Abstract.
Artemisinin-based combination therapies are the frontline treatment of Plasmodium falciparum malaria. The circulation of falsified and substandard artemisinin-based antimalarials in Southeast Asia has been a major predicament for the malaria elimination campaign. To provide an update of this situation, we purchased 153 artemisinin-containing antimalarials, as convenience samples, in private drug stores from different regions of Myanmar. The quality of these drugs in terms of their artemisinin derivative content was tested using specific dipsticks for these artemisinin derivatives, as point-of-care devices. A subset of these samples was further tested by high-performance liquid chromatography (HPLC). This survey identified that > 35% of the collected drugs were oral artesunate and artemether monotherapies. When tested with the dipsticks, all but one sample passed the assays, indicating that the detected artemisinin derivative content corresponded approximately to the labeled contents. However, one artesunate injection sample was found to contain no active ingredient at all by the dipstick assay and subsequent HPLC analysis. The continued circulation of oral monotherapies and the description, for the first time, of falsified parenteral artesunate provides a worrisome picture of the antimalarial drug quality in Myanmar during the malaria elimination phase, a situation that deserves more oversight from regulatory authorities.
INTRODUCTION
The circulation of falsified and substandard artemisinin medicines seriously threatens malaria control and elimination campaigns.1 Poor-quality artemisinin-containing antimalarials (ACAs) might result in increased morbidity and mortality and foster drug resistance development.2,3 Previous surveys conducted in the Greater Mekong Subregion (GMS) showed that high proportions of ACAs were of poor quality, usually falsified.4,5 Two earlier surveys of artesunate samples in five GMS countries found that 38% and 53% did not contain artesunate.5,6 A stratified random survey performed later in Lao PDR identified 88% of the drug outlets selling oral artesunate had falsified artesunate with no drug content.7 Although the situation has since improved greatly,8 a recent survey conducted in Lao PDR in 2012 found that 25.4% of the antimalarials collected were substandard with active pharmaceutical ingredient (API) outside the 90–110% pharmacopeia limits of the label claim.9 In Myanmar, the problem of oral artemisinin monotherapy was far more serious. In recognition of this widespread problem in Myanmar, an oral artemisinin monotherapy replacement project was implemented in eastern Myanmar, which aimed to remove these drugs from the market through price competition and increased availability of quality-assured artemisinin-based combination therapy (ACT).10,11 Despite these efforts, the recent cross-sectional surveys from the ACTwatch project still detected that oral artemisinin monotherapies were available in close to 20% of private-sector drug outlets.12,13 Besides, the quality of the ACAs was generally not tested. Therefore, increased surveillance and coordinated regulatory efforts are urgently needed.
A regional malaria elimination campaign is unfolding in the GMS with an aim to achieve this goal by 2030. Given that ACT is a cornerstone of malaria elimination, World Health Organization (WHO) has urged regulatory authorities in malaria-endemic countries to take measures to halt the production and marketing of these oral artemisinin monotherapies since 2007.14 The recent emergence of resistance in Plasmodium falciparum parasites to the artemisinin family antimalarials in the GMS raised further concerns on the circulation of oral artemisinin monotherapies.15 Within the GMS, Myanmar (Burma) has the heaviest malaria burden.16,17 Earlier surveys of antimalarial drug quality in the GMS also identified Myanmar as having the most serious problem.4,6 Thus, we aimed to assess the quality of ACAs in Myanmar during the malaria elimination phase. To facilitate ACA quality surveys in remote endemic settings, we developed antibody-based lateral-flow dipsticks as point-of-care devices for qualitative and semi-quantitative measurement of artemisinin derivatives (artemether, artesunate, and dihydroartemisinin) in ACAs.18–22
METHODS
Collection of drug samples.
Samples of all commercial ACAs were purchased as convenience samples from private drug stores in Myanmar between March and September 2015 following the MEDQUARG guidelines.23 This effort was a special project with the Myanmar Medical Association, which organized the collection of antimalarial drugs from local general practitioners in 70 malaria-endemic townships. In each township, 1–5 shops were sampled. Although ACAs were mostly in blister packs (except artesunate and artemether injections), chloroquine and primaquine were loose tablets. Only ACA samples were analyzed in this study. Samples were either analyzed on-site within a week of collection using appropriate dipsticks or stored in desiccated containers at 4°C until analysis by high-performance liquid chromatography (HPLC) in October 2015. Our analysis of the samples focused on the claimed API contents of the commercial drugs, whereas no special attention was given to the packaging appearance and dissolution.
