Abstract.
Entomological measures of transmission are important metrics specified by the World Health Organization to document the suppression and interruption of transmission of Onchocerca volvulus, the causative agent of onchocerciasis. These metrics require testing of large numbers of vector black flies. Black fly collection has relied on human landing collections, which are inefficient and potentially hazardous. As the focus of the international community has shifted from onchocerciasis control to elimination, replacement of human landing collections has become a priority. The Esperanza window trap (EWT) has shown promise as an alternative method for collection of Simulium damnosum s.l., the primary vector of O. volvulus in Africa. Here, we report the results of a community-based trial of the EWT in northern Uganda. Traps operated by residents were compared with human landing collections in two communities over 5 months. Three traps, when operated by a single village resident, collected over four times as many S. damnosum as did the two-men collection team. No significant differences were noted among the bait formulations. The results suggest that EWTs may be effectively operated by community residents and that the trap represents a viable alternative to human landing collections for entomological surveillance of O. volvulus transmission.
INTRODUCTION
Onchocerca volvulus is the causative agent of onchocerciasis, or river blindness. Historically, onchocerciasis was second only to polio as an infectious disease in terms of its socioeconomic impact on the afflicted communities.1–5 Before the 1980s, there was no safe and effective treatment for onchocerciasis. However, at that time, clinical trials demonstrated that Mectizan®, or ivermectin, was a safe and effective treatment of this scourge.6–8 Early studies also demonstrated that mass treatment of an afflicted population with ivermectin could reduce parasite transmission.9–11 Based on the dramatic effect of ivermectin on O. volvulus, Merck (Kenilworth, NJ), the manufacturer of ivermectin, announced that they would provide the drug free of charge for the treatment of onchocerciasis, “as much as needed for as long as needed.”12 As a result of this generous donation, several large international programs were begun to either control or eliminate onchocerciasis, using a strategy of mass drug administration (MDA) of ivermectin to the afflicted communities. Most notably, these included the African Programme for Onchocerciasis Control in Africa and the Onchocerciasis Elimination Program of the Americas (OEPA). OEPA, using a strategy of semiannual or quarterly distribution of ivermectin with high coverage rates, has succeeded in eliminating onchocerciasis in four of the six formerly endemic countries in Latin America (Colombia, Ecuador, Mexico, and Guatemala).13 Similar success stories have been recently recorded in Africa, including Mali and Senegal,14 Sudan,15 and several foci in Uganda.16–20 These successes resulted in the need to develop standardized metrics that can be used by the international community to verify that onchocerciasis has been eliminated. The World Health Organization (WHO) responded to this need, recently issuing a revised set of guidelines outlining the type of data that must be collected for a country to be verified as having eliminated onchocerciasis.21 These guidelines rely heavily on the use of entomological indicators both for determining that O. volvulus transmission has been interrupted and MDA may cease, and for certifying that transmission has been eliminated, following an intensive evaluation 3–5 years after treatment has been discontinued.21 Large numbers of flies need to be collected and tested to satisfy the guidelines. Pool screen polymerase chain reaction methods have been developed that permit the rapid testing of large numbers of flies for the presence of infective larvae (L3)22,23; however, collecting such a large number of flies remains problematic.
Historically, human landing collectors (HLCs) have been used to collect onchocerciasis vectors for entomological surveillance of transmission.24–26 This procedure has been criticized because of the potential risk of exposure to O. volvulus in the collectors.27 Apart from this potential danger, it can be difficult for the HLCs to capture the large number of flies needed to demonstrate that transmission has been interrupted. Thus, devising a more efficient replacement for human landing collections has become an important goal as the focus of the onchocerciasis community shifts from control to elimination.28 Recently, an alternative to HLCs for the collection of the black fly vectors of O. volvulus, known as the Esperanza window trap (EWT), has been developed.29,30 The EWT consists of an inexpensive platform made from locally available materials.30 It is baited with carbon dioxide produced from a sugar–yeast mixture, as well as baits prepared from components of human sweat shown to be attractive to Simulium damnosum s.l. and Simulium ochraceum s.l., the most important vectors of O. volvulus in Africa and Latin America, respectively. For the EWT to represent an economically feasible alternative to HLCs, it will have to be successfully deployed by minimally trained members of the endemic communities. When operated by trained entomologists, collected numbers of vector flies approximated those collected by an HLC team, when tested both in Burkina Faso and in Mexico.29,30 However, in a test of the ability of community members to operate the EWTs conducted in Mexico, the number of flies collected by the traps was found to be significantly less than when the traps were operated by trained entomologists. Despite this, the traps, when operated by the community members, were effective enough to collect a sufficient number of flies to certify the elimination of transmission in two communities.31 However, the effectiveness of the EWT, when operated by community members, has not been evaluated in Africa. Here, we report the results of a trial to evaluate the effectiveness of the EWT when operated by members of an endemic community in an onchocerciasis focus in northern Uganda.
