Abstract.
Strongyloides stercoralis is widely distributed in the tropics and subtropics. The aim of this study was to determine the prevalence of S. stercoralis and other intestinal parasites and identify the risk factors for infection with S. stercoralis in a rural area of Angola. A cross-sectional study was conducted in school-age children (SAC) in Cubal, Angola. A questionnaire collecting clinical and epidemiological variables was used, and two stool samples were collected. A concentration technique (Ritchie) and a technique for detection of larvae migration (Baermann) were performed. Of 230 SAC, 56.1% were female and the mean age was 9.3 years (SD 2.45). Severe malnutrition, according to body mass index (BMI)-for-age, was observed in 20.4% of the SAC, and anemia was found in 59.6%. Strongyloides stercoralis was observed in 28 of the 230 (12.8%) SAC. Eggs of other helminths were observed in 51 (22.2%) students: Hymenolepis spp. in 27 students (11.7%), hookworm in 14 (6.1%), Schistosoma haematobium in four (1.7%), Enterobius vermicularis in four (1.7%), Ascaris lumbricoides in three (1.3%), Taenia spp. in two (0.9%), and Fasciola hepatica in one (0.4%). Protozoa were observed in 17 (7.4%) students. Detection of S. stercoralis was higher using the Baermann technique versus using formol-ether (11.3 vs. 3%). Overall prevalence of S. stercoralis in the school population of 16 studied schools in the municipal area of Cubal was greater than 10%. This fact must be considered when designing deworming mass campaigns. The use of specific tests in larvae detection is needed to avoid overlooking this parasite.
BACKGROUND
Intestinal parasites affect more than one billion people in the world, mainly impacting impoverished populations without access to adequate water and sanitation.1,2 Strategies to control these infections have been focused in four species: Ascaris lumbricoides, Trichuris trichiura, Ancylostoma duodenale, and Necator americanus. Strongyloides stercoralis, a widely-distributed soil-transmitted helminth (STH) (Central and South America, sub-Saharan Africa, and South and Southeast Asia), has been historically neglected in the epidemiological maps.3,4 It has been estimated that 30–100 million people are currently infected with S. stercoralis, but the real prevalence is probably underestimated.5–9 The main reason for underestimation is poor sensitivity of most diagnostic tests.10–13 Infection is acquired through direct skin contact with contaminated soil and the main risk factors for the infection include walking barefoot and poor sanitation conditions. The principal difference from the rest of the STHs is its ability for autoinfection that allows it to remain in the host for long periods of time. In most cases, chronic infection is asymptomatic, although gastrointestinal and cutaneous manifestations have been documented.14 Some conditions such as the use of immunosuppressive treatment, HTLV-1 infection, and malnutrition may predispose individuals to potentially life-threatening conditions due to a massive invasion by filariform larvae, with mortality rates up to 85%.15–17
The World Health Organization recommends regular treating of 75% of preschool and SAC living in the areas where the prevalence of STH is estimated to be more than 20% with albendazole or mebendazole; however, these drugs do not have significant activity against S. stercoralis in single doses.18 Consequently, there are no public health strategies to control strongyloidiasis.
In Angola, information about intestinal parasites is scarce and there are no data about the prevalence of S. stercoralis. To provide information about the epidemiology and identify the risk factors related to S. stercoralis infection, a cross-sectional study was conducted in 16 primary schools in Cubal, a rural area in South Angola.
METHODS
A cross-sectional study was conducted between January 2015 and May 2015 in the municipality of Cubal.
Study setting and population.
The municipality of Cubal is situated in southwestern Angola. It has an estimated population of 322,000 people, with 151,000 (47%) of children under 15 years of age.19 It has four different districts: Cubal Center, Tumbulo, Yambala, and Capupa. The sample size calculation was based on the estimated prevalence registered in previous studies carried out in Africa.9,20–22 Based on the school-age population and considering a prevalence of 30%, the sample size was estimated for 378 children with an estimation error of 2.5% (CI 95%). The number of children recruited from each district was proportional to the population size of the district. We recruited children of 5–14 years in 16 schools across four districts in Cubal. Schools with the highest number of SAC in each district were chosen. Once in the school, we were given a list of all the enrolled children in alphabetical order. We then chose one in every four consecutively (i.e., the first of the list was chosen and the next three were discarded, the fifth was then chosen and so on).
