Abstract.
Babesiosis is an emerging tick-borne disease transmitted by the hard tick Ixodes scapularis, which also transmits Lyme disease. Better gradation of prognostic indicators are needed to determine which patients may develop serious complications requiring hospitalization, and to provide early guidance on appropriate therapy. In this study, we evaluated 128 patients with smear or real time polymerase chain reaction-confirmed Babesia microti infections over a period of 16 years. Patients with asplenia or immunocompromising conditions were more likely to have severe infection (P < 0.01), require hospitalization (P < 0.01), or receive prolonged courses of antimicrobials (P < 0.01). Nausea or vomiting (P < 0.01) and diarrhea (P < 0.01) along with hyperbilirubinemia (P < 0.01) were predictive of severe infection, hospitalization, and prolonged antimicrobial therapy. Patients with concurrent Lyme disease were less likely to require hospitalization and had similar severity of disease and length of antibiotic treatment compared with those without Lyme disease.
INTRODUCTION
Babesiosis is an emerging tick-borne disease of global significance transmitted by members of the apicomplexan genus Babesia.1,2 Babesia microti is the most common species producing human disease in the United States, although Babesia divergens, Babesia Duncani, and other species are also responsible.1–3 The primary vector for the parasite is the black-legged tick, Ixodes scapularis, which also transmits Lyme disease, the Ehrlichia muris-like (EML) agent, and Anaplasma phagocytophilum.2,4 Babesia spp. can also be transmitted by blood transfusions or vertically from mother to fetus.2,5,6
The incidence of reported cases of babesiosis has been steadily increasing, possibly from the geographic expansion of I. scapularis.7–9 In the United States, 95% of cases occur in seven states in the northeast and upper Midwest: Connecticut, Massachusetts, Minnesota, New Jersey, New York, Rhode Island, and Wisconsin.2,7 A marked seasonality of babesiosis is observed in the United States; most cases occur during summer, fall, and early winter, when people are active outdoors.2,7
Clinical manifestations of babesiosis include nonspecific symptoms such as fever, fatigue, malaise, weakness, diaphoresis, and myalgias, usually with gradual onset. Though most infections are self-limited or cured with a short course of antimicrobial agents, some patients experience prolonged illness despite therapy and may develop severe hemolytic anemia and thrombocytopenia along with jaundice, elevation of transaminases, acute respiratory distress syndrome (ARDS), or shock and renal failure which may be fatal.2,10–16 The duration of therapy ranges from 7 to 10 days for mild illness to more than 6 weeks for immunocompromised patients.2,15,17 Although prior studies have focused on hospitalized patients and attempted to predict those at risk for development of severe disease, underlying risk factors for hospitalization or prolonged use of antimicrobial therapy have not been described.10,16,18 Better gradation of prognostic indicators is needed to determine which patients may develop serious complications requiring hospitalization, and to provide early guidance on appropriate therapy. In this study we evaluated 128 patients with smear or real time polymerase chain reaction (RT-PCR)-confirmed Babesia infections over a period of 16 years to look for prognostic indicators indicating severe infection, hospitalization, and/or receipt of prolonged antimicrobial therapy.
MATERIALS AND METHODS
Patients with babesiosis were identified by query of the Marshfield Clinic Health System electronic health records (EHRs) for International Classification of Diseases (ICD) 9 and ICD 10 codes for babesiosis and by query of Marshfield Laboratories databases for Babesia spp. positive blood smears or RT-PCR testing results from 1999 to 2015. Both inpatients and outpatients were included. Marshfield Clinic is located in northern Wisconsin, an area endemic for tick-borne pathogens.1,8,9 Patients were included if blood smear and/or the RT-PCR assay was positive for Babesia and clinical information was available in the EHR. Babesia RT-PCR was available as a send-out assay prior to 2012 and became an in-house diagnostic test in 2012. It is specific for B. microti.19 Because positive serologic tests fail to differentiate between active infections and previous exposure, patients with only serologic evidence of babesiosis were not included. Patient records were evaluated for concurrent Lyme disease, anaplasmosis, and EML infection when available data permitted. Lyme disease status was determined using the 2017 Centers for Disease Control and Prevention (CDC) criteria and patients were classified as having suspected, probable, or confirmed infection using the CDC case definitions.20 Blood smear and/or PCR results were used as evidence of concurrent Anaplasma or EML infection.
Demographic, epidemiologic, clinical, and laboratory data were abstracted into a REDCap Electronic Database (Marshfield Clinic, Marshfield, WI).21 Statistical analysis was completed using SAS, version 9.4 (SAS Company, Cary, NC). Outcome measures included hospitalization, severe infection, > 14 days of antimicrobial therapy and mortality. Characterization of severe infection included admission to an intensive care unit, intubation, presence of shock or heart failure, splenic rupture, ARDS, or the need for a red blood cell exchange transfusion. The laboratory defined upper limit of normal range was used as the categorical cutoff for all laboratory variables. Categorical data were compared using χ2 or Fisher’s exact tests. Medians were compared using Wilcoxon rank–sum tests and means were compared using t tests. Significance was defined as a P < 0.05 by two-tailed tests and relative risks (RRs) and 95% confidence intervals (CIs) were determined. Step-wise multivariate logistic regression was completed for all variables found to be significant on univariate analyses.
Research protocols were reviewed and approved by the Marshfield Clinic Research Institute Institutional Review Board.
