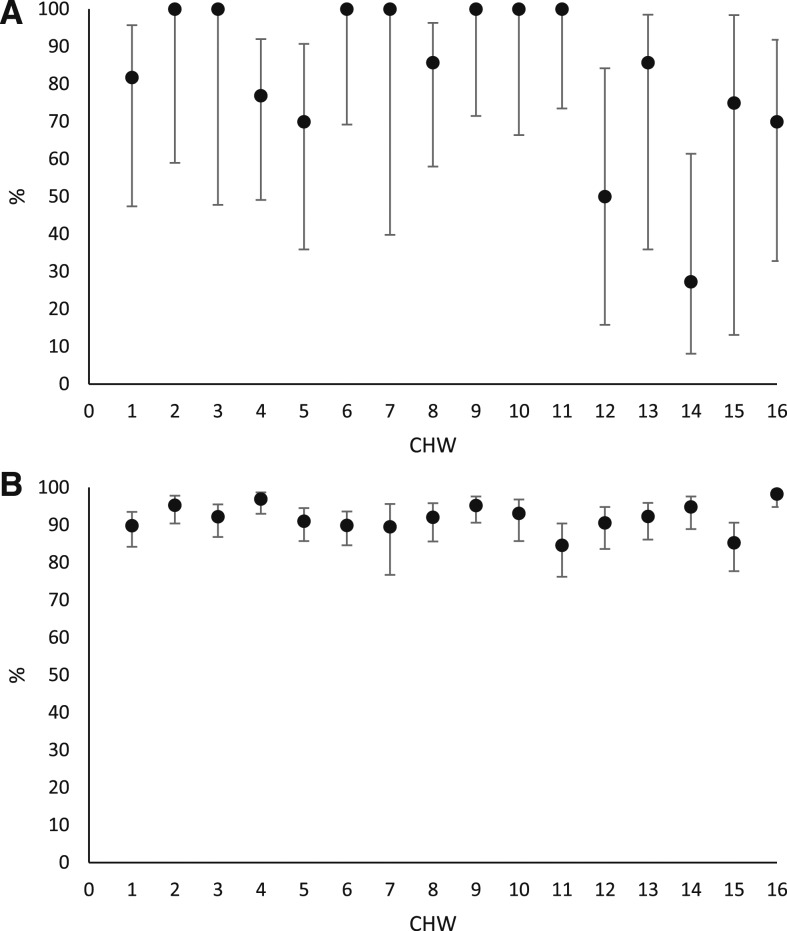
Individual differences between CHWs in RDT sensitivity and specificity are presented in Figure 3A and B. Sensitivity ranged from 27.3% up to 100% and specificity from 84.6% to 98.3%. Variance between CHWs was significant for both sensitivity and specificity with intracluster coefficients of 0.37 (P < 0.001) and 0.05 (P < 0.001), respectively. In particular, two CHWs (numbers 12 and 14) showed poor sensitivity (50.0%, 95% CI 15.8–84.2 and 27.3%, 95% CI 8.1–61.4, respectively). Due to the low overall prevalence the NPV remained high, 95.6% (95% CI 89.1–98.3) and 93.2% (95% CI 86.3–96.7) for CHW 12 and 14, respectively. If the two CHWs were excluded from the analyses, the overall sensitivity increased to 88.2% (95% CI 79.8–93.4), whereas the specificity remained similar.
Figure 3.
(A) Sensitivity of rapid diagnostic test (RDT) vs. microscopy stratified by community health worker (CHW) (% and 95% confidence intervals). (B) Specificity of RDT vs. microscopy stratified by CHW (% and 95% confidence intervals).
Discrepancies between RDT and microscopy results were further explored (Tables 4 and 5). Parasite densities in microscopy positive slides (reference test) were compared between positive (true positives) and negative RDTs (false negatives); for the former, the median parasite density was 2019.3 parasites/μL (interquartile range [IQR] 703.5–4994.0), whereas it was 104 parasites/μL (IQR 72.0–530.5) for the latter (P < 0.001) (Table 4). Almost half of the women (48.9%, 88/180) with RDT positive and microscopy negative results (false positives) had taken antimalarial treatment (artemether-lumefantrine, SP, or quinine) within the 2 weeks before the RDT testing was performed; this was 62.6% (113/180) when considering the previous 4 weeks. When including reported but unproven treatment, the proportions increased to 56.1% (101/180) and 72.8% (131/180), respectively (Table 5).
Table 4.