Field procedures.
A survey was conducted by a local health worker using a standardized questionnaire in both Portuguese and the local language of the children in the presence of their parents or guardians during daily classes. Participation in the study was voluntary and required informed consent forms to be signed by parents or legal guardians. Children who received an antiparasitary drug within the three previous months were excluded. The following information was recorded for each enrolled child: age, gender, school and district, living conditions, personal hygiene, and history of previous diseases and treatments with albendazole/mebendazole or praziquantel within the three previous months. Height and weight was obtained for all children to assess their nutritional status. Weight was recorded using an electronic scale and height was recorded using a rigid height measurement. Nutritional status was assessed and quantified using the latest WHO growth chart (2006) based on the Multicentre Growth Reference Study (MGRS) and by the BMI-for-age and height-for-age. Body mass index was calculated as weight (kg) divided by height squared (m2). As per the WHO classification, a score of −2 to −3 indicates moderate malnutrition and a score of less than −3 indicates severe malnutrition.23 A finger prick blood sample was obtained from each participant. Hemoglobin (Hb) concentration was measured using a HemoCueH photometer (HemoCue 201 + system, HemoCue, Angelholm, Sweden). Anemia was diagnosed when Hb was lower than 11 gr/dL—according to the Hb thresholds set by WHO.24 Two stool samples from two consecutive days were obtained from all children. After the interview, each participant was given an empty, prelabeled plastic container. When possible, a filled stool container was collected the same day. If the participant could not get a sample the same day, the filled stool container was collected the next day. A second empty, prelabeled container was given the next day, and the same method was used. The samples were brought to the laboratory at ambient temperature the same day they were collected.
Laboratory procedures.
Both stool samples were examined with a classic formol-ether concentration (FEC) method and Baermann method. For FEC purposes, a hazelnut-sized stool sample was mixed with 10 mL of formalin. After filtration, 2 mL of ethylic ether was added. The eluent was then centrifuged and the sediment was observed with an optic microscope for detection of both helminth eggs and protozoal cysts detection. To perform the Baermann technique, the rest of the stool sample was mixed with activated charcoal and then placed in a Petri dish over a double layer of paper towel. The sample was then covered with a single layer of paper towel. After incubating for 18 hours, the Petri dish was turned over a strainer placed over a funnel attached to a clamped rubber tube. The funnel was filled with warm water. After an hour, the content of the rubber tube was centrifuged and the sediment observed with an optic microscope for detection of S. stercoralis and hookworm larvae.25 the larvae were differentiated based on their morphology. Each stool sample was examined for a minimum of 20 minutes by two blinded and experienced researchers. If discrepancies between researchers were present, a third laboratory technician examined the sample.
Thirty percent of the slides were randomly selected and re-examined by two qualified technicians from Vall d’Hebron University Hospital Microbiology Department, Spain, for external quality control.
Comparison between techniques.
To determine which technique was better for identifying the presence of S. stercoralis and if the two samples yielded a higher diagnosis of S. stercorarlis infection versus examining only one sample, we only included the children who brought two stool samples. The stool samples where we could not perform both techniques were discarded.
Ethics approval and consent to participate.
The study was approved by the Vall d’Hebron Research Institute Ethics Committee and by the local institutions. All relevant authorities (village chiefs, school teachers, and community leaders) were informed about the purpose and procedures of the study. Written informed consent was obtained from all participants’ parents or guardians, and the children participating provided oral assent. Every student diagnosed with intestinal parasites received appropriate treatment.
Statistical analysis.