MATERIALS AND METHODS
Study site.
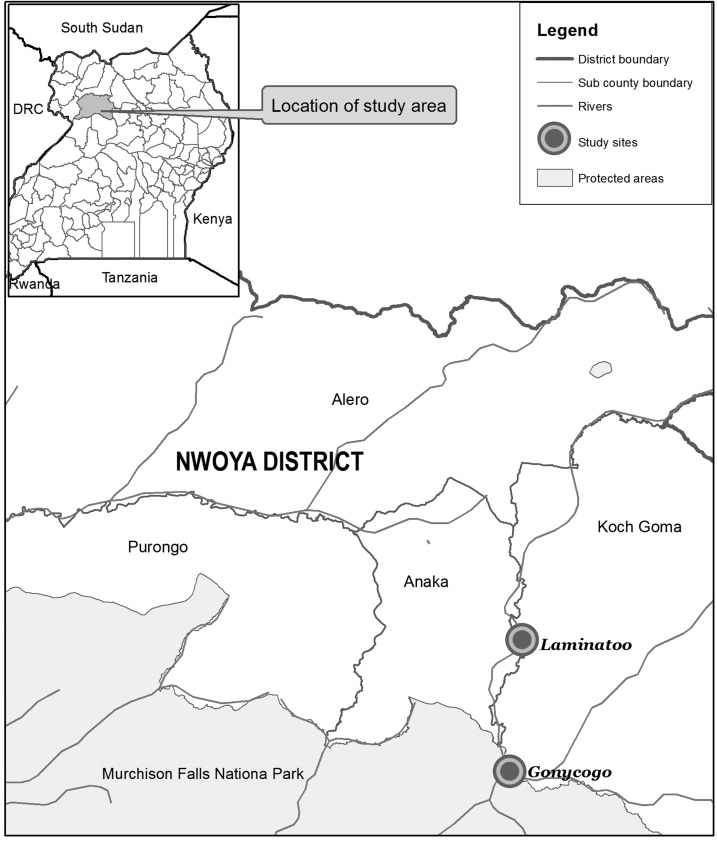
These studies were conducted in the Madi-Mid North focus of onchocerciasis in northwestern Uganda (Figure 1). Two communities, Laminatoo and Gonycogo, located along the Ayago River were included in the study. Both communities are endemic for onchocerciasis and both are located within 3 km of a breeding site for Simulium damnosum s.s., the major vector of O. volvulus in this focus and in the savanna bioclimes of Africa as a whole.32 Breeding sites were identified by use of a remote sensing model as previously described33 and verified both by ground inspection and by consultations with members of the communities. Laminatoo and Gonycogo are separated by a distance of approximately 10 km.
Figure 1.
Location of the communities involved in this study.
Trap construction.
The EWTs consisted of a 1 m2 of a blue plastic tarpaulin affixed to a steel frame. The center third of the trap surface on both sides was painted black with latex paint. Trap surfaces were coated with a thin layer of Tangle-Trap® insect glue (Scotts Miracle-Gro, Marysville, OH), which was renewed as needed. The frame was designed so the legs could be pushed into the ground, holding the trap upright with the bottom of the tarpaulin maintained roughly 5 cm from the ground (Figure 2). All traps were baited with CO2 that was produced by dissolving 500 g of table sugar and 100 g of baker’s yeast in 3 L of water contained in a 4-L plastic jug, as previously described.30 Two jugs were used per trap. CO2 from the jugs was directed to the top of the traps through a plastic tube (Figure 2). In addition to CO2, the individual traps were baited with three different human sweat attractants: 1) sweat-impregnated socks worn by one of the volunteers for 3 days before use; 2) the BG Sweetscents human bait lure (Biogents AG, Regensburg, Germany); and 3) aroma beads saturated with a mixture of human sweat components shown to be attractive to S. damnosum s.l., as previously described.34
Figure 2.
Deployment of the Esperanza window trap (EWT): A typical deployment of the EWT is shown, in a ca. 3-m-diameter clearing prepared in the brush. The arrow highlights the sweat compound bait (in this case aroma beads encased in a nylon stocking). This figure appears in color at www.ajtmh.org.
Selection and training of community volunteers.
Four days before the trials began, team members visited each of the two communities and consulted with the village leadership regarding the work to be undertaken. Once the consent of the leaders was obtained, the population of the village was asked to convene at a central location. The purpose of the study was then explained to the population and volunteers enlisted to help with the study. Two days later, the volunteers from both communities convened at a central school, where the investigators conducted training sessions on the activities to be undertaken (e.g., setup and maintenance of the traps, collection and storage of flies from the trap surfaces, and how to conduct human landing collections). In the following week, the volunteers were supervised by the study personnel while they conducted the first two collections. The volunteers were then permitted to conduct the remaining collections without direct supervision.
Collection procedures.