False-negative RDT results (microscopy as reference test): real-time PCR results and parasite density
| Discrepancy | N | PCR positive | Parasite density* |
|---|---|---|---|
| False-negative RDT (ref microscopy) | 27 (1.1%) | 2/6 (33.3%) | 16 had parasite density < 200 p/μL (59.3%) |
| Median: 104, IQR: 72–530.5 p/μL |
IQR = interquartile range; PCR = polymerase chain reaction; RDT = rapid diagnostic test.
By microscopy.
Table 5.
False positive RDT results (microscopy as reference test): real-time PCR results and recent anti-malarial treatment
| Discrepancy | N | PCR positive (HV1) | AL treatment | IPTp-SP | Any treatment |
|---|---|---|---|---|---|
| False positive RDT (ref microscopy) | 180 (7.4%) | 6/24 (25%) | 50 had received AL in last 14 days (27.8%) | 49 had received SP in last 14 days (27.2%) | 88 had received AL, SP or quinine in last 14 days (48.9%) |
| 72 had received AL in last 28 days (40.0%) | 64 had received SP in last 28 days (35.6%) | 113 had received AL, SP or quinine in last 28 days (62.8%) | |||
| 47 reported being under treatment with AL | 101 reported or received AL, SP or quinine in last 14 days (56.1%) | ||||
| 131 reported or received AL, SP or quinine in last 28 days (72.8%) |
AL = artemether-lumefantrine; HV1 = first home visit; IPTp-SP = Intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine; PCR = polymerase chain reaction; RDT = rapid diagnostic test.
RDT versus real-time PCR.
From first home visit, 628 RDTs were available for analyses. Malaria prevalence by real-time PCR was 6.0% (95% CI 4.3–8.1%). When taking real-time PCR as reference test and after correction for clustering, RDT sensitivity was 75.7% (95% CI 66.2–83.2) and specificity 96.6% (95% CI 94.2–98.0) (Table 3).
Median parasite density (by real-time PCR) was 34.65 parasites/μL (IQR 10.4–111.3) in true-positive RDTs and 1.12 parasites/μL (IQR 0.5–20.6) in false-negative RDTs (P = 0.04) (Table 6). Of women with a false-positive RDT, 40% (8/20) had used antimalarial treatment within the last 4 weeks, when including reported but unproven treatment this increased to 55% (11/20) (Table 7).
Table 6.
False-negative RDT results (real-time PCR as reference test): microscopy results and parasite density
| Discrepancy | N | Microscopy positive | Parasitemia* by qPCR |
|---|---|---|---|
| False-negative RDT (ref real-time PCR) | 9 (1.4%) | 2/8 (25%) | All had parasitemia < 200 p/μL |
| Median: 1.1, IQR: 0.47–20.6 p/μL |
IQR = interquartile range; qPCR = quantitative polymerase chain reaction; RDT = rapid diagnostic test.
By qPCR.
Table 7.
False positive RDT results (real-time PCR as reference test): microscopy results and recent antimalarial treatment
| Discrepancy | N | Microscopy positive | AL treatment | IPTp-SP | Any treatment |
|---|---|---|---|---|---|
| False positive RDT (ref real-time PCR) | 20 (3.2%) | 2/20 (10%) | 4 had received AL in last 14 days (20%) | 4 had received SP in last 14 days (20%) | 8 had received AL or SP in last 14 days (40%) |
| 4 had received AL in last 28 days (20%) | 4 had received SP in last 28 days (20%) | 8 had received AL or SP in last 28 days (40%) | |||
| 6 reported being under treatment with AL | 11 reported or received AL, SP or quinine in last 14 days (55%) |
AL = artemether-lumefantrine; IPTp-SP = intermittent preventive treatment with sulfadoxine-pyrimethamine; PCR = polymerase chain reaction; RDT = rapid diagnostic test.
Adherence to test results by CHWs and pregnant women.
Of RDT positive women 79.1% (239/302) were treated with artemether–lumefantrine by the CHW. The most common reason for not giving treatment despite a positive RDT was ongoing treatment (77.9%). Furthermore, in four cases the CHW reported that he/she had no artemether–lumefantrine in stock. For the remaining 11 cases, the reason for nonadherence to the treatment protocol is unknown (Table 8). Full adherence to the drug regimen by pregnant women was 96.5% (no antimalaria tablets left after 3 days). Reasons for nonadherence were side-effects (3/7), the woman forgot to take the medication (1/7) or the woman was already under treatment (1/7) (Table 8).
Table 8.