Data were analyzed using STATATM version 11.0 (College Station, TX). To assess the prevalence of intestinal parasites and risk factors related with S. stercoralis infection, the analysis was restricted to those children who provided two stool samples and both techniques were performed in both samples. The estimated prevalence of strongyloidiasis was calculated overall and stratified by school. Absolute and relative frequencies were calculated for qualitative variables. For quantitative variables, mean and standard deviation or median and interquartile range were estimated. The normal distribution of quantitative variables was tested through the Shapiro–Wilk test. Differences of normally distributed variables between groups were evaluated using t tests and χ2 tests. For the non-normally distributed variables, the differences were evaluated using Mann–Whitney U test. Demographics, hygiene and sanitation conditions, water source, and district were selected as potential risk factors of S. stercoralis infection. A final model was developed using a multivariate logistic regression analysis including the infection with S. stercoralis as a dependent variable (S. stercoralis infection yes/no as a dichotomous variable). Variables with a significance < 0.2 in the univariate analysis and variables considered to be clinically important were included as independent variables. The results have been described with odds ratios, their 95% confidence intervals (95% CI), and P values. P values < 0.05 were considered statistically significant.
RESULTS
Study sample and compliance.
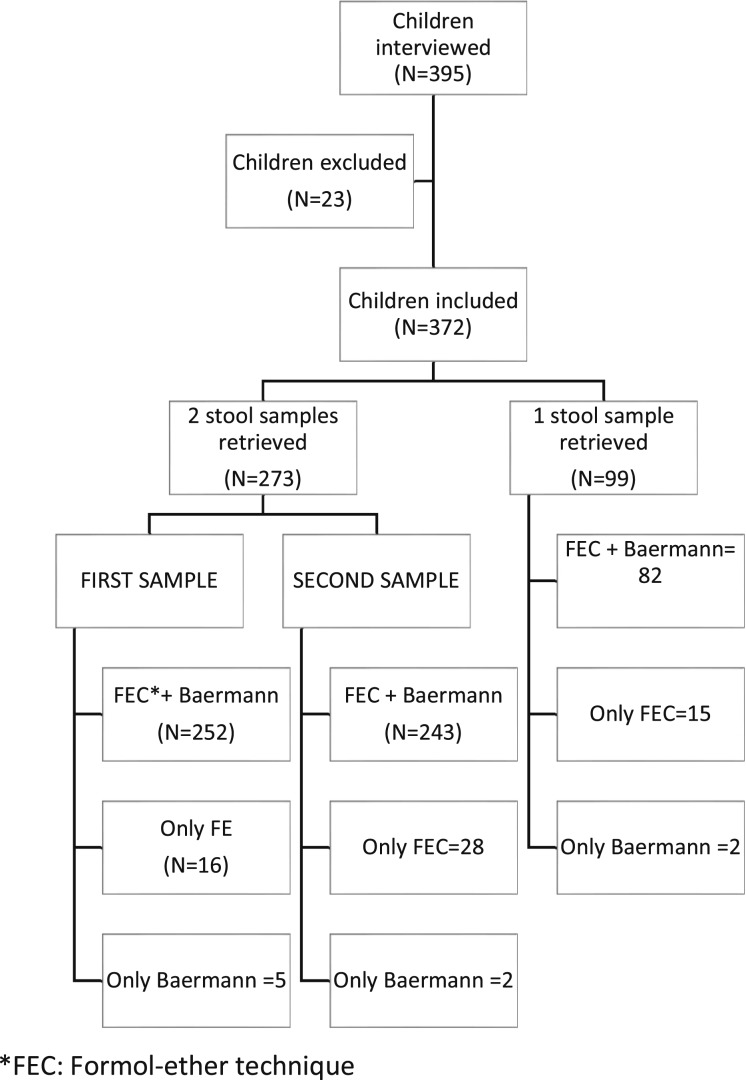
A total of 395 SAC were interviewed. Fifteen were excluded because of having taken antiparasitary drugs during the last three months and eight because of not providing any stool samples. Of 372 students included, 214 (57.5%) were female and mean age was 9.30 years (SD 2.41). A total of 273 students provided two stool samples, and 99 students provided a single stool sample. In some cases, owing to insufficient stool material, either FEC or Baermann techniques were not performed in all the stool samples. Figure 1 shows the distribution of the number of samples provided and the number of laboratory techniques performed in each sample. Epidemiological data, hygiene and sanitation conditions, and clinical information are shown in Table 1.