Preparation of dipsticks and drug analysis.
The colloidal gold-based lateral-flow dipsticks specific for artesunate, artemether, and dihydroartemisinin were prepared as described.19,21 Standard artesunate, artemether, and dihydroartemisinin were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Stock solutions of these standards were prepared in ethanol at 1 mg/mL. Artemether and dihydroartemisinin standard solutions were used to make final concentrations of 20, 50, 100, 200, 500, 1,000, and 2,000 ng/mL in distilled water. Artesunate standard solution was diluted to 62.5, 125, 250, 500, 1,000, 2,000, and 4,000 ng/mL in distilled water. These standards were used to determine the sensitivity or detection limits of the dipsticks, which are defined as the lowest concentration of the target analyte at which the test line is not visible by visual inspection. Briefly, 80 μL of the standard solution were added dropwise into the sample well of a dipstick. The color of the test and control lines was visually examined within 5–10 minutes.
For on-site analysis of the collected ACA samples, tablets were ground in a clean mortar to fine powder. The drug powder was extracted with 50 mL of tap water and mixed thoroughly. The capsules were diluted to 2 mg/mL based on the labeled API content using ethanol. Based on the labeled API content, the sample extract was diluted to the concentration of the detection limit with tap water and proceeded immediately for the dipstick assay. Each sample was analyzed in triplicates. We used dipsticks for artemether, artesunate, and dihydroartemisinin, which had detection limits of 1,000, 2,000, and 4,000 ng/mL, respectively, corresponding approximately to 0.001–0.01% of an ACA tablet. With the dipsticks, color development was observed within 5–10 minutes after adding the drug samples diluted with tap water to the concentrations of the detection limits of the respective dipsticks based on the labeled drug contents. The control line was used to indicate the validity of the dipsticks and water was included as negative control for each batch of drugs tested. If no band was detected on the test line at concentrations of the detection limits of the dipsticks, it indicated that the ACA concentrations in the stock solutions were above 100% of the labeled API contents and the drugs were considered passed. For those samples where the test line appeared after testing at the drug concentrations corresponding to the detection limits of the dipsticks, they were tested again at higher drug concentrations (lower dilutions). If the test line disappeared at higher drug concentrations, the drugs were considered substandard. If the test line persisted at higher drug concentrations, the drugs were considered falsified.
Quantitation of API by HPLC.
Commercial drug samples were prepared according to an established procedure.24 Injection drugs were transferred quantitatively into a flask and dissolved in 10 mL of ethanol, whereas drug capsules were dissolved directly in ethanol. Drug tablets were ground in a clean mortar to fine powder, and the powdered drugs were dissolved in ethanol. All drug stock solutions were made to 2 mg/mL based on the labeled artemisinin derivative contents. After thorough mixing, dihydroartemisinin stocks were further sonicated for 30 minutes (SB5200, Branson, Shanghai, China). Twenty-eight drugs randomly sampled were analyzed by HPLC according to procedures described previously.25,26 Artemether and artesunate were quantified using a Shimadzu LC-20A HPLC system consisting of two LC-20AT solvent delivery units, an SIL-20A auto sampler, an SPD-M20A photodiode array detector, and a CBM-20Alite system controller (Kyoto, Japan). Dihydroartemisinin was measured using an HPLC system consisting of a Waters 600E multisolvent delivery system and a Waters 2487 dual absorbance detector (Milford, MA), and the data were collected and analyzed by using Waters Millennium 32 software. The mobile phase, standards, and drug extracts were filtered through a 0.5 μm syringe filter before HPLC analysis. A C18 column (250 × 4.6 mm, 5 μm particle size; Thermo, Vantaa, Finland) was used to separate artemether, artesunate, and dihydroartemisinin. The injection volume for artesunate, artemether, and dihydroartemisinin was 20, 20, and 10 μL, respectively. A flow rate of 1 mL/minutes was used with the mobile phase consisting of acetonitrile-water at 60:40, 80:20, and 40:60 (v/v) for artesunate, artemether, and dihydroartemisinin, respectively. Detection wavelength was set at 210 nm. The HPLC standard curves were obtained using standard solutions of the drugs at concentrations of 0, 0.5, 1, 2, and 4 mg/mL, and the detection limits for all drugs were 0.02 mg/mL. The amount of active ingredient determined by HPLC within 85–115% of that stated on the label was considered acceptable based on the U.S. Pharmacopeia Convention (http://www.pharmacopeia.cn/v29240/usp29nf24s0_c905.html).