Collections were conducted twice per week, the interval decided upon by the volunteers themselves as most compatible with their weekly schedules. The study commenced on May 25, 2016 (near the end of the dry season) and continued through October 27, 2016 (the end of the rainy season). The collections were carried out at breeding sites located close to each village. HLCs were conducted by a pair of volunteers working from sunrise to sunset (circa 7 am to 6 pm). Each volunteer alternated collecting with their partner at hourly intervals throughout the day.
A single individual was responsible for operating the traps at each site on each collection day. Three individual EWTs were placed in partially shaded clearings located in the bush surrounding each breeding site. The clearings were approximately 3 m in diameter (Figure 2). Each EWT was located at least 30 m away from the other traps and from the site where the HLCs were done. Each trap was baited with a fresh solution of yeast and sugar in the morning and with one bait (dirty socks, BG Sweetscents lure, or aroma beads saturated with sweat compounds). Each of the three traps at each location was baited with a different sweat bait. Traps were rotated among the three positions daily.
Collections began every morning as soon after sunrise as the traps could be set up and baited, which took about 1 hour. Thus, the traps were active from approximately 8 am to 6 pm every day. At the end of the day, flies were removed from the sticky surface of the trap by solubilizing the glue holding the fly with a small drop of white spirits (odorless mineral spirits). The flies were transferred to a small vial containing white spirits and agitated gently to solubilize the remaining glue. They were then rinsed once in isopropanol to remove the mineral spirits and stored in a vial of isopropanol at room temperature. The traps were covered with a piece of plastic each evening to prevent night flying insects from becoming stuck to the traps during the evening hours. The preserved flies were retained by the volunteers and turned over to the study team for morphological confirmation as S. damnosum s.l.
Statistical analysis.
The data were first analyzed using a frequentist approach, which asked the question, “How often were the number of flies collected by a given trap/bait combination equal to or greater that those collected by the HLC team?” In conducting this first analysis, data from all collection days were included.
Prior to developing models to further analyze the data, the data were analyzed using methods of exploratory data analysis (EDA) to identify collection days that exhibited extreme values relative to those seen on more typical collection days. These values were dropped from the analysis used to construct the models used to predict average daily collections and to relate the trap collections to the HLC data.
The initial analysis of the data with the outliers removed indicated that the data were overdispersed. The data were therefore analyzed for differences among the different baits and the HLCs using the SAS/GENMOD program package (SAS Institute, Cary, NC) and specifying a negative binomial model, applying Tukey’s adjustment for multiple comparisons. The negative binomial was also used to develop a model relating the number of flies collected by the traps to the HLC.
RESULTS
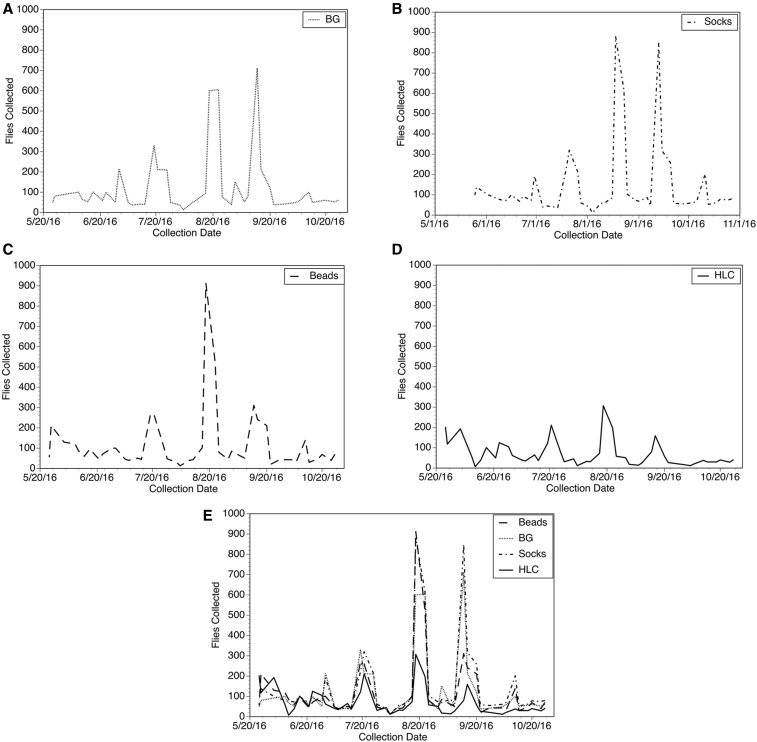
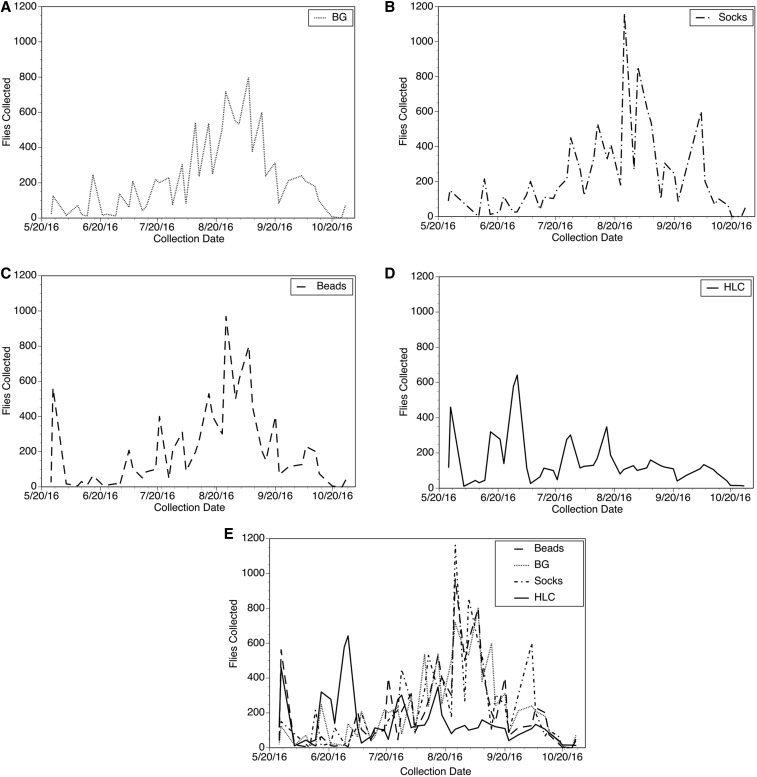