Figure 1.
Flow chart detailing compliance for stool examination.
Table 1.
Demographics, hygiene conditions, and clinical information of school-aged children.
| Characteristics | N (%) Overall: 372 |
|---|---|
| Demographics | |
| Gender (female) | 129 (56.1) |
| Age, mean (SD)* | 9.3 (2.4) |
| District | |
| Cubal | 85 (37) |
| Yambala | 51 (22.2) |
| Tumbulo | 75 (32.6) |
| Capupa | 19 (8.3) |
| Hygiene-sanitation conditions | |
| Child use to walk barefoot (yes) | 151 (65.7) |
| Latrine at home (yes) | 96 (41.7) |
| Water source | |
| River | 95 (41.3) |
| Well | 94 (40.9) |
| Rain | 4 (1.7) |
| Tap water | 35 (15.2) |
| Nutritional status | |
| BMI-for-age between −2 and −3 Z score | 33 (14.3) |
| BMI-for-age < −3 Z score | 47 (20.4) |
| Height-for-age between −2 and −3 Z score | 37 (16.1) |
| Height-for-age < −3 Z score | 21 (9.1) |
| Anemia (Hb < 11 gr/dL) | 137 (59.6) |
SD = standard deviation; BMI = body mass index; Hb = hemoglobin.
Prevalence of S. stercoralis and other intestinal parasites.
Strongyloides stercoralis was observed in 28 of the 230 (12.2%) SAC. Eggs of other helminths were observed in 51 (22.2%) students, and protozoa were observed in 17 (7.4%) SAC. Twenty-four (6.4%) children were infected with more than one intestinal parasite. Table 2 summarizes the prevalence of intestinal parasites observed.
Table 2.
Prevalence of helminths and protozoa
| Intestinal parasites | N = 230 |
|---|---|
| Intestinal helminths | |
| Strongyloides stercoralis | 28 (12.2%) |
| Hymenolepis spp. | 27 (11.7%) |
| Hookworm | 14 (6.1%) |
| Enterobius vermicularis | 4 (1.7%) |
| Ascaris lumbricoides | 3 (1.3%) |
| Taenia spp | 2 (0.9%) |
| Fasciola hepatica | 1 (0.4%) |
| Protozoa | |
| Giardia lamblia | 12 (5.2%) |
| Balantidium coli | 5 (1.2%) |
| Entamoeba histolytica/dispar | 3 (1.3%) |
The prevalence of S. stercoralis using FEC was 2.6%, whereas the prevalence rose to 11.7% using the Baermann technique. Diagnosis yield increased from 3% to 12.2% after analyzing two consecutive samples. These results are shown in Table 3.
Table 3.
Formol-ether and Baermann technique for the diagnosis of Strongyloides stercoralis
| Formol-ether technique | Baermann technique | Both formol-ether and Baermann techniques | |
|---|---|---|---|
| One sample | 5/230 (2.1%) | 16/230 (6.9%) | 16/230 (6.9%) |
| Two samples | 7/230 (3%) | 26/230 (11.3%) | 28/230 (12.2%) |
Quality control was performed as mentioned in methods section, and we found a 70% match in the results between the local and the external technicians. False positives were observed in 8.7% of the samples, and false negatives were observed in 20.3% of the samples.
Characteristics of SAC infected with S. stercoralis and the risk factors associated with S. stercoralis infection.
When we compared the group of children infected with S. stercoralis with the group of children not infected with S. stercoralis, we observed that the presence of other intestinal parasites was more frequent in SAC infected with S. stercoralis than SAC not infected with S. stercoralis (14.3% vs. 6.4%. P = 0.027). Children without a latrine at home were numerically more likely to be infected with S. stercoralis, but this association was not statistically significant. Characteristics of infected and not infected SAC are summarized in Table 4. We found large variability between schools. We found a prevalence of more than 30% in one school from the Yambala district. In contrast, there were no SAC infected with S. stercoralis in another school from the same district and in two schools from Capupa district.
Table 4.