RESULTS
Description of samples.
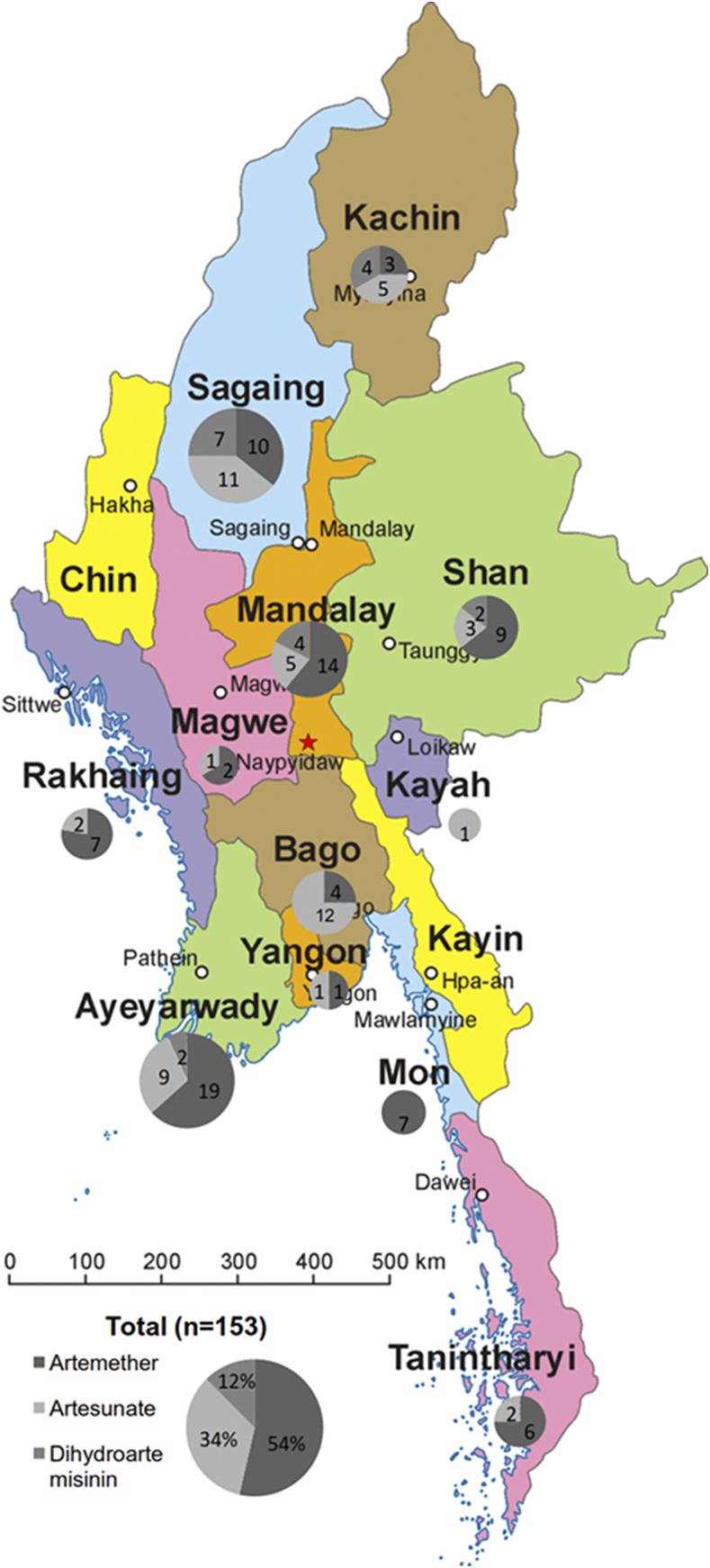
A total of 153 ACA samples were bought from private drug stores located in 11 divisions and states (Figure 1). Most of the ACAs had stated manufacturers from Southeast and South Asia and distributed by at least five trading companies in Myanmar (Table 1). Among the ACA samples, artemether, artesunate, and dihydroartemisinin-containing antimalarials accounted for 54% (82/153), 34% (52/153), and 12% (19/153), respectively. The most common ACAs were artemether-lumefantrine, artesunate monotherapy, and dihydroartemisinin-piperaquine phosphate (Table 1). Despite WHO’s urge to ban oral monotherapies of artemisinin family drugs,14 oral artesunate (33%, 50/153) and artemether (4%, 6/153) monotherapies still accounted for a considerable proportion of the ACAs collected during this survey. Injectable artesunate and artemether monotherapies, presumably used to treat severe malaria, accounted for 1% (2/153) and 5% (7/153), respectively. Furthermore, 8% (12/153) of drugs were found to have expired at the time of purchase, whereas 10% (15/153) of drugs had no clear labels giving the expiry dates. We did not observe an association between stated brand names and whether expired.
