Abstract
Background
In China, the essential oil of the fruit, Fructus Alpiniae zerumbet (FAZ), is used to treat cardiovascular diseases. Recent in vitro studies have shown that the essential oil of FAZ (EOFAZ) can protect endothelial cells from injury. Because of the prevalence of diabetes mellitus and its effects on the cardiovascular system, the aim of this study was to investigate the mechanism of the effects of EOFAZ on human umbilical vein endothelial cells (HUVECs) treated with high levels of glucose in vitro.
Material/Methods
The lactate dehydrogenase (LDH) leakage assay was used to detect HUVEC injury. Tumor necrosis factor-alpha (TNF-α), interleukin-8 (IL-8), and nuclear transcription factor-kappa B (NF-κB) p65 subunit DNA-binding activity was detected. The expression of NF-κB pathway-associated proteins, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) was studied by Western blotting. The cellular location of NF-κB in HUVECs was evaluated using immunofluorescence.
Results
Cell viability and LDH leakage assays showed that high glucose-induced HUVEC injury was reduced by EOFAZ. High glucose-induced secretion of IL-8, TNF-α, ICAM-1, and VCAM-1 was reduced, and translocation of the p65 subunit of NF-κB to the endothelial cell nucleus was inhibited by EOFAZ. Western blotting confirmed that EOFAZ blocked the activation of NF-κB induced by high glucose levels. EOFAZ reduced high glucose-induced p65/DNA binding to inhibit NF-κB activation.
Conclusions
The findings of this in vitro study showed that treatment of HUVECs with EOFAZ had a protective role against the effects of high glucose levels via the NF-κB signaling pathway.
MeSH Keywords: Glucose, Human Umbilical Vein Endothelial Cells, NF-kappa B
Background
Endothelial cell dysfunction is considered to be the basis of the pathogenesis of vascular disease associated with diabetes mellitus, which is also associated with vascular inflammation that may be involved in the progression of the complications of diabetes [1,2]. Hyperglycemia is an initiator of vascular endothelial cell injury and dysfunction, leading to vascular disease [3]. Hyperglycemia can also cause vascular inflammation, which is a key component in the development of diabetic vascular disease [4,5]. Also, exposure of endothelial cells to high levels of glucose can result in the generation of proinflammatory cytokines and chemokines, including nitric oxide (NOS) and cell adhesion molecules that promote inflammatory responses [6]. The cell adhesion molecules, intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin are also important inflammatory mediators in vascular disease [7,8]. In particular, the activation of the nuclear transcription factor-kappa B (NF-κB) signaling pathway can promote the expression of ICAM-1 and VCAM-1 [9,10].
There is increasing evidence from published studies that NF-κB signaling cascades can participate in both inflammation and apoptosis [11–13]. Also, NF-κB induces the expression of inflammatory cytokines, leading to the amplification of the inflammatory cascade that results in endothelial cell damage [14,15]. In physiologically normal conditions, the binding of NF-κB inhibitor to NF-κB remains inactive, and NF-κB is present in the endothelial cell cytoplasm. However, during endothelial cell injury, the NF-κB inhibitor becomes ubiquitinated, phosphorylated and degraded by activated kinases, which leads to the activation of NF-κB. Activated NF-κB is transferred into the nucleus to initiate inflammatory cytokine transcription to induce inflammation [16,17]. Studies have shown that hyperglycemia can activate NF-κB, followed by the upregulation of VCAM-1 and ICAM-1 expression, and ultimately leads to cellular damage [18].
In southeast and southwest China, the essential oil of the fruit, Fructus Alpiniae zerumbet (FAZ), is used to treat cardiovascular diseases and diseases of the digestive tract [19]. FAZ is a local Miao traditional Chinese medicine, extensively used in Guizhou Province [19]. Recent in vitro studies have shown that the essential oil of FAZ (EOFAZ) can protect endothelial cells from injury, and exhibits a wide range of pharmacological properties, including anti-inflammatory effects, protection against cardiovascular diseases, and antioxidant activity [20,21]. Also, we have previously shown that EOFAZ could reduce vascular endothelial cell injury using a variety of injurious agents [22].
Because of the prevalence of diabetes mellitus and its effects on the cardiovascular system, the aim of this in vitro study was to investigate the mechanisms of the effects of EOFAZ on human umbilical vein endothelial cells (HUVECs) treated with high levels of glucose and to examine the role of the NF-κB signaling pathways.