Characteristics of school-aged children infected and not infected with Strongyloides stercoralis
| Presence of S. stercoralis (N = 28) | No presence of S. stercoralis (N = 202) | P | |
|---|---|---|---|
| Gender (female) | 15 (53.6) | 114 (56.4) | 0.775 |
| Age, mean (SD)* | 9.5 (2.46) | 9.3 (2.45) | 0.637 |
| District | |||
| Cubal | 9 (32.14) | 76 (37.6) | 0.120 |
| Yambala | 11 (39.3) | 40 (19.8) | – |
| Tumbulo | 7 (25) | 68 (33.7) | – |
| Capupa | 1 (3.6) | 18 (8.9) | – |
| Hygiene and sanitation conditions | |||
| Child use to walk barefoot (yes) | 17 (60.7) | 134 (66.3) | 0.557 |
| Latrine at home (yes) | 9 (32.1) | 87 (43.1) | 0.272 |
| Nutritional status | |||
| BMI-for-age < −2 Z score | 8 (33.3) | 72 (35.6) | 0.823 |
| Height-for-age < −2 Z score | 72 (26.9) | 51 (25.2) | 0.854 |
| Anemia (Hb < 11 gr/dL) | 13 (54.2) | 124 (61.7) | 0.475 |
| Recent medical history | |||
| Abdominal pain | 15 (53.6) | 106 (52.7) | 0.934 |
| Nausea/Vomiting | 5 (17.9) | 39 (19.4) | 0.846 |
| Itchy skin | 10 (35.7) | 63 (31.5) | 0.654 |
| Antiparasitary drugs (more than 3 months before) | 3 (10.7) | 33 (16.3) | 0.461 |
| Other intestinal helminths | 13 (46.4) | 53 (26.2) | 0.027 |
SD = standard deviation; Hb = hemoglobin; BMI = body mass index.
In the multivariate regression model, we did not observe any associations with S. stercoralis infection. These findings are summarized in Table 5.
Table 5.
Risk factors for Strongyloides stercoralis infection determined using a multivariate logistic regression model
| Risk factors | OR (95 CI%) | P |
|---|---|---|
| District | ||
| Yambala | 2.45 (0.99–6.05) | 0.053 |
| Hygiene-sanitation conditions | ||
| Child use to walk barefoot (yes) | 0.68 (0.29–1.59) | 0.374 |
| Latrine at home (yes) | 0.76 (0.30–1.96) | 0.572 |
OR = odds ratios.
DISCUSSION
This is the first study to assess the prevalence of S. stercoralis in Angola, and it shows that more than 10% of SAC are infected with this helminth in the 16 studied schools. This prevalence increased to 30% in one of the schools.
Published data from other African countries shows a heterogeneous infection rate. Cote d’Ivoire, Sudan, and Sierra Leona have reported infection rates below 5%;26–28 however, studies from Ghana and Nigeria have reported a prevalence of 17.9% and 35%, respectively.20,29,30 Tanzania and Ethiopia have reported prevalence similar to our study.31–36 A recent study on symptomatic children under 5 years of age in Angola showed a prevalence of S. stercoralis of 3.5%.22 However, a comparison of these results is difficult because of the use of different laboratory techniques and study population characteristics.
There are several risk factors related to S. stercoralis infection, including poor hygiene and sanitation conditions.9 Khieu et al.37 observed that not using shoes or the absence of a latrine increased the incidence of S. stercoralis infection. In our study, none of the poor hygiene conditions previously described were associated with S. stercoralis. This lack of association could be explained because of the method used for data collection and verification. Family members may over-report desirable hygiene conditions which are different from the real household environment. Direct verification methods would have been necessary to avoid this possible bias.
We found large variability between schools, with one school showing a prevalence of more than 30%. We could not identify any differences in demographical data or hygiene conditions between districts or schools, so it is unknown why SAC from some schools had higher rate of infection. However, we did not assess differences in environmental factors between districts and school areas. Previous studies have reported that temperature, rainfall, and altitude are associated with the patterns of intestinal parasite infection. Differences in environmental factors between districts could explain our finding.