RESULTS
Epidemiology.
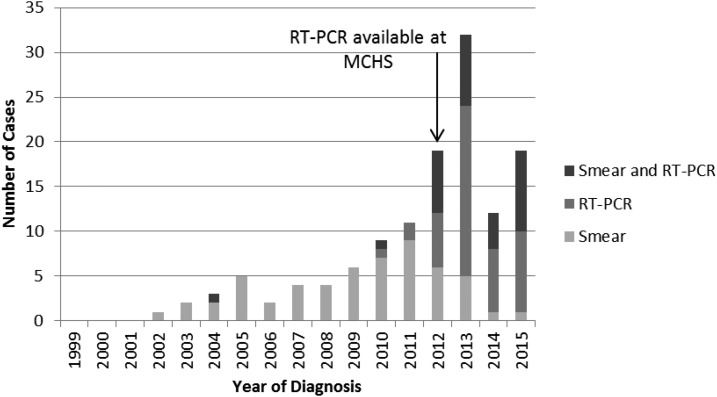
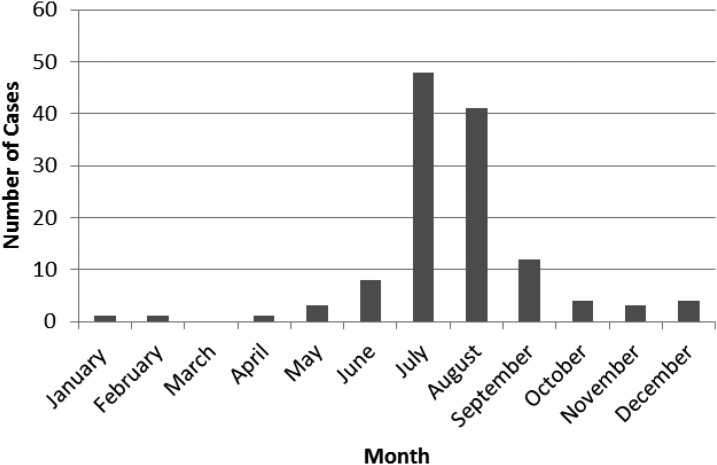
A total of 128 babesiosis cases were diagnosed between 1999 and 2015 (Figure 1). Frequency of babesiosis remained low (less than five cases per year) until 2008. In 2009, cases of babesiosis began to increase sharply to a peak of 32 cases per year in 2013. The majority of cases (80.5%) were diagnosed between July and September (Figure 2).
Figure 1.
Number of babesiosis cases per year from 1999–2015 at Marshfield Clinic Health System (MCHS). * Reverse Transcriptase Polymerase Chain Reaction.
Figure 2.
Number of babesiosis cases by month of diagnosis from 1999–2015.
Clinical presentation.
Among the 128 patients, 66.4% were male, 61.7% were white, 0.8% were African or African American, and 37.5% were documented as “other.” The median age was 64.9 (range: 19.0 to > 89) years. Attachment of a tick was reported by 41.4% of patients. Chronic medical conditions were present in 99 (77.3%) patients including 14 (10.9%) with asplenia and 12 (9.4%) that were immunocompromised. The immunocompromised cohort included six patients on chemotherapy for active cancer, five patients on chronic immunosuppressive medication for autoimmune disorders, and one patient with x-linked agammaglobulinemia. Autoimmune disorder, which ranged from hypothyroidism to systemic lupus erythematosus were present in 11 (8.6%) patients. Cardiac conditions were present in 51 (40%) patients including 17 patients with arrhythmia, 25 patients with coronary artery disease, 11 patients with congestive heart failure or cardiomyopathy, and four patients with valvular disease.
Of patients with B. microti, evidence of additional infections was found in 47 (36.7%) of patients including 38 (29.7%) patients with confirmed or probable Lyme disease, six (4.5%) with anaplasmosis, and three (2.3%) with both evidence of Lyme disease and anaplasmosis. Of patients with Lyme disease five (12%) had confirmed infection and 36 (72%) had probable infection based on CDC case definitions. When comparing clinical and laboratory features of patients with and without concurrent Lyme disease only differences in the presence of rash (36.2% versus 11.1%, P < 0.01) reached statistical significance. RR for hospitalization for coinfected patients was 0.73 (CI: 0.53–0.99, P = 0.03).
Clinical and laboratory findings are summarized in Table 1. The most common presenting features were fatigue and fever, observed in greater than 80% of patients. Myalgia or arthralgia and chills were each present in greater than 60% of patients. Less common symptoms reported were cough or shortness of breath (33.6%), anorexia (32.8%), headaches (31.2%), and night sweats (30.5%). Gastrointestinal symptoms including anorexia (32.8%), nausea and/or vomiting (33.6%), and diarrhea (15.6%) were also reported.
Table 1.