The daily collections from Laminatoo are summarized in Figure 3, whereas those from Gonycogo are summarized in Figure 4. A total of 44 collections (with each collection consisting of three traps and an HLC team) were conducted over the study period in each village. In general, the collection pattern from the traps paralleled those obtained by the HLC team, with high trap collections on days when collections by the HLC team were high. However, this association was stronger in the collections from Laminatoo than in Gonycogo. A frequentist analysis of the collection data is presented in Table 1. This analysis asks the question, “How often did a particular trap collection exceed the collection obtained by the HLC team?” For example, the trap baited with the dirty socks collected more S. damnosum s.l. than the HLC team 77% of the time in Laminatoo and 59% of the time in Gonycogo. The three traps each consistently collected more black flies than the HLC team in both villages. When combined, the three traps together collected more S. damnosum s.l. than the HLC 98% of the time in Laminatoo and 86% of the time in Gonycogo (Table 1).
Figure 3.
Collections from the three traps and human landing collectors in the community of Laminatoo: (A) Daily collections of Simulium damnosum from the trap baited with the BG Sweetscents lure. (B) Daily collections of S. damnosum from the trap baited with the sweat-impregnated socks. (C) Daily collections of S. damnosum from the trap baited with aroma beads. (D) Daily collections of S. damnosum from the HLC team. (E) Collections by all methods overlaid to show concordance among the collections.
Figure 4.
Collections from the three traps and human landing collectors in the community of Gonycogo: (A) Daily collections of Simulium damnosum from the trap baited with the BG Sweetscents lure. (B) Daily collections of S. damnosum from the trap baited with the sweat-impregnated socks. (C) Daily collections of S. damnosum from the trap baited with aroma beads. (D) Daily collections of S. damnosum from the HLC team. (E) Collections by all methods overlaid to show concordance among the collections.
Table 1.
Frequentist analysis of collection data from Laminatoo and Gonycogo
| Trap bait | Laminatoo* | Gonycogo* |
|---|---|---|
| Socks | 34/44 (77) | 26/44 (59) |
| Aroma beads | 33/44 (75) | 25/44 (57) |
| BG lure | 35/44 (79) | 26/44 (59) |
| Combined traps | 43/44 (98) | 38/44 (86) |
Figures represent the number of times over the total number of collections when trap collections exceeded those of the human landing collector team (percentage in brackets.).
The data were then analyzed using a negative binomial model as described in the Materials and Methods section. The estimated average number of flies collected each day for each of the trap methods and the HLC at each of the study sites is presented in Table 2, whereas the probabilities that these values were different from one another are presented in Table 3. As suggested by the frequentist analysis, the trap collections exceeded those obtained by the HLC team, though the differences were statistically significant in only some of the comparisons. In contrast, the analysis suggested that the collections obtained by the different traps baited with the three different baits were not significantly different (Tables 2 and 3).
Table 2.
Estimated average daily collections obtained by different collection methods
| Collection method | Laminatoo | Gonycogo |
|---|---|---|
| Sock trap | 76.23 | 178.48 |
| BG trap | 64.17 | 180.91 |
| Aroma bead trap | 71.24 | 150.37 |
| HLC | 51.82 | 100.20 |
HLC = human landing collector.
Table 3.