Figure 1.
Distribution of collected artemisinin-based antimalarial drugs in Myanmar. This figure appears in color at www.ajtmh.org.
Table 1.
Types of artemisinin-based antimalarials collected in Myanmar
| Ingredients (mg) | Stated trade name | Stated manufacturer | Stated distributing company in Myanmar | Total number |
|---|---|---|---|---|
| Artemether/lumefantrine (20/120) | SUPA ARTE | Unknown | AA Medical Products Ltd., Yangon | 39 |
| Artel+ | Unknown | Poly Gold Company, Yangon | 26 | |
| MALARTE-AL SS | Global Pharma Healthcare Pvt. Ltd., Chennai, India | Unknown | 1 | |
| PAI COTOKIN | Ningbo Shuangwei Pharmaceutical Co., Ltd., Nigbo, China | Pai Brothers’ Int’l trading Co., Ltd. | 2 | |
| Lumartem | CIPLA LTD. MIDC, Patalganga, India | Unknown | 1 | |
| Artemether (80) | ARTEM injection | Kunming Pharmaceutical Corp., Kunming, China | Poly Gold Company, Yangon | 4 |
| Artemether (80) | Artemether injection | Unknown | Unknown | 2 |
| Artemether (80) | Malareight injection | Unknown | Unknown | 1 |
| Artemether (50) | AA-ARTEMETHER (tablets) | AA Medical Products Ltd., Vietnam | Unknown | 1 |
| Artemether (80) | ARTEM (capsules) | Kunming Pharmaceutical Corp., China | Poly Gold Company, Yangon | 3 |
| Artemether (50) | ARTEMETHER (tablets) | Kunming Pharmaceutical Corp., China | Unknown | 2 |
| Artesunate (50) | Artesunate (tablets) | Mediplantex, Hanoi, Vietnam | Liberty Group Trading Ltd. | 48 |
| Artesunate (50) | Traphasunat (tablets) | Traphaco Joint-stock Company, Vietnam | M/S Sandar Myaing Company Ltd. | 2 |
| Artesunate (60) | Artesun (injection) | Guilin Pharmaceutical Co. Ltd., China | Unknown | 2 |
| Dihydroartemisinin/piperaquine phosphate (40/320) | D-ARTEPP | Guilin Pharmaceutical Co., Ltd., China | Unknown | 7 |
| Duo-Cotecxin | Zhejiang Holley Nanhu Pharmaceutical Co., Ltd., Jiaxing, China | Unknown | 9 | |
| Darplex | Kunming Pharmaceutical Corp., China | Unknown | 3 |
On-site dipstick testing of the collected samples.