Presenting clinical and laboratory features of patients with babesiosis
| Signs and symptoms | N = 128 n (%) |
|---|---|
| Fatigue | 109 (85.2) |
| Fever* | 105 (82.0) |
| Myalgia or arthralgia | 85 (66.4) |
| Chills | 80 (62.5) |
| Nausea or vomiting | 43 (33.6) |
| Cough or shortness of breath | 43 (33.6) |
| Anorexia | 42(32.8) |
| Headache | 40 (31.2) |
| Night sweats | 39 (30.5) |
| Lightheadedness or dizziness | 38 (29.7) |
| Rash | 26 (20.3) |
| Abdominal pain | 21(16.4) |
| Diarrhea | 20 (15.6) |
| Hepatosplenomegaly | 6 (4.7) |
| Laboratory studies (n)† | Median (Minimum–Maximum) |
| Hemoglobin (g/dL) (113) | 12.2 (3.4–16.2) |
| WBC (×103/μL) (113) | 5.5 (1.6–19.7) |
| Platelet count (×103/μL) (113) | 68 (3–703) |
| CRP (mg/dL) (40) | 10.3 (0.5–45.3) |
| ESR (mm/hour) (20) | 40 (7–117) |
| Procalcitonin (ng/mL) (12) | 1.72 (0.16–46.48) |
| Sodium (mmol/L) (105) | 134 (120–145) |
| Creatinine (mg/dL) (104) | 1.1 (0.58–7.6) |
| ALP (U/L) (95) | 88 (29–344) |
| ALT (U/L) (98) | 45 (12–299) |
| AST (U/L) (101) | 57 (16–702) |
| Total bilirubin (mg/dL) (95) | 1.3 (0.3–18.4) |
| Albumin (g/dL) (96) | 3.1 (1.6–4.6) |
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; WBC = white blood cell.
Documented temperature > 100.4 °F at home or in clinic or fever reported by the physician.
Laboratory studies were not available for all patients.
Hemoglobin (Hb) values of < 12 gm/dL were recorded in 44.2% of patients at diagnosis. Median platelet count at diagnosis was 68 (3–703) × 103/μL with 69.9% of patients having a platelet count less than 100,000 × 103/μL. Inflammatory markers were elevated in most patients, including 92.5% of patients with C-reactive protein (CRP; median 10.24 [0.5–45.3] mg/dL) obtained, 100% of patients with procalcitonin (median 1.72 [0.16–46.48] ng/mL) obtained and 70% of patients with erythrocyte sedimentation rate ( median 40 [7–117] mm/hour) obtained. Median total bilirubin at presentation was 1.3 (0.3–18.4) mg/dL.
Outcomes.
A total of 63.3% patients were hospitalized with a median length of hospitalization of 5.0 (1.0–65.0) days. Of hospitalized patients, 59.3% had a diagnosis of babesiosis made prior to or at the time of admission severe infections occurred in 20.3% of patients and included five (3.9%) with shock, eight (6.3%) with ARDS, and 12 (9.4%) who required an exchange transfusion; no deaths were reported (Table 2). Relapsing disease occurred in 12 (9.3%) patients. Of patients with relapsing disease six were immunocompetent, five had asplenia, and one had x-linked agammaglobulinemia. Administration of antimicrobial agents for more than 14 days occurred in 21.3% of patients and median length of administration was 10 (2–444) days. The most common agents prescribed for babesiosis included azithromycin (87.5%) paired with atovaquone (84.4%), followed by clindamycin (24.2%) paired with quinine (22.7%) with some patients receiving both combinations of medication alternately during their treatment course. Doxycycline was prescribed for 50.8% of patients, usually in combination with atovaquone and azithromycin.
Table 2.
Babesiosis patients with severe infection by complication type
| Patients with severe infection*† | 26 |
| Required ICU care | 15 |
| Intubation | 4 |
| Tracheostomy | 4 |
| Shock | 5 |
| Heart failure | 4 |
| Acute respiratory distress syndrome | 8 |
| Dialysis | 11 |
| Exchange transfusion | 12 |
Characterization of severe infection included admission to an intensive care unit (ICU), intubation, presence of shock or heart failure, splenic rupture, acute respiratory distress syndrome (ARDS), or the need for a red blood cell exchange transfusion.
Some patients had more than one complication.
Risk factors for hospitalization are summarized in Table 3. Age greater than 75 years (RR = 1.44, CI: 1.14–1.82; P = 0.01), underlying cardiac conditions (RR = 1.40, CI: 1.09–1.81; P = 0.01), asplenia (RR = 1.70, CI: 1.46–1.98; P < 0.01), or autoimmune disorders (RR = 1.50, CI: 1.18–1.90; P = 0.05) increased the risk for hospitalization. Clinical features associated with increased risk for hospitalization included: nausea or vomiting (RR = 1.50, CI: 1.18–1.92, P < 0.01), diarrhea (RR = 1.65, CI: 1.38–2.00; P < 0.01), lightheadedness or dizziness (RR = 1.63, CI: 1.29–2.05; P < 0.01), and fatigue (RR = 1.84, CI: 1.01–3.37; P < 0.01). After adjusting for covariates diarrhea (odds ratio [OR] = 34.42, CI: 1.30–912.52; P = 0.03) and lightheadedness (OR = 8.08, CI: 1.67–39.28; P < 0.01) remained significant predictors of hospitalization.
Table 3.