Adherence to treatment guidelines after a positive RDT by CHWs and pregnant women
| RDT positive | AL given | Reasons nonadherence CHW | AL course completed | Reasons nonadherence full AL course |
|---|---|---|---|---|
| N = 307 | 239/302 (79.1%) | 4/46 no AL available | 195/202 (96.5%) | 3/7 medicine made woman feel ill |
| 53/68 already under treatment (77.9%) | 1/7 forgot to take medicine | |||
| 1/7 was already under treatment with AL | ||||
| 2/7 unknown |
AL = artemether-lumefantrine; CHW = community health worker; RDT = rapid diagnostic test.
DISCUSSION
CHW are able to screen pregnant women for malaria with RDTs and treat them adequately if positive. CHWs were able of performing RDTs with a fair sensitivity of 81.5% (95% CI 67.9–90.2) and specificity of 92.1% (95% CI 89.9–93.9). Previous studies on HRP2-based RDTs performed by professional health care showed higher sensitivity (94%, 95% CI 91–96) on average, but lower specificity (81%, 95% CI 71–88) in pregnant women (reviewed in Kattenberg and others).23 These differences can be related to RDT brands used, endemic settings, or skills in execution of RDTs. The latter could also be the cause of CHWs not doing equally well in terms of RDT sensitivity in this study, with two CHWs (12 and 14) performing unsatisfactorily (sensitivities of 50% and 27%, respectively). However, the CHWs were supervised at a regular basis during the study and no failures in RDT execution were reported by the field supervisors. Therefore, it remains unclear if this was a problem of RDT execution, or if there are other reasons. Previous studies have shown that mistakes in RDT execution are often related to the volume of blood and buffer used, the timing of reading, and/or incorrect interpretation of faint bands or invalid results.17
False-negative RDT results may be explained by the detection threshold of the test (around 200 parasites/μL),24,25 as almost 60% of all false negatives had a lower density. The large majority (8/11) of RDT false negatives above the 200 parasites/μL threshold, were missed by the two poorer performing CHWs, again suggesting mistakes in the execution of the tests. However, even if only well performing CHWs would screen pregnant women for malaria, it means that some women with low parasite densities would be left untreated. This is unfortunate since it has been shown that infections with low parasite densities are also related to maternal anemia, low birth weight, and premature births.22 Furthermore, the sensitivity of RDTs was calculated against microscopy of peripheral blood, while both these methods (as well as real-time PCR of peripheral blood) may miss placental infections.23 Therefore, the number of women with a malaria infection but not identified by a RDT is likely higher than presented here.
Most false-positive RDT results can probably be attributed to prolonged antigen circulation after clearance of a P. falciparum infection. Although microscopy detects live parasites that are usually cleared within a few days after treatment, it has been shown that HRP2 antigens can persist in the circulation for at least 4 weeks after treatment in pregnant women.20 This explains the decreasing specificity over successive home visits; it reflects the increased chance of women having experienced a malaria infection from which HRP2 antigens are still circulating. In our study, 72.6% of the women had used or reported use of antimalarial treatment in the 28 days preceding a false-positive RDT. For the remaining false-positive results, it could be that reporting antimalarial treatment was not always accurate, resulting in a recall bias. It could also be that some false-positive results were actually true positives, but missed by microscopy reading. This seems to be the case for some positive RDTs at home visit 1 that were tested negative by microscopy but positive by real-time PCR.
The comparisons of RDT with real-time PCR resulted in a lower sensitivity 75.7% (95% CI 66.2–83.2) than with microscopy as reference test. Given that real-time PCR can detect even lower parasite densities than microscopy (∼20 parasites/mL versus 50–100 parasites/μL, respectively), this is to be expected.21,25 Compared with two other studies in Burkina Faso in which pregnant women were tested at antenatal care visits by professional health-care workers, the sensitivity was high compared with one (sensitivity 55.8%, 95% CI 50.0–62.4),26 but low compared with the other (90.9%, 95% CI 87.5, 93.6).27 However, both used a different test for comparison (nested PCR and not real-time PCR) which could impact on sensitivity; in addition, the latter study used both PCR and microscopy as reference test. The observation that parasite densities were significantly lower for false-negative RDT samples than for true-positive RDT samples in our study, confirmed the idea that the major bottleneck was the detection limit of RDTs.
The specificity of RDTs compared with real-time PCR was high (96.6%, 95% CI 94.2–98.0) and fairly similar to specificity found in the two previous studies in Burkina Faso (99.3%, 95% CI 98.4–99.7 and 94.1%, 95% CI 89.4, 97.1).26,28 Antigen persistence of a cleared infection may again be the cause of the few false-positive RDT results, because in contrast to antigen, DNA from dead Plasmodium parasites seems to be rapidly cleared from the bloodstream.29
Adherence to test results by CHWs was excellent, given the fact that almost 80% of the women were treated after a positive RDT, and that the most common reason for not giving treatment was that the woman was already under treatment or had just finished treatment. This shows that CHWs were well trained in treatment guidelines and unlikely to overtreat the pregnant women. Good adherence to positive test results was shown in previous studies on CHWs.17 Furthermore, the high adherence of pregnant women to the full course of AL shows the high trust in the CHWs and the test results, at least within this trial context.