Material and Methods
Chemical and herbal materials
The fruit, Fructus Alpiniae zerumbet (FAZ), was collected in Zhenfeng County, Guizhou Province, China. The content of the essential oil from FAZ (EOFAZ) was assessed by Associate Professor Chen Zuyun, and the voucher specimen (Number: 20151026) was stored in the Key Laboratory of Optimal Utilization of Natural Medicinal Resources, the High Educational Key Laboratory of Guizhou Province for Natural Medicinal Pharmacology and Druggability, Guizhou Medical University [19]. The essential oil was extracted from the fruit of Alpinia zerumbet by steam distillation, and the yield was 1.3%. Analysis of the chemical constituents of the essential oil was performed by gas chromatography-mass spectrometry (GC-MS), as previously described [23].
Materials
D-glucose (Lot No. 405A0918) and D-mannitol (Lot No. 1126F038), and a bicinchoninic acid (BCA) assay kit were obtained from Beijing Solarbio Science & Technology Co., Ltd. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and pyrrolidine dithiocarbamate (PDTC) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Nuclear transcription factor-kappa B (NF-κB) p65 rabbit polyclonal antibody, phospho-NF-κB p65 (Ser536) antibody, IκBα (NF-κB inhibitor) antibodies, NF-κB activation-nuclear translocation assay kit and lysis buffer were all obtained from the Beyotime Institute of Biotechnology (Jiangsu, China). The lactate dehydrogenase (LDH) activity assay kit was acquired from the Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China). Antibodies to VCAM-1 and ICAM-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
Primary human umbilical vein endothelial cells (HUVECs) and selected endothelial cell culture medium, 1% endothelial cell growth supplement, 1% penicillin/streptomycin solution and 5% fetal bovine serum were obtained from ScienCell Research Laboratories (San Diego, CA, USA). Cultures were maintained in a humidified environment, 37°C and 5% CO2. HUVECs between three and five passages were used in all experiments.
MTT colorimetric assay for assessing cell metabolic activity and cell viability
MTT was used to assess the viability of HUVECs. Cells were seeded into 96-well plates and cultured until they reached 80% confluence. The cells were then exposed to the indicated concentrations of glucose for 24 h or pre-incubated with the indicated concentrations of EOFAZ for 1 h. The supernatant was discarded and 20 μl of 5 mg/ml MTT were added to each well for 4 h. Then, 100μl dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals and shaken for 10 min. The ELx800 microplate reader (General Electric, Fairfield, CT, USA) was used to determine the optical density (OD) with the spectrometric absorbance of 490 nm. The cell survival rate (%) was calculated using the following equation:
Lactate dehydrogenase (LDH) leakage assay
LDH release, or leakage, is related to the permeability of cell membranes and can be measured as a biomarker of cell injury. For the LDH leakage assay, following HUVEC treatment, the culture media was collected and centrifuged for 10 min at 1000×g, and the LDH activity in the supernatant was measured as the level of extracellular LDH activity. LDH activity was measured by determining the absorbance at 405 nm using the ELx800 microplate reader (General Electric, Fairfield, CT, USA), in accordance with the manufacturer’s instructions.
Enzyme-linked immunosorbent assay (ELISA)
Following treatment with EOFAZ, the cell supernatant was collected to detect the concentration of tumor necrosis factor-α (TNF-α) and interleukin (IL)-8 using commercial ELISA kits, and according to the manufacturer’s instructions (Boster Biological Technology, Wuhan, China).
NF-κB nuclear translocation by immunofluorescence
The major subunit p65 of NF-κB was detected by immunofluorescence staining to confirm whether NF-κB was activated in the HUVECs, as shown by nuclear translocation. The cells were transferred to six-well plates, the culture medium was removed, and the cells were washed twice with phosphate buffered saline (PBS). The HUVECs were fixed in 4% paraformaldehyde for 10 min, washed three times with PBS, and blocked with a solution of PBS containing 5% bovine serum albumin (BSA) for 1 h at 37°C. After discarding the BSA, the cells and the primary antibody against NF-κB p65 were incubated at room temperature for 1 h, then washed three times with PBS and incubated with Cy3-labeled secondary antibody for 2 h. The cells were washed twice with PBS and stained with 4′, 6-diamidino-2- phenylindole (DAPI). After adding anti-fade medium, the cellular pattern of immunofluorescence staining was evaluated using an Eclipse Ti-U fluorescence microscope (Nikon Corporation, Tokyo, Japan).