Risk factors for hospitalization in patients with babesiosis
| (N = 128) | Hospitalization | |||
|---|---|---|---|---|
| Univariate analyses | ||||
| Yes (81) | No (47) | RR (95% CI) | P value | |
| Age > 75 years | 25 | 5 | 1.44 (1.141.82) | 0.01 |
| Male | 54 | 31 | 0.99 (0.75–1.31) | 0.93 |
| Comorbidities | 70 | 29 | 0.54 (0.33–0.87) | < 0.01 |
| Cardiac | 39 | 12 | 1.40 (1.09–1.81) | 0.01 |
| Asplenia | 14 | 0 | 1.70 (1.46–1.98) | < 0.01 |
| Autoimmune | 10 | 1 | 1.50 (1.18–1.90) | 0.05 |
| Any Coinfection | 24 | 23 | 0.73 (0.53–0.99) | 0.03 |
| Signs and symptoms | ||||
| Fever | 73 | 32 | 0.50 (0.28–0.89) | < 0.01 |
| Fatigue | 74 | 35 | 1.84 (1.01–3.37) | 0.01 |
| Chills | 56 | 24 | 1.34 (0.99–1.83) | 0.04 |
| Myalgia or arthralgia | 46 | 39 | 0.66 (0.52–0.85) | < 0.01 |
| Nausea or vomiting | 35 | 8 | 1.50 (1.18–1.92) | < 0.01 |
| Lightheaded or dizzy | 33 | 5 | 1.63 (1.29–2.05) | < 0.01 |
| Diarrhea | 19 | 1 | 1.65 (1.38–2.00) | < 0.01 |
| Rash | 11 | 15 | 0.62 (0.39–0.98) | 0.01 |
| History of tick bite | 25 | 28 | 0.63 (0.46–0.86) | < 0.01 |
| Parasitemia > 10% (n) | 8 (28) | 0 (2) | 1.10 (0.96–1.26) | 1.0 |
| WBC × 103/μL (n) | (75) | (38) | ||
| < 5.0 | 32 | 15 | 1.04 (0.80–1.36) | 0.74 |
| > 10.0 | 7 | 1 | 1.35 (1.00–1.82) | 0.19 |
| Hb < 12.0 g/dL (n) | 45 (76) | 9 (37) | 1.59 (1.21–2.08) | < 0.01 |
| Platelets < 150 × 103/μL (n) | 66 (74) | 25 (39) | 1.99 (1.13–3.52) | < 0.01 |
| Na < 135 mmol/L (n) | 50 (73) | 11(32) | 1.57 (1.15–2.13) | < 0.01 |
| Creatinine > 1.2 mg/dL (n) | 23 (72) | 4 (32) | 1.34 (1.06–1.69) | 0.04 |
| ALT > 54 U/L (n) | 2 (69) | 5 (29) | 0.39 (0.12–1.26) | 0.01 |
| AST > 40 U/L (n) | 52 (69) | 19 (32) | 1.29 (0.92–1.82) | 0.10 |
| ALP > 147 units/L (n) | 11 (65) | 7 (30) | 0.87 (0.59–1.29) | 0.57 |
| Total bilirubin > 1.9 mg/dL (n) | 21 (65) | 2 (30) | 1.49 (1.19–1.87) | 0.01 |
| Albumin < 3.4 g/dL (n) | 51 (66) | 11 (30) | 1.86 (1.26–2.77) | < 0.01 |
| CRP > 3.0 mg/dL (n) | 30 (32) | 5 (8) | 2.14 (0.73–6.32) | 0.05 |
| Multivariate analyses | ||||
| OR (95% CI) | P value | |||
| Coinfection | 0.22 (0.06–0.81) | 0.03 | ||
| Diarrhea | 34.42 (1.30–912.52) | 0.03 | ||
| Myalgia or arthralgia | 0.19 (0.04–0.88) | 0.03 | ||
| Lightheaded or dizzy | 8.08 (1.67–39.28) | < 0.01 | ||
| Albumin < 3.4 g/dL | 5.60 (1.54–20.34) | < 0.01 | ||
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CRP = C-reactive protein; CI = confidence interval; Hb = hemoglobin; OR = odds ratio; RR = relative risk; WBC = white blood cell.
Bold values indicate significance.
Coinfection with Lyme disease or anaplasmosis (RR = 0.73, CI: 0.53–0.99; P = 0.03), known tick bite (RR = 0.63, CI: 0.46–0.86; P < 0.01), rash (RR = 0.62, CI: 0.39–0.98; P = 0.01), and fever (RR = 0.50, CI: 0.28–0.89; P < 0.01) reduced risk for hospitalization. Numerous laboratory factors including Hb < 12 gm/dL (RR = 1.59, CI: 1.21–2.08, P = <0.01), platelets < 150 × 103/μL (RR = 1.99, CI: 1.13–3.52; P < 0.01), sodium < 135 mmol/L (RR = 1.57, CI: 1.15–2.13, P < 0.01), creatinine > 1.2 mg/dL (RR = 1.34, CI: 1.06–1.69), total bilirubin > 1.9 mg/dL (RR = 1.49, CI: 1.19–1.87; P = 0.01), albumin < 3.4 g/dL (RR = 1.86, CI: 1.26–2.77, P < 0.01), and CRP > 3.0 μg/dL RR = 2.14, CI: 0.73–6.32; P = 0.05) increased the risk for hospitalization. After multivariate analyses Albumin < 3.4 g/dL (OR = 5.59, CI: 1.54–20.36; P < 0.01) remained a significant predictor of hospitalization and coinfection remained protective against hospitalization (OR = 0.22, CI: 0.062–0.81; P = 0.02).