Detection of S. stercoralis was higher using the Baermann technique for larvae detection in comparison to FEC (80% of diagnostic yield increase). Most techniques used to diagnose STH based on egg detection have low sensitivity in detecting S. stercoralis. This is one reason for the potential global underestimation of prevalence in other studies. Several studies have shown an increase in the diagnosis of S. stercoralis using Baermann and Harada Mori techniques.38,39 However, low and irregular elimination of S. stercoralis larva in feces makes it difficult to detect in a single stool sample, in comparison with several samples, as we observed in our results.40 Molecular methods primarily based on polymerase chain reaction are also useful in diagnosing S. stercoralis, but we did not consider them owing to their cost and sophistication. Other diagnostic methods such as serological techniques have shown to be an alternative to diagnose S. stercoralis.9,41 However, sensitivity and specificity may differ between the different tests commercialized and the infrastructure required to perform them is more complex.
Other intestinal parasites were detected in our study, but the prevalence was lower than the data from studies performed in other areas of Angola.42 However, our data are similar to a recent study performed in the same area.43 An interesting finding was that the prevalence of Hymenolepis spp. was much higher than the prevalence of other intestinal helminths. This may be because of more extended use of both albendazole and mebendazole in comparison with praziquantel because of lower cost. A recent article from Angola highlights the importance of Hymenolepis spp. in this country, especially in areas with poor hygiene conditions.44
Mass drug administration (MDA) to SAC from areas with a prevalence of STH higher than 20% is the principal WHO strategy to diminish these infections.16,18 However, common drugs used are albendazole or mebendazole and not ivermectin which is the first-line treatment of S. stercoralis. Single-dose albendazole or mebendazole does not demonstrate significant activity against S. stercoralis. A recent review assessed the effects of ivermectin versus benzimidazoles for treating chronic S. stercoralis infection, and showed a higher parasitological cure with ivermectin compared with albendazole.45 Ivermectin has been extensively used in MDA for filarial worms Onchocerca volvulus and Wuchereria bancrofti. Regular ivermectin MDA for this purpose has showed an important impact on the prevalence of S. stercoralis.46 In Cubal, MDA campaigns for the control of lymphatic filariasis, onchocerciasis, soil-transmitted helminthiasis control, or schistosomiasis have not been performed. Findings derived from our study suggest that strategies to control intestinal parasites should be implemented in some areas of Cubal and should include ivermectin in combination with other drugs. Beyond strongyloidiasis, the addition of ivermectin would help control other neglected tropical diseases such as lymphatic filarias, scabies, and soil-transmitted helminthiasis.47 Programs to control intestinal parasite infections should include interventions to promote health and nutrition, facilitate access to safe water, and educate individuals on personal hygiene measures along with MDA campaigns. The observed spatial heterogeneity should help target these campaigns to areas with higher prevalence of intestinal infections.
Both malnutrition and anemia were highly prevalent in the studied population. Studies from different areas of Angola have recently published similar data.37 Although malnutrition and anemia were related to STH infection, an association between S. stercoralis infection and malnutrition was not observed in our study. However, these data provide evidence of a health problem in SAC that should be investigated in future studies.
Our study has several limitations. The main limitation was having a smaller sample size than expected. This was because of a considerable number of children not submitting the two stool samples requested. Children who submitted only one stool sample were excluded with the purpose of obtaining more comparable and robust data. Second, random sampling was not used in data collection, making it possible that our findings do not adequately reflect the prevalence of intestinal parasites in the area. Third, we only used fecal-based methods and not any serological techniques. Although serological tests may overestimate the prevalence of S. Strongyloides because of cross-reactivity with other nematode infections,48 we believe that the combination of fecal and serological tests would increase the detection of S. stercoralis infection. However, the infrastructure required to perform these techniques were not available.
CONCLUSIONS
In conclusion, the prevalence of S. stercoralis is 12.2% in the 16 studied schools, with relevant foci more than 30%. Data from similar studies are necessary to assess the real impact of mass deworming campaigns. The use of a combination of specific tests in larvae detection and analysis of multiple stool samples increases the probability of detection of S. stercoralis and should be used to avoid S. stercoralis misdiagnosis.