Probabilities that the null hypothesis of no difference between trapping methods is correct
| Laminatoo | Gonycogo | ||||||
|---|---|---|---|---|---|---|---|
| Beads | Socks | HLC | Beads | Socks | HLC | ||
| BG | 0.80 | 0.52 | 0.31 | BG | 0.87 | 0.99 | 0.07 |
| Beads | 0.97 | 0.04 | Beads | 0.89 | 0.35 | ||
| Socks | 0.01 | Socks | 0.08 | ||||
Because the analysis indicated that there were no significant differences among the traps baited with the different baits, the data from all traps and the HLCs from both communities were then combined to produce a model relating the trap collections to the HLCs. In developing this model, the extreme observations identified by the EDA were excluded, leaving a total of 68 data points. The final model took the following form:
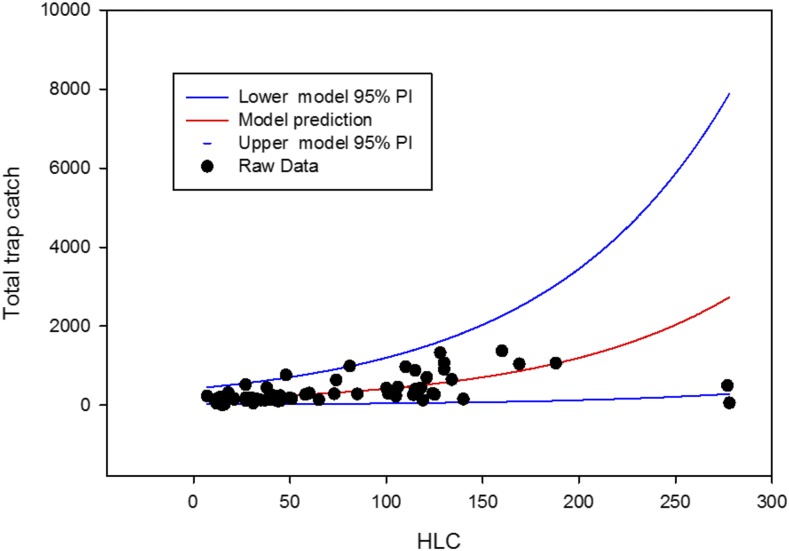
The fitted model (and associated 95% prediction intervals) is presented in Figure 5. All but one of the 68 paired data points were found to fall within the 95% confidence intervals predicted by the model. Based on this model, the estimated overall average daily collection from the HLC teams was 76.117 flies/day, whereas the three traps operated at each site produced an estimated average daily collection of 321.32 flies/day (P < 0.001).
Figure 5.
Performance of model relating human landing collector to sock-baited trap collections. This figure appears in color at www.ajtmh.org.
DISCUSSION
The data presented earlier demonstrate that individual EWTs, when operated by community members, equaled or exceeded the performance of HLC teams working in parallel. Furthermore, although the HLC teams required two individuals to carry out one day’s collection, a single individual was able to easily operate three EWTs. The individual operating the traps collected an average of 4.2 times as many flies per day as did the HLC team. Thus, if the HLC teams were reassigned to trap operation, they should be able collect over eight times as many flies as they would if they were carrying out traditional HLCs. In this regard, it is important to note that the three traps together collected a total of 17,055 vectors in Laminatoo and 27,119 vectors in Gonycogo in 44 collection days. These numbers far exceed the minimum of 6,000 flies per community required to meet the current WHO guidelines for verifying suppression and interruption of transmission.21 Therefore, the data suggest that the EWT will be able to replace HLC teams for entomological surveillance activities in onchocerciasis foci in Uganda, where S. damnosum s.s. is the vector. The traps should prove quite useful to the Ugandan Onchocerciasis Elimination Program in this context. Uganda has historically had 17 isolated foci of onchocerciasis transmission.35,36 In most of these foci, Simulium neavei, a phoretic species of black fly found in East Africa, has been the major vector. It is very susceptible to larvicides and tends to breed in small streams that are easily treated using backpack sprayers.37 Furthermore, the range of S. neavei has been shrinking due to the disappearance of its natural phoretic crab hosts.38 Thus, a combination of ivermectin MDA and larviciding has proven very effective in rapidly interrupting O. volvulus transmission in the majority of S. neavei vector onchocerciasis foci in Uganda. Currently, the last major large focus in which transmission is still ongoing in Uganda is the Madi-Mid North focus, where S. damnosum s.s is the vector, and within which this study was carried out. As the Ugandan Onchocerciasis Elimination Program intensifies its effort to suppress transmission in this focus, the ability to capture large numbers of vectors using the traps should help collect the entomological data necessary to accomplish this goal.