Risk factors for severe infection are summarized in Table 4. Patients with asplenia (RR = 3.00, CI: 1.54–5.84; P < 0.01) and autoimmune disorders (RR = 2.53, CI: 1.19–5.38; P = 0.04) were more likely to have severe infections. Among clinical features, only nausea or vomiting (RR = 2.70, CI: 1.38–5.35; P < 0.01) and diarrhea (RR = 3.37, CI: 1.80–6.34; P < 0.01) were associated with severe disease. Parasitemia > 10% at diagnosis (RR = 2.14, CI: 1.21–3.77; P = 0.04), white blood cell (WBC) count > 10.0 × 103/μL (RR = 2.14, CI: 1.21–3.77; P = 0.04), creatinine > 1.2 mg/dL (RR = 2.04, CI: 1.03–4.03; P = 0.04), and total bilirubin > 1.9 mg/dL (RR = 4.87, CI: 2.43–9.74; P < 0.01) were predictive of severe infection. After adjusting for covariates total bilirubin > 1.9 mg/dL remained highly predictive of severe infection (OR = 11.39, CI: 3.34–37.73; P < 0.01) and WBC count < 5.0 × 103/μL was highly protective of severe infection (OR = 0.18, CI: 0.04–0.77; P = 0.02).
Table 4.
Risk factors for severe infection in patients with babesiosis
| (N = 128) | Severe infection | |||
|---|---|---|---|---|
| Univariate analyses | ||||
| Yes (26) | No (102) | RR (95% CI) | P value | |
| Age > 75 years | 7 | 23 | 1.67 (0.54–2.50) | 0.69 |
| Male | 18 | 67 | 0.88 (0.42–1.86) | 0.73 |
| Comorbidities | 22 | 77 | 0.62 (0.23–1.66) | 0.32 |
| Cardiac | 11 | 40 | 1.11 (0.55–2.21) | 0.77 |
| Asplenia | 7 | 7 | 3.00 (1.54–5.84) | < 0.01 |
| Autoimmune | 5 | 6 | 2.53 (1.19–5.38) | 0.04 |
| Any Coinfection | 10 | 37 | 1.08 (0.53–2.18) | 0.84 |
| Signs and symptoms | ||||
| Fever | 22 | 83 | 0.83 (0.32–2.18) | 1.0 |
| Fatigue | 26 | 83 | 0.76 (0.69–0.85) | 0.01 |
| Chills | 17 | 63 | 1.13 (0.55–2.34) | 0.73 |
| Myalgia or arthralgia | 14 | 71 | 0.59 (0.30–1.16) | 0.13 |
| Nausea or vomiting | 15 | 28 | 2.70 (1.38–5.35) | < 0.01 |
| Lightheaded or dizzy | 10 | 28 | 1.48 (0.74–2.96) | 0.28 |
| Diarrhea | 10 | 10 | 3.37 (1.80–6.34) | < 0.01 |
| Rash | 6 | 20 | 1.18 (0.53–2.63) | 0.69 |
| History of tick bite | 7 | 46 | 0.52 (0.24–1.15) | 0.99 |
| Length from symptom onset to treatment start > 7 days | 19 | 51 | 2.35 (1.01–5.48) | 0.03 |
| Parasitemia > 10% (n) | 7 | 1 | 2.14 (1.21–3.77) | 0.04 |
| WBC × 103/μL (n) | (24) | (89) | ||
| < 5.0 | 4 | 43 | 0.28 (0.10–0.77) | < 0.01 |
| > 10.0 | 4 | 4 | 2.14 (1.21–3.77) | 0.04 |
| Hb < 12.0 g/dL (n) | 14 (25) | 40 (88) | 1.39 (0.69–2.79) | 0.35 |
| Platelets < 150 × 103/μL (n) | 22 (26) | 69 (87) | 2.02 (0.55–7.48) | 0.29 |
| Na < 135 mmol/L (n) | 18 (24) | 43 (81) | 2.16 (0.94–5.01) | 0.06 |
| Creatinine > 1.2 mg/dL (n) | 10 (24) | 17 (80) | 2.04 (1.03–4.03) | 0.04 |
| ALT > 54 U/L (n) | 1 (23) | 6 (75) | 0.59 (0.09–3.76) | 0.55 |
| AST > 40 U/L (n) | 17 (23) | 54 (78) | 1.20 (0.52–2.74) | 0.67 |
| ALP > 147 units/L (n) | 4 (22) | 14 (73) | 0.95 (0.37–2.47) | 1.0 |
| Total bilirubin > 1.9 mg/dL (n) | 14 (23) | 9 (72) | 4.87 (2.43–9.74) | < 0.01 |
| Albumin < 3.4 g/dL (n) | 18 (23) | 44 (73) | 1.97 (0.80–4.85) | 0.14 |
| CRP > 3.0 mg/dL (n) | 12 (12) | 23 (28) | 0.66 (0.52–0.83) | 0.30 |
| Multivariate analyses | ||||
| OR (95% CI) | P value | |||
| WBC < 5.0 × 103/μL | 0.18 (0.04–0.77) | 0.02 | ||
| Total bilirubin > 1.9 mg/dL | 11.37 (3.43–37.73) | < 0.01 | ||
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CRP = C-reactive protein; CI = confidence interval; Hb = hemoglobin; OR = odds ratio; RR = relative risk; WBC = white blood cell.
Bold values indicate significance.