Acknowledgments:
We acknowledge the sixth National Plan (PN) of Research + Development + Innovation (I+D+I) 2008-2011, ISCIII-General Division Networks, and Cooperative Research Centers + Collaborative Research Network on Tropical Diseases (RICET):RD12/0018/0020 and RD12/0018/0001. This article is the result of a coordinated effort with local authorities that enabled the study’s success. This collaboration is tremendously important for future interventions aimed at decreasing STH infection and associated morbidity. All authors read and approved the final version of the manuscript.
REFERENCES
- 1.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ, 2014. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L, 2003. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 19: 547–551. [DOI] [PubMed] [Google Scholar]
- 3.Olsen A, , et al. , 2009. Strongyloidiasis—the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg 103: 967–972. [DOI] [PubMed] [Google Scholar]
- 4.Krolewiecki AJ, , et al. , 2013. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Negl Trop Dis 7: e2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genta RM, 1989. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis 11: 755–767. [DOI] [PubMed] [Google Scholar]
- 6.Utzinger J, , et al. , 2012. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly 142: w13727. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen T, Montresor A, Savioli L, 1996. Effectively controlling strongyloidiasis. Parasitol Today 12: 164. [DOI] [PubMed] [Google Scholar]
- 8.Puthiyakunnon S, , et al. , 2014. Strongyloidiasis—an insight into its global prevalence and management. PLoS Negl Trop Dis 8: e3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schär F, , et al. , 2013. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 7: e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisoffi Z, , et al. , 2014. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8: e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knopp S, , et al. , 2014. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg 90: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sultana Y, Jeoffreys N, Watts MR, Gilbert GL, Lee R, 2013. Real-time polymerase chain reaction for detection of Strongyloides stercoralis in stool. Am J Trop Med Hyg 88: 1048–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levenhagen MA, Costa-Cruz JM, 2014. Update on immunologic and molecular diagnosis of human strongyloidiasis. Acta Trop 135: 33–43. [DOI] [PubMed] [Google Scholar]
- 14.Khieu V, Srey S, Schär F, Muth S, Marti H, Odermatt P, 2013. Strongyloides stercoralis is a cause of abdominal pain, diarrhea and urticaria in rural Cambodia. BMC Res Notes 6: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mejia R, Nutman TB, 2012. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis 25: 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keiser PB, Nutman TB, 2004. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev 17: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho EM, Da Fonseca Porto A, 2004. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunol 26: 487–497. [DOI] [PubMed] [Google Scholar]
- 18.WHO , 2006. Preventive Quemotherapy in Human helminthiasis: Coordinated Use of Antihelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 19.Administraçao Municipal do Cubal EdALE , 2009. Perfil do Município do CUBAL, Província de Benguela 2009. [Google Scholar]
- 20.Agi PI, 1997. Comparative helminth infections of man in two rural communities of the Niger Delta, Nigeria. West Afr J Med 16: 232–236. [PubMed] [Google Scholar]
- 21.Becker SL, , et al. , 2015. Real-time PCR for detection of Strongyloides stercoralis in human stool samples from Côte d’Ivoire: diagnostic accuracy, inter-laboratory comparison and patterns of hookworm co-infection. Acta Trop 150: 210–217. [DOI] [PubMed] [Google Scholar]
- 22.Gasparinho C, , et al. , 2016. Etiology of diarrhea in children younger than 5 years attending the Bengo general hospital in Angola. Pediatr Infect Dis J 35: e28–e34. [DOI] [PubMed] [Google Scholar]
- 23.Group WMGRS, 2006. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl 450: 76–85. [DOI] [PubMed] [Google Scholar]
- 24.WHO , 2001. Iron Deficiency Anaemia: Assessment, Prevention and Control. A Guide for Programme Managers. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 25.Garcia LS. Diagnostic Medical Parasitology. Washington, DC: American Society for Microbiology, 1–172.