The performance of the EWTs in the community-run trial in Uganda was much better than was previously reported in a similar study of community-directed use of the traps in Mexico.31 In that study, the traps collected roughly 20% of the number of flies obtained by HLC teams working in parallel. There are two possible reasons for the superior performance of the EWT in Uganda when compared with Mexico. First, the EWTs, when operated by trained entomologists in Mexico during their initial development, collected 50–75% of the number of flies obtained by the HLC team.29 In contrast, the EWTs’ performance was not significantly different from that of an HLC team in its initial evaluations in Burkina Faso,30 in an S. damnosum savanna focus similar to the one here. It is therefore possible that the EWT platform is inherently more efficient for the collection of S. damnosum s.s. than S. ochraceum. Second, it is clear from the studies we have conducted that proper placement of the EWT is critical to its success. Movement of the trap even 10 m can result in dramatic differences in trap performance. In the Mexican study, the traps were placed within the communities themselves and in an adjoining coffee finca,31 in an area of heavy bush, which was not cleared as it was here. This reduced the visibility of the traps, perhaps contributing to their relative lack of performance. Given that the performance of the traps is highly dependent on placement, it is likely that if these are to be deployed widely, it will be necessary to conduct preliminary studies with the traps to optimize their placement to ensure that the trap’s performance is as good as possible.
The current WHO guidelines are based on meeting a simple benchmark of demonstrating that the prevalence of flies carrying L3 must be less than the upper bound of a 95% confidence interval of 1/2,000.21 However, a more accurate measure of transmission intensity, and one that is typically used in modeling transmission breakpoints, is the annual transmission potential (ATP), which is the number of L3 a typical individual residing in an endemic area is exposed to in 1 year. The ATP is the product of the prevalence of flies carrying L3, the average number of L3 per positive fly, and the number of bites a resident is exposed to in a year, or annual biting rate (ABR). The ABR is usually calculated from HLC data obtained over the course of the transmission season. As described earlier, it was possible to construct a negative binomial model that can relate trap collections to the HLCs, and thereby to the daily biting rate. It should thus be possible to use this model to indirectly estimate ABRs from the trap collections. These data, when coupled with the prevalence of flies carrying L3, should allow one to estimate ATPs and their associated 95% confidence intervals, data that can be used in many of the models of O. volvulus transmission available today.39–41
This study evaluated three different baits—the commercial BG Sweetscents lure, an aroma bead–based lure containing six compounds that have been shown to be attractive to S. damnosum s.s.,34 and worn sweat-impregnated socks. The results suggested that all three baits were equally effective. This is perhaps not surprising, as the Sweetscents and aroma bead lures contain some compounds in common34,42 and both use compounds that are present in human sweat, the presumed active ingredient in the socks. Given that all three baits produced satisfactory results, it is tempting to recommend the use of sweat-impregnated socks as the preferred bait for the EWT. Dirty socks have the advantage of being locally available, easily produced, inexpensive, and easily renewed. However, both the BG lure and the aroma beads are based on compounds that are universally found in human sweat. It is well known that individuals differ widely in their attractiveness to blood-seeking insects.43,44 It might, therefore, be possible to improve on the existing baits by identifying the compounds that make certain individuals particularly attractive to black flies and incorporating these into a bait formulation.
Finally, it is important to note that the initial evaluations of the EWT in Burkina Faso and the community-based study described here, while being carried out on opposite sides of the African continent, both targeted the same savanna-dwelling species of S. damnosum s.l. Although this is the major vector of O. volvulus throughout most of sub-Saharan Africa, there are at least five other sibling species of S. damnosum s.l. in West Africa that are important vectors of the parasite.32 Similarly, there are several other Simulium species that are competent vectors for O. volvulus in East and Northern Africa.35,45 The performance of the EWT has not been evaluated on any of these species. It will be necessary to carefully evaluate the performance of the EWT in areas endemic for these other vector species before attempting to use the EWT to replace HLCs continent wide.
Acknowledgments:
We sincerely thank the Nwoya District Local government for allowing this study to be conducted in their district and the communities of Laminatoo and Gonycogo for their enthusiastic participation in this project. We also thank Edson Byamukama for assistance with figure preparation and Eddie W. Cupp for critically reading the manuscript.
REFERENCES
- 1.Prost A, 1986. The burden of blindness in adult males in the savanna villages of West Africa exposed to onchocerciasis. Trans R Soc Trop Med Hyg 80: 525–527. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Expert Committee, 1995. Onchocerciasis and Its Control. Report no. 852. Geneva, Switzerland: World Health Organization.