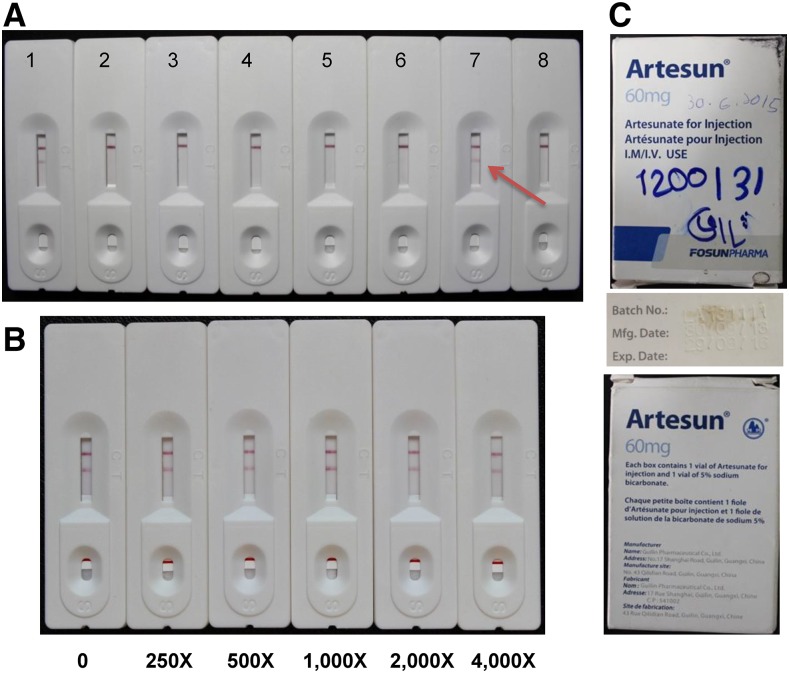
For all drugs tested on-site with the dipsticks, all artemether- and dihydroartemisinin-containing ACAs did not show the test lines when tested at the detection limits of the dipsticks, indicating that the detected API contents matched approximately their labeled API contents. However, one artesunate injection collected from Bago showed the test line at the dilution corresponding to 2,000 ng/mL, suggesting that this drug had artesunate content below the labeled content (Figure 2A). When this drug was tested at much higher concentrations, the test line still persisted, suggesting that this drug contained minimal, if any, API (Figure 2B). This drug with a lot number of LA131111 and labeled as the product of Guilin Pharmaceutical Co., Ltd. was not past the expiration date (Figure 2C). Comparison of the packaging with a genuine sample obtained directly from the manufacturer did not yield any unambiguous clues of falsification.
Figure 2.
Artesunate dipstick test of artemisinin-based antimalarial drugs. (A) Test of artesunate-containing drugs at 2,000 ng/mL. 1) water (negative control); 2) artesunate standard (2,000 ng/mL, positive control); 3) Artesunate (203514); 4) Traphasunat (02/090412); 5) Artesunate (216214); 6) Artesunate (212414); 7) Artesun injection (LA131111); 8) Artesunate (210514). Arrow points to the test line shown in sample 7. (B) Testing of the Artesun injection sample (LA131111) at higher concentrations. The stock solution of LA131111 (2 mg/mL) was diluted for 0–4,000 times and tested on the artesunate dipsticks. (C) The appearance of the package of the Artesun injection (LA131111) showing the expiration date of the drug. This figure appears in color at www.ajtmh.org.
HPLC validation of the dipstick testing results.
Since the dipsticks only offered us an estimate of the API contents in the tested drugs, we selected 28 drugs that passed the dipstick tests and the failed artesunate injection drug for confirmation by HPLC. We first classified the drugs according to their trade names and chose one or more from each type for analysis. The HPLC result showed that all tested drugs except one artesunate tablet sample had HPLC-confirmed API contents falling within the 85–115% range of the labeled contents of the respective drugs (Table 2), indicating that the field performance of the dipsticks was satisfactory. However, HPLC analysis did not detect any API content in the artesunate injection drug. In fact, no peaks were detected within the entire HPLC spectrum. Examination of the 5% sodium bicarbonate vial in the packet using broad-range pH indicator paper showed that the pH value of the content was close to seven. Dropwise addition of 1 M HCl to the sodium bicarbonate vial did not produce bubbles as compared with the vial from the genuine packet obtained from the manufacturer, suggesting that no bicarbonate was contained in that vial. This result was communicated with Guilin Pharmaceutical Co., Ltd, who informed us that they had found falsification of this drug in Myanmar before. The Myanmar MRA and WHO Rapid Alert (https://www.quamed.org/en/news/who-rapid-alert-system.aspx) were informed.
Table 2.