Western blotting
HUVECs were harvested, and protein was extracted using a lysis buffer containing protease and phosphatase inhibitors. Cell lysates were prepared in lysis buffer and spun at 13,000 rpm at 4°C for 20 min. A commercial BCA protein assay kit was used to measure the protein concentration of the cell lysates. Samples were analyzed by 10% stacking sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% nonfat dried milk powder in TBS containing 0.1% Tween 20 for 1 h at room temperature and incubated overnight at 4°C with primary antibodies directed against phosphorylated NF-κB p65 (1: 1000), IkBα (1: 1000), ICAM-1 (1: 1000), VCAM-1 (1: 800), and GAPDH (1: 10,000). After washing the blots to remove excessive primary antibody binding, the membranes were incubated for 1 h with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1: 5000) at room temperature, followed by enhanced chemiluminescence (ECL) detection (Amersham Biosciences, UK). Densitometric measurements of the Western blot band analysis were performed using Syngene Gel Imaging System (Bio-Rad).
NF-κB DNA-binding assay
To evaluate NF-κB DNA-binding, the cellular nuclear proteins were extracted from HUVECs using a nuclear extraction kit (Beyotime Institute of Biotechnology). The NF-κB p65 subunit DNA-binding activity was then studied in the nuclear extract obtained using an NF-κB p65 transcription factor assay kit, according to the manufacturer’s protocol (Cayman Chemical Company, Ann Arbor MI, USA). The NF-κB in the cellular nuclear proteins was specifically bound to the NF-κB responsive element in the sequence of specific double-stranded DNA. Incubation at room temperature for 1.5 h with a primary antibody against NF-κB (1: 500) was performed. After washing, HRP-conjugated goat anti-rabbit IgG (1: 1000) was incubated to supply a sensitive colorimetric readout at 450 nm using the ELx800 microplate reader (General Electric, Fairfield, CT, USA)
Statistical analysis
Data were obtained from a minimum of three to five independent experiments and expressed as the mean ± standard deviation (SD). Statistical analysis was undertaken using a Student’s t-test for two groups, and a one-way ANOVA followed by Scheffe’s test for multiple comparisons. P<0.05 was considered to be statistically significant.
Results
Essential oil of Fructus Alpiniae zerumbet (EOFAZ) protected human umbilical vein endothelial cells (HUVECs) from high glucose-induced injury
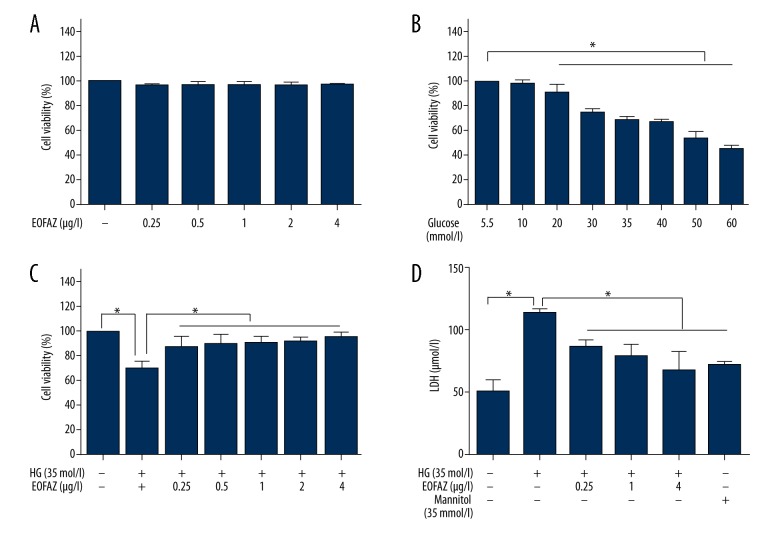
The viability of human umbilical vein endothelial cells (HUVECs) measured using an MTT assay, showed that cell viability remained stable and was not significantly altered by treatment with EOFAZ at 0.25−4 μg/mL for 24 h (Figure 1A). Treatment of HUVECs with glucose (5.5–75 mmol/L) reduced cell viability in a dose-dependent manner (Figure 1B). Also exposure to 35 mmol/L glucose for 24 h significantly reduced cell viability to 68.7%±3.4% (P<0.01). Therefore, a high concentration of glucose (35 mmol/L, with exposure for 24 h) was used as the model condition in subsequent experiments. Also, prevention of high glucose-induced HUVEC injury by essential oil of Fructus Alpiniae zerumbet (EOFAZ) was dose-dependent (Figure 1C).
Figure 1.