- 3.Murdoch ME, Asuzu MC, Hagan M, Makunde WH, Ngoumou P, Ogbuagu KF, Okello D, Ozoh G, Remme J, 2002. Onchocerciasis: the clinical and epidemiological burden of skin disease in Africa. Ann Trop Med Parasitol 96: 283–296. [DOI] [PubMed] [Google Scholar]
- 4.Mathers CD, Ezzati M, Lopez AD, 2007. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis 1: e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauerbrey M, 2008. The Onchocerciasis Elimination Program for the Americas (OEPA). Ann Trop Med Parasitol 102 (Suppl 1): 25–29. [DOI] [PubMed] [Google Scholar]
- 6.Greene BM, et al. , 1985. Comparison of ivermectin and diethylcarbamazine in the treatment of onchocerciasis. N Engl J Med 313: 133–138. [DOI] [PubMed] [Google Scholar]
- 7.White AT, Newland HS, Taylor HR, Erttmann KD, Keyvan-Larijani E, Nara A, Aziz MA, D’Anna SA, Williams PN, Greene BM, 1987. Controlled trail and dose finding study of ivermectin for the treatment of onchocerciasis. J Infect Dis 156: 463–470. [DOI] [PubMed] [Google Scholar]
- 8.Remme J, Baker RHA, DeSole G, Dadzie KY, Walsh JF, Adams MA, Alley ES, Avissey HSK, 1989. A community trial of ivermectin in the onchocerciasis focus of Asubende, Ghana. I. Effect on the microfilarial reservoir and the transmission of Onchocerca volvulus. Tropenmed Parasitol 40: 367–374. [PubMed] [Google Scholar]
- 9.Cupp EW, Bernardo MJ, Kiszewski AE, Collins RC, Taylor HR, Aziz MA, Greene BM, 1986. The effects of ivermectin on transmission of Onchocerca volvulus. Science 231: 740–742. [DOI] [PubMed] [Google Scholar]
- 10.Cupp EW, Ochoa AO, Collins RC, Ramberg FR, Zea G, 1989. The effect of multiple ivermectin treatments on infection of Simulium ochraceum with Onchocerca volvulus. Am J Trop Med Hyg 40: 501–506. [DOI] [PubMed] [Google Scholar]
- 11.Trpis M, Childs JE, Fryauff DJ, Greene BM, Williams PN, Munoz BE, Pacque MC, Taylor HR, 1990. Effect of mass treatment of a human population with ivermectin on transmission of Onchocerca volvulus by Simulium yahense in Liberia, West Africa. Am J Trop Med Hyg 42: 148–156. [DOI] [PubMed] [Google Scholar]
- 12.Colatrella B, 2008. The Mectizan Donation Program: 20 years of successful collaboration: a retrospective. Ann Trop Med Parasitol 102 (Suppl 1): 7–11. [DOI] [PubMed] [Google Scholar]
- 13.Carter TC, 2015. Onchocerciasis Elimination Program for the Americas. Available at: http://www.cartercenter.org/health/river_blindness/oepa.html. Accessed May 7, 2015.
- 14.Diawara L, et al. , 2009. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLOS Neg Tropl Dis 3: e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarroug IM, et al. , 2016. The first confirmed elimination of an onchocerciasis focus in Africa: Abu Hamed, Sudan. Am J Trop Med Hyg 27: 1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakwo TL, et al. , 2013. The disappearance of onchocerciasis from the Itwara focus, western Uganda after elimination of the vector Simulium neavei and 19 years of annual ivermectin treatments. Acta Trop 126: 218–221. [DOI] [PubMed] [Google Scholar]
- 17.Katabarwa M, et al. , 2014. Transmission of Onchocerca volvulus by Simulium neavei in Mount Elgon focus of eastern Uganda has been interrupted. Am J Trop Med Hyg 90: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakwo TL, et al. , 2015. Successful interruption of the transmission of Onchocerca volvulus in Mpamba-Nkusi focus, Kibaale District, mid-western Uganda. East Afr Med J 92: 401–407. [Google Scholar]
- 19.Katabarwa MN, et al. , 2016. The imaramagambo onchocerciasis focus in southwestern Uganda: interruption of transmission after disappearance of the vector Simulium neavei and its associated freshwater crabs. Am J Trop Med Hyg 95: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakwo T, et al. , 2017. Interruption of the transmission of Onchocerca volvulus in the Kashoya-Kitomi focus, western Uganda by long-term ivermectin treatment and elimination of the vector Simulium neavei by larviciding. Acta Trop 167: 128–136. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization, 2016. Guidelines for Stopping Mass Drug Administration and Verifying Elimination of Human Onchocerciasis: Criteria and Procedures. Document no. WHO/HTM/NTD/PCT/2016.1. Geneva, Switzerland: WHO.