A limitation of this study was the quality of filter paper samples. Although most CHWs seemed sufficiently trained in performing RDTs, the correct preparation of filter papers turned out to be more difficult. For most blood spots the amount of blood was less than the requested 50 μL for which the extraction and real-time PCR were validated. The lack of sufficient blood may have led to wrong estimations of parasite density and to false-negative real-time PCR results if the parasite density was already low. However, because specificity was high for RDTs compared with real-time PCR as reference test, it is unlikely that the latter was an issue.
Furthermore, it is unclear whether the screen and treat intervention by CHWs would work as well if it was implemented in the regular health-care system, as the current results were obtained during a trial setting in which stock supply was carefully managed and CHWs were in close contact with field supervisors. This is something that should be evaluated after implementation. This study has highlighted the qualities and the issues of screening pregnant women with relatively simple diagnostics for malaria by CHWs. CHWs can perform RDTs with acceptable sensitivity and high specificity and have shown good adherence to treatment guidelines. The biggest area for improvement, before implementing this intervention, would be thorough examination of correct execution of RDTs by all CHWs. Due to the intrinsic limitations of the current RDTs, cases with low parasite densities will nevertheless be missed. A new simple diagnostic point-of-care test, that can detect lower parasite densities and that is preferably less sensitive to antigen persistence, could therefore further improve overall performance. In any case, this study has shown that CHWs can be trained and instructed for innovative purposes, which might present new opportunities for other public health issues.
Supplementary Material
Acknowledgments:
We would like to thank all study participants of the COSMIC trial and the research and field staff of the Unité de Recherche Clinique de Nanoro. Also we would like to thank K. Peeters Grietens and A. Compaore for critically reviewing the manuscript. Furthermore, we would like to thank MR4 for providing us with malaria parasites contributed by ATCC®.
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD, 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 7: 93–104. [DOI] [PubMed] [Google Scholar]
- 2.WHO Global Malaria Programme, 2012. Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP) Available at: http://www.who.int/malaria/publications/atoz/who_iptp_sp_policy_recommendation/en/. Accessed May 1, 2013.
- 3.Eisele TP, Larsen DA, Anglewicz PA, Keating J, Yukich J, Bennett A, Hutchinson P, Steketee RW, 2012. Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect Dis 12: 942–949. [DOI] [PubMed] [Google Scholar]
- 4.Van Eijk AM, Hill J, Larsen DA, Webster J, Steketee RW, Eisele TP, ter Kuile FO, 2013. Coverage of intermittent preventive treatment and insecticide-treated nets for the control of malaria during pregnancy in sub-Saharan Africa: asynthesis and meta-analysis of national survey data, 2009–11. Lancet Infect Dis 13: 1029–1042. [DOI] [PubMed] [Google Scholar]
- 5.Thiam S, Kimotho V, Gatonga P, 2013. Why are IPTp coverage targets so elusive in sub-Saharan Africa? A systematic review of health system barriers. Malar J 12: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grietens KP, Gies S, Coulibaly SO, Ky C, Somda J, Toomer E, Muela Ribera J, D’Alessandro U, 2010. Bottlenecks for high coverage of intermittent preventive treatment in pregnancy: the case of adolescent pregnancies in rural Burkina Faso. PLoS One 5: e12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill J, et al. , 2015. Access and use of interventions to prevent and treat malaria among pregnant women in Kenya and Mali: a qualitative study. PLoS One 10: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidoo I, Roper C, 2011. Drug resistance maps to guide intermittent preventive treatment of malaria in African infants. Parasitology 138: 1469–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geiger C, Compaore G, Coulibaly B, Sie A, Dittmer M, Sanchez C, Lanzer M, Jänisch T, 2014. Substantial increase in mutations in the genes pfdhfr and pfdhps puts sulphadoxine-pyrimethamine-based intermittent preventive treatment for malaria at risk in Burkina Faso. Trop Med Int Health 19: 690–697. [DOI] [PubMed] [Google Scholar]
- 10.Mockenhaupt FP, Bedu-Addo G, Eggelte TA, Hommerich L, Holmberg V, von Oertzen C, Bienzle U, 2008. Rapid increase in the prevalence of sulfadoxine-pyrimethamine resistance among Plasmodium falciparum isolated from pregnant women in Ghana. J Infect Dis 198: 1545–1549. [DOI] [PubMed] [Google Scholar]