Effects of the essential oil of the fruit, Fructus Alpiniae zerumbet (EOFAZ) on glucose-induced cell viability and injury of human umbilical vein endothelial cells (HUVECs). (A) The viability of HUVECs during treatment with various concentrations of EOFAZ for 24 hours. Cell viability was assessed using an MTT assay (n=6). (B) HUVECs were incubated with different concentrations of glucose for 24 h. Cell viability was assessed using an MTT assay (n=6). (C) HUVECs were pretreated with various concentrations of EOFAZ for 1 h before stimulation with high glucose (35 mmol/L) for 24 h. Cell viability was assessed via an MTT assay (n=6). (D) The expression of lactate dehydrogenase (LDH) leakage in culture supernatants of HUVECs was measured by enzyme-linked immunosorbent assay (ELISA) (n=5). Mannitol was used as an osmotic control. Data are presented as the mean ± standard deviation (SD). * P<0.01.
We chose three doses of EOFAZ (0.25, 1.0, and 4.0 μg/mL) to evaluate its protective effect. Lactate dehydrogenase (LDH) leakage can be used as a biomarker of cell membrane damage. The degree of LDH leakage was significantly increased in HUVECs exposed to 35 mmol/L of glucose alone. However, preincubation with EOFAZ for 1 h reduced the increased levels of LDH leakage. To eliminate the effect of osmotic pressure of high glucose levels on LDH leakage, cells were exposed to D-mannitol with the same osmotic pressure as high glucose. it was confirmed that D-mannitol did not cause a significant increase in LDH leakage (Figure 1D).
EOFAZ reduces the secretion of inflammatory cytokines
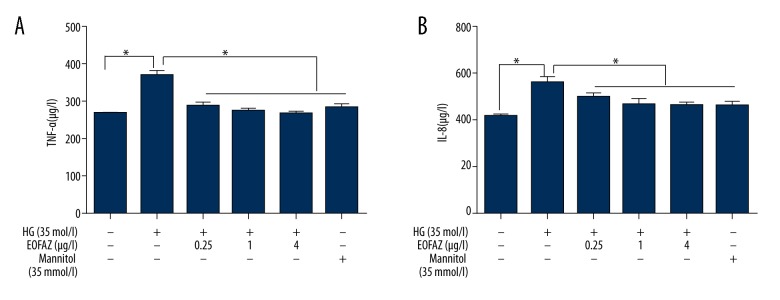
To determine whether EOFAZ inhibited the expression of inflammatory cytokines in 35 mmol/L glucose-stimulated HUVECs, the levels of TNF-α and IL-8 were measured using an enzyme-linked immunosorbent assay (ELISA). Figure 2 showed that high glucose increased TNF-α and IL-8 expression, and EOFAZ pretreatment reduced high glucose-induced TNF-α and IL-8 expression in a dose-dependent manner.
Figure 2.
The essential oil of the fruit, Fructus Alpiniae zerumbet (EOFAZ) reduced the high glucose-induced secretion of tumor necrosis factor (TNF)-α and interleukin (IL)-8 in human umbilical vein endothelial cells (HUVECs). Cells were pre-protected with EOFAZ (0.25, 1.0, and 4.0 μg/mL) for 1 h, then exposed to high glucose levels (35 mmol/L) for 24 h. (A) The expression of TNF-α and (B) IL-8 on culture supernatants of human umbilical vein endothelial cells (HUVECs) were measured by enzyme-linked immunosorbent assay (ELISA) (n=5). Data are presented as the mean ±SD. * P<0.01.
EOFAZ downregulated the expression of cell adhesion molecules in high glucose-induced HUVEC injury
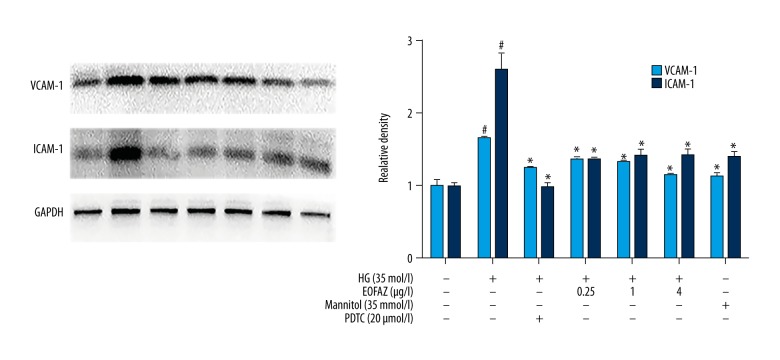
The inhibitor of nuclear transcription factor-kappa B (NF-κB), pyrrolidine dithiocarbamate (PDTC) was used to detect cell adhesion molecule expression in high glucose-induced HUVECs via NF-κB signaling. The Western blot analysis showed that high glucose increased VCAM-1 and ICAM-1 production in HUVECs. PDTC inhibited the effects of high glucose on ICAM-1 and VCAM-1 expression (Figure 3). These results indicated that NF-κB might be indispensable to the high glucose-induced expression of ICAM-1and VCAM-1 in HUVECs. We pretreated HUVECs with EOFAZ at the indicated concentrations (0.25, 1.0, and 4.0 μg/mL). As shown in Figure 3, the pretreatment of HUVECs with EOFAZ significantly inhibited the high glucose-induced effect on the levels of ICAM-1 and VCAM-1.