- 22.Katholi CR, Toe L, Merriweather A, Unnasch TR, 1995. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J Infect Dis 172: 1414–1417. [DOI] [PubMed] [Google Scholar]
- 23.Gopal H, Hassan HK, Rodríguez-Pérez MA, Toé LD, Lustigman S, Unnasch TR, 2012. Oligonucleotide based magnetic bead capture of Onchocerca volvulus DNA for PCR pool screening of vector black flies. PLOS Neg Trop Dis 6: e1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh JF, Davies JB, LeBerre R, Garms R, 1978. Standardization of criteria for assessing the effects of Simulium control in onchocerciasis control programmes. Trans R Soc Trop Med Hyg 72: 675–676. [DOI] [PubMed] [Google Scholar]
- 25.Davies JB, Seketeli A, Walsh JF, Barro T, Sawadogo R, 1981. Studies on biting Simulium damnosum s.l. at a breeding site in the Onchocerciasis Control Programme area during and after an interruption of insecticidal treatments. Tropenmed Parasitol 32: 17–24. [PubMed] [Google Scholar]
- 26.Porter CH, Collins RC, 1988. Seasonality of adult black flies and Onchocerca volvulus transmission in Guatemala. Am J Trop Med Hyg 38: 153–167. [DOI] [PubMed] [Google Scholar]
- 27.Jacobi CA, Enyong P, Renz A, 2010. Individual exposure to Simulium bites and intensity of Onchocerca volvulus infection. Parasit Vectors 3: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cupp EW, Sauerbrey M, Richards F, 2011. Elimination of human onchocerciasis: history of progress and current feasibility using ivermectin (Mectizan®) monotherapy. Acta Trop 120 (Suppl 1): S100–S108. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Pérez MA, et al. , 2013. Development of a novel trap for the collection of black flies of the Simulium ochraceum complex. PLoS One 8: e76814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toé LD, et al. , 2014. Optimization of the Esperanza Window Trap for the collection of the African onchocerciasis vector Simulium damnosum sensu lato. Acta Trop 137: 39–43. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Pérez MA, Adeleke MA, Rodríguez-Luna IC, Cupp EW, Unnasch TR, 2014. Evaluation of a community-based trapping program to collect Simulium ochraceum sensu lato for verification of onchocerciasis elimination. PLOS Neg Trop Dis 8: e3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vajime C, Quillevere D, 1978. The distribution of the Simulium damnosum complex in West Africa with particular reference to the onchocerciasis control programme area. Tropenmed Parasitol 29: 473–481. [PubMed] [Google Scholar]
- 33.Jacob BG, Novak RJ, Toe L, Sanfo M, Griffith DA, Lakwo TL, Habomugisha P, Katabarwa MN, Unnasch TR, 2013. Validation of a remote sensing model to identify Simulium damnosum s.l. breeding sites in sub-Saharan Africa. PLOS Neg Trop Dis 7: e2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young RM, et al. , 2015. Identification of human semiochemicals attractive to the major vectors of onchocerciasis. PLOS Neg Trop Dis 9: e3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raybould JN, White GB, 1979. The distribution, bionomics and control of onchocerciasis vectors (Diptera: Simuliidae) in eastern Africa and the Yemen. Tropenmed Parasitol 30: 505–547. [PubMed] [Google Scholar]
- 36.Ndyomugyenyi R, 1998. The burden of onchocerciasis in Uganda. Ann Trop Med Parasitol 92 (Suppl 1): S133–S137. [DOI] [PubMed] [Google Scholar]
- 37.Garms R, Lakwo TL, Ndyomugyenyi R, Kipp W, Rubaale T, Tukesiga E, Katamanywa J, Post RJ, Amazigo UV, 2009. The elimination of the vector Simulium neavei from the Itwara onchocerciasis focus in Uganda by ground larviciding. Acta Trop 111: 203–210. [DOI] [PubMed] [Google Scholar]
- 38.Katabarwa M, et al. , 2012. Transmission of onchocerciasis in Wadelai focus of northwestern Uganda has been interrupted and the disease eliminated. J Parasitol Res 2012: 748540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basanez MG, Collins RC, Porter CH, Little MP, Brandling-Bennett D, 2002. Transmission intensity and the patterns of Onchocerca volvulus infection in human communities. Am J Trop Med Hyg 67: 669–679. [DOI] [PubMed] [Google Scholar]
- 40.Lamberton PH, et al. , 2015. Onchocerciasis transmission in Ghana: persistence under different control strategies and the role of the simuliid vectors. PLoS Negl Trop Dis 9: e0003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basanez MG, Walker M, Turner HC, Coffeng LE, de Vlas SJ, Stolk WA, 2016. River blindness: mathematical models for control and elimination. Adv Parasitol 94: 247–341. [DOI] [PubMed] [Google Scholar]
- 42.AG B, 2016. BG Sweetscent Saftey Data Sheet. Regensburg, Germany: Biogents AG. [Google Scholar]
- 43.Mukabana WR, Takken W, Coe R, Knols BG, 2002. Host-specific cues cause differential attractiveness of Kenyan men to the African malaria vector Anopheles gambiae. Malar J 1: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukabana WR, Takken W, Killeen GF, Knols BG, 2004. Allomonal effect of breath contributes to differential attractiveness of humans to the African malaria vector Anopheles gambiae. Malar J 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higazi TB, Boakye DA, Wilson MD, Mahmoud BM, Baraka OZ, Mukhtar MM, Unnasch TR, 2000. Cytotaxonomic and molecular analysis of Simulium (Edwardsellum) damnosum Theobald sensu lato from Abu Hamed, Sudan. J Med Entomol 37: 547–553. [DOI] [PubMed] [Google Scholar]