Risk factors for prolonged antimicrobial use are summarized in Table 5. As with severe infection, asplenia (OR = 6.61, CI: 3.88–11.27; P < 0.01), and autoimmune disorders (OR = 3.36, CI: 1.77–6.39; P < 0.01) were more likely to receive greater than 14 days of therapy, even when correcting for covariates. There were two patients with asplenia that received only 10 days of antimicrobials with no relapses. One of these patients did receive an exchange transfusion early in the treatment course. Patients who presented with nausea or vomiting (RR = 2.13, CI: 1.09–4.15; P = 0.03), diarrhea (RR = 2.41, CI: 1.23–4.73; P = 0.02), WBC count > 10.0 × 103/dL (RR = 3.26, CI: 1.66–6.38; P < 0.01), total bilirubin > 1.9 mg/dL (RR = 3.09, CI: 1.63–5.86; P < 0.01), and albumin < 3.4 g/dL (RR = 2.71, CI: 1.01–7.25; P = 0.04) were more likely to receive > 14 days of antimicrobials. Patients with a known history of tick bite were less likely to receive prolonged antimicrobials (RR = 0.42, CI: 0.18–0.97; P = 0.03).
Table 5.
Risk factors for receiving more than 14 days of antibiotic therapy in patients with babesiosis
| (N = 128) | Length of therapy > 14 days | |||
|---|---|---|---|---|
| Univariate analyses | ||||
| Yes (26) | No (96) | RR (95% CI) | P value | |
| Age > 75 years | 7 | 22 | 1.22 (0.57–2.63) | 0.61 |
| Male | 16 | 67 | 1.33 (0.67–2.66) | 0.42 |
| Comorbidities | 24 | 71 | 0.29 (0.07–1.16) | 0.05 |
| Cardiac | 9 | 38 | 0.84 (0.41–1.74) | 0.64 |
| Asplenia | 12 | 2 | 6.61 (3.88–11.27) | < 0.01 |
| Autoimmune | 6 | 4 | 3.36 (1.77–6.39) | < 0.01 |
| Any coinfection | 11 | 33 | 1.30 (0.66–2.58) | 0.45 |
| Signs and symptoms | ||||
| Fever | 22 | 78 | 0.83 (0.32–2.16) | 0.78 |
| Fatigue | 24 | 80 | 2.08 (0.54–8.04) | 0.36 |
| Chills | 16 | 61 | 0.94 (0.46–1.88) | 0.85 |
| Myalgia or arthralgia | 11 | 71 | 0.36 (0.18–0.71) | < 0.01 |
| Nausea or vomiting | 13 | 26 | 2.13 (1.09–4.15) | 0.03 |
| Lightheaded or dizzy | 9 | 24 | 1.43 (0.71–2.88) | 0.33 |
| Diarrhea | 8 | 11 | 2.41 (1.23–4.73) | 0.02 |
| Rash | 4 | 22 | 0.67 (0.25–1.78) | 0.41 |
| History of tick bite | 6 | 45 | 0.42 (0.18–0.97) | 0.03 |
| Length from symptom onset to treatment start | 16 | 22 | 1.33 (0.64–2.77) | 0.44 |
| Parasitemia > 10% (n) | 6 | 2 | 2.25 (1.09–4.65) | 0.09 |
| WBC × 103/μL (n) | (24) | (83) | ||
| < 5.0 | 4 | 40 | 0.29 (0.11–0.78) | < 0.01 |
| > 10.0 | 5 | 3 | 3.26 (1.66–6.38) | < 0.01 |
| Hb < 12.0 g/dL (n) | 15 (26) | 34 (81) | 1.61 (0.83–3.18) | 0.16 |
| Platelets < 150 × 103/μL (n) | 20 (24) | 66 (83) | 1.29 (0.39–4.27) | 0.68 |
| Na < 135 mmol/L (n) | 15 (24) | 43 (75) | 1.18 (0.57–2.43) | 0.65 |
| Creatinine > 1.2 mg/dL (n) | 9 (24) | 17 (74) | 1.66 (0.83–3.33) | 0.16 |
| ALT > 54 U/L (n) | 1 (24) | 5 (69) | 0.63 (0.10–3.90) | 1.0 |
| AST > 40 U/L (n) | 20 (24) | 46 (72) | 2.27 (0.85–6.07) | 0.08 |
| ALP > 147 units/L (n) | 6 (24) | 10 (66) | 1.54 (0.73–3.26) | 0.35 |
| Total bilirubin > 1.9 mg/dL (n) | 12 (24) | 10 (66) | 3.09 (1.63–5.86) | < 0.01 |
| Albumin < 3.4 g/dL (n) | 20 (24) | 39 (67) | 2.71 (1.01–7.25) | 0.04 |
| CRP > 3.0 mg/dL (n) | 13 (14) | 22 (94) | 1.86 (0.31–11.29) | 0.64 |
| Multivariate analysis | ||||
| OR (95% CI) | P value | |||
| Autoimmune disease | 5.781 (1.10–30.40) | 0.04 | ||
| Asplenia | 40.06 (6.74–238.03) | < 0.01 | ||
| Myalgia or arthralgia | 0.21 (0.06–0.72) | 0.01 | ||
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CRP = C-reactive protein; CI = confidence interval; Hb = hemoglobin; OR = odds ratio; RR = relative risk; WBC = white blood cell.
Bold values indicate significance.