Comparison of the dipstick and high-performance liquid chromatography (HPLC) method for the quantitation of artemisinins in commercial drugs
| Stated trade name* | SM/DC | Lot no. | Region | Dipstick results | HPLC results† (mg/mL) |
|---|---|---|---|---|---|
| Artel+ | DC: Poly Gold Company | Unknown | Ayeyawaddy | Passed | 2.09 ± 0.05 |
| Unknown | Bago | Passed | 1.95 ± 0.06 | ||
| AA-ARTEMTHER | SM: AA Medical Products Ltd., Vietnam | Unknown | Mandalay | Passed | 1.95 ± 0.06 |
| MALARTE-AL SS | SM: Global Pharma Healthcare Pvt. Ltd., India | IB465 | Sagaing | Passed | 2.04 ± 0.03 |
| SUPA ARTE | DC: AA Medical Products Ltd. | Unknown | Rakhine | Passed | 1.95 ± 0.01 |
| ARTEM injection | SM: Kunming Pharmaceut. Corp., China | 20130211 | Mandalay | Passed | 1.98 ± 0.01 |
| DC: Poly Gold Company | Unknown | Ayeyawaddy | Passed | 2.21 ± 0.03 | |
| 20130209 | Sagaing | Passed | 2.07 ± 0.01 | ||
| Artemether injection | Unknown | Unknown | Mandalay | Passed | 1.97 ± 0.01 |
| Unknown | Unknown | Mandalay | Passed | 2.01 ± 0.05 | |
| Unknown | Unknown | Ayeyawaddy | Passed | 1.97 ± 0.02 | |
| PAI COTOKIN | SM: Ningbo Shuangwei Pharmaceutical Co., Ltd., China | 0801 | Kachin | Passed | 1.81 ± 0.01 |
| DC: Pai Brothers’ Int’L trading Co., Ltd. | Ayeyawaddy | Passed | 1.84 ± 0.04 | ||
| Lumartem | SM: CIPLA LTD. MIDC Patalganga, India | M.L.845 | Bago | Passed | 2.08 ± 0.05 |
| ARTEM (capsules) | SM: KPC Group. | Unknown | Ayeyawaddy | Passed | 1.87 ± 0.02 |
| DC: Poly Gold Company | 120224 | Tanintharyi | Passed | 1.80 ± 0.15 | |
| ARTEMETHER (tablets) | SM: Kunming Pharmaceutical Corp., China | Unknown | Tanintharyi | Passed | 1.98 ± 0.04 |
| Artesunate (tablets) | SM: Mediplantex, Hanoi, Vietnam DC: Liberty Group Trading Ltd. | 216214 | Ayeyawaddy | Passed | 2.08 ± 0.08 |
| 264613 | Bago | Passed | 1.80 ± 0.04 | ||
| Kayah | Passed | 2.30 ± 0.05 | |||
| 212514 | Mandalay | Passed | 2.22 ± 0.05 | ||
| Bago | Passed | 2.31 ± 0.09 | |||
| 210514 | Rakhine | Passed | 2.04 ± 0.09 | ||
| Artesun (injection) | SM: Guilin Pharmaceutical Co,. Ltd. | LA131111 | Bago | Failed | 0 |
| D-ARTEPP | SM: Guilin Pharmaceutical Co., Ltd., China | SQ140102 | Sagaing | Passed | 1.73 ± 0.04 |
| Duo-Cotecxin | SM: Zhejiang Holley Nanhu Pharmaceutical Co., Ltd., China | 130635 | Mandalay | Passed | 1.85 ± 0.01 |
| 19032014 | Shan | Passed | 1.90 ± 0.04 | ||
| 24062013 | Kachin | Passed | 1.89 ± 0.03 | ||
| 07112014 | Sagaing | Passed | 1.86 ± 0.02 |
SM = Stated manufacturer; DC = Stated distributing Company. Drugs were all ground and diluted to 2 mg/mL based on the labeled contents. The artesunate tablet that had the active pharmaceutical ingredient (API) content determined by HPLC outside the 85–115% of the labeled API content was highlighted in bold.
Each sample was analyzed in triplicate.
Data represent mean ± SD.