- 26.Gbakima AA, Sahr F, 1995. Intestinal parasitic infections among rural farming communities in eastern Sierra Leone. Afr J Med Med Sci 24: 195–200. [PubMed] [Google Scholar]
- 27.Yapi YG, Briet OJ, Vounatsou P, 2006. Prevalence of geohelminths in savana and forest areas of Côte d’Ivoire. West Afr J Med 25: 124–125. [DOI] [PubMed] [Google Scholar]
- 28.Magambo JK, Zeyhle E, Wachira TM, 1998. Prevalence of intestinal parasites among children in southern Sudan. East Afr Med J 75: 288–290. [PubMed] [Google Scholar]
- 29.Verweij JJ, , et al. , 2009. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg 103: 342–346. [DOI] [PubMed] [Google Scholar]
- 30.Dada-Adegbola HO, Bakare RA, 2004. Strongyloidiasis in children five years and below. West Afr J Med 23: 194–197. [DOI] [PubMed] [Google Scholar]
- 31.Knopp S, , et al. , 2008. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis 2: e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontanet AL, , et al. , 2000. Epidemiology of infections with intestinal parasites and human immunodeficiency virus (HIV) among sugar-estate residents in Ethiopia. Ann Trop Med Parasitol 94: 269–278. [DOI] [PubMed] [Google Scholar]
- 33.Assefa S, Erko B, Medhin G, Assefa Z, Shimelis T, 2009. Intestinal parasitic infections in relation to HIV/AIDS status, diarrhea and CD4 T-cell count. BMC Infect Dis 9: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knopp S, , et al. , 2009. Changing patterns of soil-transmitted helminthiases in Zanzibar in the context of national helminth control programs. Am J Trop Med Hyg 81: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 35.Getaneh A, Medhin G, Shimelis T, 2010. Cryptosporidium and Strongyloides stercoralis infections among people with and without HIV infection and efficiency of diagnostic methods for Strongyloides in Yirgalem Hospital, southern Ethiopia. BMC Res Notes 3: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai K, Saathoff E, Antelman G, Msamanga G, Fawzi WW, 2009. Geophagy (Soil-eating) in relation to Anemia and Helminth infection among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg 80: 36–43. [PMC free article] [PubMed] [Google Scholar]
- 37.Khieu V, , et al. , 2014. High prevalence and spatial distribution of Strongyloides stercoralis in rural Cambodia. PLoS Negl Trop Dis 8: e2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddiqui AA, Berk SL, 2001. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 33: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 39.Sato Y, Kobayashi J, Toma H, Shiroma Y, 1995. Efficacy of stool examination for detection of Strongyloides infection. Am J Trop Med Hyg 53: 248–250. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen PB, Mojon M, 1987. Improved diagnosis of Strongyloides stercoralis by seven consecutive stool specimens. Zentralbl Bakteriol Mikrobiol Hyg [A] 263: 616–618. [DOI] [PubMed] [Google Scholar]
- 41.Luvira V, , et al. , 2016. Comparative diagnosis of strongyloidiasis in immunocompromised patients. Am J Trop Med Hyg 95: 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sousa-Figueiredo JC, , et al. , 2012. Epidemiology of malaria, schistosomiasis, geohelminths, anemia and malnutrition in the context of a demographic surveillance system in northern Angola. PLoS One 7: e33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bocanegra C, , et al. , 2015. Epidemiology of schistosomiasis and usefulness of indirect diagnostic tests in school-age children in Cubal, Central Angola. PLoS Negl Trop Dis 9: e0004055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soares Magalhães RJ, , et al. , 2013. Extending helminth control beyond STH and schistosomiasis: the case of human hymenolepiasis. PLoS Negl Trop Dis 7: e2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Jr, Terashima A, Samalvides F, Pérez-Molina JA, Plana MN, 2016. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev 1: CD007745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anselmi M, , et al. , 2015. Mass administration of ivermectin for the elimination of onchocerciasis significantly reduced and maintained low the prevalence of Strongyloides stercoralis in Esmeraldas, Ecuador. PLoS Negl Trop Dis 9: e0004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albonico M, , et al. , 2016. StrongNet: an international network to improve diagnostics and access to treatment for strongyloidiasis control. PLoS Negl Trop Dis 10: e0004898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J, 2013. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 7: e2002. [DOI] [PMC free article] [PubMed] [Google Scholar]