DISCUSSION
Similar to CDC data, the number of cases of babesiosis increased significantly after 2010.22 This may be secondary to the expanding territory of I. scapularis, increased prevalence of B. microti in I. scapularis populations, or due to increased availability, ease, and accuracy of diagnostic testing.4,8,9,23 The frequency reported here is likely an underestimate as patients with serologic testing results only were excluded and many patients with mild disease likely self-recover and do not seek medical attention.2 As would be expected, the majority of cases occurred from July to September. It is important to note that no patients in this cohort had a documented blood transfusion in the 2 years preceding a diagnosis of babesiosis. In most patients (60%) a history of tick bite was lacking and presenting symptoms were nonspecific. A high index of suspicion is therefore required for timely diagnosis and initiation of treatment, particularly since length of time to diagnosis > 7 days was significantly associated with severe infection (RR = 2.35, CI: 1.01–5.48; P = 0.03).
Lyme disease was the most likely coinfection detected in 29.7% of patients. Despite controlling for covariates including length of time to diagnosis, patients with Lyme disease (P = 0.02) were at less risk for hospitalization when compared with patients without Lyme disease. There were no differences in severity of infection or need for prolonged duration of therapy in patients with and without Lyme coinfection. Prior studies based on serologic data as well as murine studies looking at severity of infection with combined babesiosis and Lyme disease have shown either increased severity of disease or no change in severity of disease with concurrent Lyme disease.11,24–26 It is possible that concurrent use of doxycycline has therapeutic benefit, though sufficient power was lacking to demonstrate a benefit of doxycycline in our analysis. Doxycycline has both in vitro and in vivo activity against Babesia gibsoni, though activity against B. microti has only been described in isolated case reports.17,27–29 Provider confidence in the diagnosis may also explain reduced hospitalization rates in this population, though the majority of patients (59.3%) had a diagnosis of babesiosis prior to admission. Further studies are needed to evaluate severity of illness with concurrent Lyme disease, taking into account geographic location and confounding medications.
Although risk for diagnosis of babesiosis and hospitalization increased with age, age itself was not predictive of severe infection or receipt of prolonged antibiotic therapy, contrary to prior studies.16,30,31 Similar to previous findings, asplenia and autoimmune disorders were associated with severe infection, hospitalization, and prolonged duration of therapy.2,10,13,14,16,17 The majority (60%) of patients with asplenia, autoimmune disease, or immunocompromised status received more than 14 days of treatment. It is unclear from these data if patients with these conditions required prolonged therapy for symptomatic disease or were given prolonged therapy merely based on current recommendations. As previously reported, cardiac comorbidities were associated with increased risk for hospitalization, though we were unable to appreciate a similar risk for severe infection or receipt of prolonged antibiotics.16 Nausea, vomiting, and diarrhea were common and significantly increased the risk for hospitalization, severe infection, and receipt of more than 14 days of antibiotic therapy. Although anemia increased the risk of hospitalization, no increased risk for a longer duration of therapy or severe infection was associated with anemia. Total bilirubin of > 1.9 mg/dL was associated with increased risk for all three outcomes, however. Elevation of hepatic transaminases did not predict need for hospitalization, infection severity, or prolonged duration of antimicrobials. Parasitemia > 10% was predictive of severe infection, though differences in the risk of hospitalization or prolonged antimicrobial therapy were not appreciated given the low number of patients with a parasitemia percentage reported. Utilization of parasitemia percentage to predict severity of infection or determine treatment length is likely to become more problematic with the shift in diagnostics from smear to RT-PCR unless quantitative PCR methods are used. It is important to note that though multivariate analyses were completed it was insufficiently powered given the size of the cohort and the univariate analyses may more accurately reflect prognostic indicators.
Similar to other studies of this type, classification of severe infection and necessity of interventions including hospitalization and exchange transfusions along with prescribed treatments relied heavily on physician diagnoses and preferences, though these may not have been consistent among all providers. It was not possible to determine which treatment courses had superior therapeutic benefit. Prior studies have shown good response of azithromycin plus atovaquone with few side effects for nonlife threatening disease; studies evaluating optimal treatment in immunocompromised or severely ill patients are limited however.15,17,32,33 It is currently recommended that severely ill patients receive quinine plus clindamycin, though nearly three quarters of patients develop severe side effects.32,33 Although limited in geographic representation, our study represents one of the only comprehensive examinations of babesiois in the upper midwest and the differences observed here may be related to regional genetic variations in the parasite that may affect virulence expression and clinical presentation of disease.3,34 Importantly, there were no mortalities in this cohort although 10% or greater mortality has been previously described for hospitalized or immunocompromised patients.2,10,16
In summary, providers should maintain a high degree of suspicion for babesiosis in patients with potential exposure in endemic areas, particularly since incidence appears to be increasing and the risk of severe illness increases with a delay to treatment start of more than 7 days. Further studies are needed to evaluate patients with concurrent B. microti and Lyme disease, and to better delineate optimal treatment of patients with asplenia, immunocompromising conditions, or severe disease.