DISCUSSION
The GMS has been afflicted with widespread circulation of falsified and substandard ACAs.4,6 Here we evaluated this situation in Myanmar, where malaria burden is the heaviest and falsified ACA problem was the most serious in the GMS, using field-deployable dipstick assays. By convenience sampling of ACAs in the private sector, we found a considerable proportion (37%) of oral artesunate and artemether monotherapies in the market. Similarly, recent surveys of representative outlets in Myanmar have identified that > 25% of drug outlets still stocked oral artemisinin monotherapies.13 A recent survey in Lao PDR also identified the presence of oral monotherapies of ACAs,9 but this problem appeared to have resolved lately with tightened governmental regulations.8 In addition, this survey also found that at least 8% of the drug samples had expired when purchased. Thus, governmental regulations need to be tightened to remove oral artemisinin monotherapies and expired drugs from the market to prevent and slow down the development and spread of resistance to artemisinin. On-site analysis using our specific dipsticks for artesunate, artemether, and dihydroartemisinin confirmed the performance of these dipsticks as convenient, simple, and cost-effective tools for point-of-care drug quality testing. At the current cost of 0.1–0.2 USD/dipstick, the dipsticks should have a promising potential for screening antimalarial quality in remote endemic sites. From this survey, all the ACA drugs passed these dipstick assays, but one artesunate injection drug contained no API content as determined by both the dipstick and HPLC analyses. We did not find earlier reports on falsified artesunate injections. Given that artesunate injection is vital in the treatment of severe falciparum malaria, the clinical consequences of “artesunate”-containing no API would be very serious. Clinical trials in severe malaria, both adults27 and children,28 suggest that in comparison with quinine, parenteral artesunate reduces mortality by 34.7% and 22.5%, respectively, and is recommended as first-line therapy by WHO for severe malaria.29 With increasing use of this life-saving drug globally, the discovery of one sample of falsified parenteral artesunate has grave implications and strongly suggests that enhanced global monitoring of the quality of these products are urgently required. In 1999 the discovery of falsified oral artesunate in Cambodia was an alert to what became a severe public health problem in mainland Asia and sub-Saharan Africa.4 Because the GMS countries are pursuing malaria elimination and ACAs are playing a critical role in this campaign, the situation of the antimalarial drug quality in Myanmar is quite worrisome and deserves more oversights from the regulatory authorities. Given the limitations associated with the convenience sampling methods, more systematic investigations of the problems of oral artemisinin monotherapies and falsification in the GMS are highly needed.
Acknowledgments:
We thank Paul Newton for his advice and critical reading of the manuscript.
Disclaimer: The funder had no role in study design, data collection, data analysis, data interpretation, writing of the report, and decision to submit the paper for publication.
REFERENCES
- 1.Nayyar GM, Breman JG, Newton PN, Herrington J, 2012. Poor-quality antimalarial drugs in southeast Asia and sub-Saharan Africa. Lancet Infect Dis 12: 488–496. [DOI] [PubMed] [Google Scholar]
- 2.Newton PN, et al. , 2006. Manslaughter by fake artesunate in Asia–will Africa be next? PLoS Med 3: e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newton PN, Caillet C, Guerin PJ, 2016. A link between poor quality antimalarials and malaria drug resistance? Expert Rev Anti Infect Ther 14: 531–533. [DOI] [PubMed] [Google Scholar]
- 4.Newton PN, et al. , 2008. A collaborative epidemiological investigation into the criminal fake artesunate trade in southeast Asia. PLoS Med 5: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondorp AM, et al. , 2004. Fake antimalarials in southeast Asia are a major impediment to malaria control: multinational cross-sectional survey on the prevalence of fake antimalarials. Trop Med Int Health 9: 1241–1246. [DOI] [PubMed] [Google Scholar]
- 6.Newton P, et al. , 2001. Fake artesunate in southeast Asia. Lancet 357: 1948–1950. [DOI] [PubMed] [Google Scholar]
- 7.Sengaloundeth S, et al. , 2009. A stratified random survey of the proportion of poor quality oral artesunate sold at medicine outlets in the Lao PDR – implications for therapeutic failure and drug resistance. Malar J 8: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phanalasy S, 2017. The malaria testing and treatment landscape in the southern Lao People’s Democratic Republic (PDR). Malar J 16: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabernero P, et al. , 2015. A repeat random survey of the prevalence of falsified and substandard antimalarials in the Lao PDR: a change for the better. Am J Trop Med Hyg 92: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khin HS, Aung T, Aung M, Thi A, Boxshall M, White C, 2016. Using supply side evidence to inform oral artemisinin monotherapy replacement in Myanmar: a case study. Malar J 15: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khin HS, Aung T, Thi A, White C, 2016. Oral artemisinin monotherapy removal from the private sector in eastern Myanmar between 2012 and 2014. Malar J 15: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thein ST, Khin HSS, Thi A, 2017. Anti-malarial landscape in Myanmar: results from a nationally representative survey among community health workers and the private sector outlets in 2015/2016. Malar J 16: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thein ST, Khin HSS, Thi A, 2017. Insights into the availability and distribution of oral artemisinin monotherapy in Myanmar: evidence from a nationally representative outlet survey. Malar J 16: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO , 2007. World Health Assembly Resolution WHA60.18 Available at: http://www.who.int/tdr/about/governance/documents/WHA60.18.pdf. Accessed July 16, 2017.