- 11.Naidoo I, Roper C, 2013. Mapping “partially resistant”, “fully resistant”, and “super resistant” malaria. Trends Parasitol 29: 505–515. [DOI] [PubMed] [Google Scholar]
- 12.Braun V, Rempis E, Schnack A, Decker S, Rubaihayo J, Tumwesigye NM, Theuring S, Harms G, Busingye P, Mockenhaupt FP, 2015. Lack of effect of intermittent preventive treatment for malaria in pregnancy and intense drug resistance in western Uganda. Malar J 14: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrington WE, Mutabingwa TK, Kabyemela E, Fried M, Duffy PE, 2011. Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance. Clin Infect Dis 53: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai M, et al. , 2016. Impact of sulfadoxine-pyrimethamine resistance on effectiveness of intermittent preventive therapy for malaria in pregnancy at clearing infections and preventing low birth weight. Clin Infect Dis 62: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chico RM, Cano J, Ariti C, Collier TJ, Chandramohan D, Roper C, Greenwood B, 2015. Influence of malaria transmission intensity and the 581G mutation on the efficacy of intermittent preventive treatment in pregnancy: systematic review and meta-analysis. Trop Med Int Health 20: 1621–1633. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization, 2005. The Roll Back Malaria Strategy for Improving Access to Treatment through Home Management of Malaria. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 17.Ruizendaal E, Dierickx S, Peeters Grietens K, Schallig HDFH, Pagnoni F, Mens PF, 2014. Success or failure of critical steps in community case management of malaria with rapid diagnostic tests: a systematic review. Malar J 13: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott S, et al. , 2014. Community-based scheduled screening and treatment of malaria in pregnancy for improved maternal and infant health in The Gambia, Burkina Faso and Benin: study protocol for a randomized controlled trial. Trials 15: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization, 2016. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 20.Kattenberg JH, Tahita CM, Versteeg IA, Tinto H, Traoré-Coulibaly M, Schallig HD, Mens PF, 2012. Antigen persistence of rapid diagnostic tests in pregnant women in Nanoro, Burkina Faso, and the implications for the diagnosis of malaria in pregnancy. Trop Med Int Health 17: 550–557. [DOI] [PubMed] [Google Scholar]
- 21.Hermsen CC, Telgt DS, Linders EH, van de Locht LA, Eling WM, Mensink EJ, Sauerwein RW, 2001. Detection of Plasmodium falciparum malaria parasites in vivo by real-time quantitative PCR. Mol Biochem Parasitol 118: 247–251. [DOI] [PubMed] [Google Scholar]
- 22.Cottrell G, Moussiliou A, Luty AJF, Cot M, Fievet N, Massougbodji A, Deloron P, Tuikue Ndam N, 2015. Submicroscopic Plasmodium falciparum infections are associated with maternal anemia, premature births, and low birth weight. Clin Infect Dis 60: 1481–1488. [DOI] [PubMed] [Google Scholar]
- 23.Kattenberg JH, Ochodo EA, Boer KR, Schallig HD, Mens PF, Leeflang MM, 2011. Systematic review and meta-analysis: rapid diagnostic tests versus placental histology, microscopy and PCR for malaria in pregnant women. Malar J 10: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization, 2012. Malaria Rapid Diagnostic Test Performance: Results of WHO Product Testing of Malaria RDTs: Round 4, Vol 4. Geneva, Switzerland: WHO. [Google Scholar]
- 25.Joanny F, Löhr SJ, Engleitner T, Lell B, Mordmüller B, 2014. Limit of blank and limit of detection of Plasmodium falciparum thick blood smear microscopy in a routine setting in Central Africa. Malar J 13: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyabayinze DJ, et al. , 2016. HRP2 and pLDH-based rapid diagnostic tests, expert microscopy, and PCR for detection of malaria infection during pregnancy and at delivery in areas of varied transmission: a prospective cohort study in Burkina Faso and Uganda. PLoS One 11: e0156954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams JE, et al. , 2016. The performance of a rapid diagnostic test in detecting malaria infection in pregnant women and the impact of missed infections. Clin Infect Dis 62: 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes S, et al. , 2016. Cost effectiveness of intermittent screening followed by treatment versus intermittent preventive treatment during pregnancy in West Africa: analysis and modelling of results from a non-inferiority trial. Malar J 15: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarra W, Snounou G, 1998. Only viable parasites are detected by PCR following clearance of rodent malarial infections by drug treatment or immune responses. Infect Immun 66: 3783–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.