REFERENCES
- 1.Spach DH, Liles WC, Campbell GL, Quick RE, Anderson DE, Jr, Fritsche TR, 1993. Tick-borne diseases in the United States. N Engl J Med 329: 936–947. [DOI] [PubMed] [Google Scholar]
- 2.Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ, 2015. Babesiosis. Infect Dis Clin North Am 29: 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemieux JE, et al. , 2016. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat Microbiol 1: 16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belongia EA, 2002. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis 2: 265–273. [DOI] [PubMed] [Google Scholar]
- 5.Joseph JT, et al. , 2012. Vertical transmission of Babesia microti, United States. Emerg Infect Dis 18: 1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moritz ED, Winton CS, Tonnetti L, Townsend RL, Berardi VP, Hewins ME, Weeks KE, Dodd RY, Stramer SL, 2016. Screening for Babesia microti in the U.S. Blood Supply. N Engl J Med 375: 2236–2245. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention, 2012. Babesiosis surveillance: 18 states, 2011. MMWR Morb Mortal Wkly Rep 61: 505–509. [PubMed] [Google Scholar]
- 8.Eisen RJ, Eisen L, Beard CB, 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol 53: 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mead PS, 2015. Epidemiology of Lyme disease. Infect Dis Clin North Am 29: 187–210. [DOI] [PubMed] [Google Scholar]
- 10.Hatcher JC, Greenberg PD, Antique J, Jimenez-Lucho VE, 2001. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis 32: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 11.Knapp KL, Rice NA, 2015. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res 2015: 587131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwayama DP, Briones RJ, 2008. Spontaneous splenic rupture caused by Babesia microti infection. Clin Infect Dis 46: e92–e95. [DOI] [PubMed] [Google Scholar]
- 13.Meldrum SC, Birkhead GS, White DJ, Benach JL, Morse DL, 1992. Human babesiosis in New York state: an epidemiological description of 136 cases. Clin Infect Dis 15: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 14.Rosner F, Zarrabi MH, Benach JL, Habicht GS, 1984. Babesiosis in splenectomized adults. Review of 22 reported cases. Am J Med 76: 696–701. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez E, Vannier E, Wormser GP, Hu LT, 2016. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA 315: 1767–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White DJ, Talarico J, Chang HG, Birkhead GS, Heimberger T, Morse DL, 1998. Human babesiosis in New York state: review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med 158: 2149–2154. [DOI] [PubMed] [Google Scholar]
- 17.Krause PJ, et al. , 2008. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 46: 370–376. [DOI] [PubMed] [Google Scholar]
- 18.Cunha BA, Raza M, Schmidt A, 2015. Highly elevated serum ferritin levels are a diagnostic marker in babesiosis. Clin Infect Dis 60: 827–829. [DOI] [PubMed] [Google Scholar]
- 19.Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S, 2012. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol 50: 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Diseae Control and Prevention, 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA 274: 937. [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention, 2016. Parasites-Babesiosis Available at: https://www.cdc.gov/parasites/babesiosis/data-statistics/maps/maps.html. Accessed September 1, 2016.
- 23.Hersh MH, Ostfeld RS, McHenry DJ, Tibbetts M, Brunner JL, Killilea ME, LoGiudice K, Schmidt KA, Keesing F, 2014. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS One 9: e99348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman JL, LeVine D, Thill C, Kuhlow C, Benach JL, 2005. Babesia microti and Borrelia burgdorferi follow independent courses of infection in mice. J Infect Dis 192: 1634–1641. [DOI] [PubMed] [Google Scholar]
- 25.Diuk-Wasser MA, Vannier E, Krause PJ, 2016. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol 32: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang TJ, Liang MH, Sangha O, Phillips CB, Lew RA, Wright EA, Berardi V, Fossel AH, Shadick NA, 2000. Coexposure to Borrelia burgdorferi and Babesia microti does not worsen the long-term outcome of lyme disease. Clin Infect Dis 31: 1149–1154. [DOI] [PubMed] [Google Scholar]
- 27.Lin MY, Huang HP, 2010. Use of a doxycycline-enrofloxacin-metronidazole combination with/without diminazene diaceturate to treat naturally occurring canine babesiosis caused by Babesia gibsoni. Acta Vet Scand 52: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuu A, Yamasaki M, Xuan X, Ikadai H, Hikasa Y, 2008. In vitro evaluation of the growth inhibitory activities of 15 drugs against Babesia gibsoni (Aomori strain). Vet Parasitol 157: 1–8. [DOI] [PubMed] [Google Scholar]
- 29.Vercammen F, De Deken R, Maes L, 1996. Prophylactic treatment of experimental canine babesiosis (Babesia canis) with doxycycline. Vet Parasitol 66: 251–255. [DOI] [PubMed] [Google Scholar]
- 30.Vannier E, Borggraefe I, Telford SR, 3rd, Menon S, Brauns T, Spielman A, Gelfand JA, Wortis HH, 2004. Age-associated decline in resistance to Babesia microti is genetically determined. J Infect Dis 189: 1721–1728. [DOI] [PubMed] [Google Scholar]
- 31.Menis M, et al. , 2015. Babesiosis occurrence among the elderly in the United States, as recorded in large Medicare Databases during 2006–2013. PLoS One 10: e0140332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krause PJ, et al. , 2000. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 343: 1454–1458. [DOI] [PubMed] [Google Scholar]
- 33.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB, 2006. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43: 1089–1134. [DOI] [PubMed] [Google Scholar]
- 34.Silva JC, et al. , 2016. Genome-wide diversity and gene expression profiling of Babesia microti isolates identify polymorphic genes that mediate host-pathogen interactions. Sci Rep 6: 35284. [DOI] [PMC free article] [PubMed] [Google Scholar]