- 15.Fairhurst RM, Dondorp AM, 2016. Artemisinin-resistant Plasmodium falciparum malaria. Microbiol Spectr 4: EI10-0013-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui L, et al. , 2012. Malaria in the greater Mekong subregion: heterogeneity and complexity. Acta Trop 121: 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewitt S, Delacollette C, Poirot E, 2013. Malaria control in the greater Mekong subregion: an overview of the current response and its limitations. Southeast Asian J Trop Med Public Health 44 (Suppl 1): 249–305, discussion 306–307. [PubMed] [Google Scholar]
- 18.Guo S, Cui Y, He L, Zhang L, Cao Z, Zhang W, Zhang R, Tan G, Wang B, Cui L, 2013. Development of a specific monoclonal antibody-based ELISA to measure the artemether content of antimalarial drugs. PLoS One 8: e79154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He L, Nan T, Cui Y, Guo S, Zhang W, Zhang R, Tan G, Wang B, Cui L, 2014. Development of a colloidal gold-based lateral flow dipstick immunoassay for rapid qualitative and semi-quantitative analysis of artesunate and dihydroartemisinin. Malar J 13: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo S, Cui Y, Wang K, Zhang W, Tan G, Wang B, Cui L, 2016. Development of a specific monoclonal antibody for the quantification of artemisinin in Artemisia annua and rat serum. Anal Chem 88: 2701–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo S, He L, Tisch DJ, Kazura J, Mharakurwa S, Mahanta J, Herrera S, Wang B, Cui L, 2016. Pilot testing of dipsticks as point-of-care assays for rapid diagnosis of poor-quality artemisinin drugs in endemic settings. Trop Med Health 44: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo S, Zhang W, He L, Tan G, Min M, Kyaw MP, Wang B, Cui L, 2016. Rapid evaluation of artesunate quality with a specific monoclonal antibody-based lateral flow dipstick. Anal Bioanal Chem 408: 6003–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newton PN, et al. , 2009. Guidelines for field surveys of the quality of medicines: a proposal. PLoS Med 6: e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo S, Zhang W, He L, Tan G, Min M, Kyaw MP, Wang B, Cui L, 2016. Rapid evaluation of artesunate quality with a specific monoclonal antibody-based lateral flow dipstick. Anal Bioanal Chem 408: 6003–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batty KT, Davis TM, Thu LT, Binh TQ, Anh TK, Ilett KF, 1996. Selective high-performance liquid chromatographic determination of artesunate and alpha- and beta-dihydroartemisinin in patients with falciparum malaria. J Chromatogr B Biomed Appl 677: 345–350. [DOI] [PubMed] [Google Scholar]
- 26.Karbwang J, Na-Bangchang K, Molunto P, Banmairuroi V, Congpuong K, 1997. Determination of artemether and its major metabolite, dihydroartemisinin, in plasma using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl 690: 259–265. [DOI] [PubMed] [Google Scholar]
- 27.Dondorp A, Nosten F, Stepniewska K, Day N, White N, 2005. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366: 717–725. [DOI] [PubMed] [Google Scholar]
- 28.Dondorp AM, et al. , 2010. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376: 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO , 2015. Guidelines for the Treatment of Malaria. 3rd edition. Geneva, Switzerland: World Health Organization, 316. [Google Scholar]