Figure 3.
The effects of the essential oil of the fruit, Fructus Alpiniae zerumbet (EOFAZ) on the high glucose-derived increased production of ICAM-1 and VCAM-1 in human umbilical vein endothelial cells (HUVECs). HUVECs were incubated with EOFAZ (0.25, 1.0, and 4.0 μg/mL) or PDTC (20 μM) for 1 h, then exposed to high glucose (35 mmol/L) for 24 h. Western blotting analysis was performed to analyze the expression level of ICAM-1 and VCAM-1 (n=4). Data are shown as the mean ±SD. # P<0.01 vs. control. * P<0.01 vs. the high glucose group.
EOFAZ blocks high glucose-induced activation of NF-κB
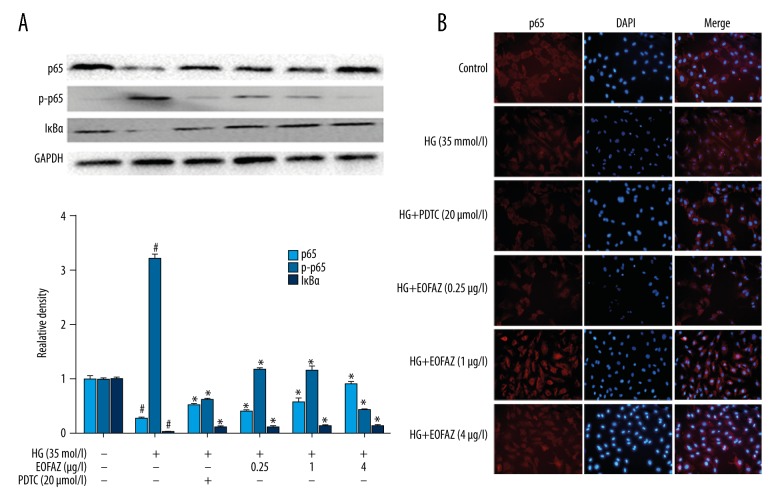
Because the NF-κB pathway is a crucial mediator of inflammation, the effect of EOFAZ on high glucose-induced NF-κB pathway activation was studied. In the immunofluorescence assay to determine whether EOFAZ could affect the high glucose-activated NF-κB pathway, p65 was found to be transferred into the cell nucleus of the HUVECs, and that EOFAZ reduced the phosphorylation of p65 in a dose-dependent manner. Also, EOFAZ pretreatment significantly inhibited the effect of high glucose by reducing the levels of p65 and IκBα (Figure 4A). The decrease in NF-κB expression induced by high glucose was mediated by an increase in NF-κB p65 translocation into the nucleus, which was also inhibited by EOFAZ. (Figure 4B). Therefore, these findings demonstrated that high glucose-mediated NF-κB activation could be inhibited by EOFAZ.
Figure 4.
The essential oil of the fruit, Fructus Alpiniae zerumbet (EOFAZ)-mediated blockade of high glucose-induced nuclear transcription factor-kappa B (NF-κB) activation. HUVECs were incubated with EOFAZ (0.25, 1.0, and 4.0 μg/mL) or pyrrolidine dithiocarbamate (PDTC) (20 μM) for 1 h, then exposed to high glucose (35 mmol/L) for 24 h. (A) NF-κB p65, p65 phosphorylation, and IκBα were examined by Western blotting (n=4). Data are presented as the mean ±SD. # P<0.01 vs. control; * P<0.01 vs. the high glucose group. (B) High glucose significantly increased the translocation of NF-κB p65 from the cytoplasm to the nucleus, and EOFAZ attenuated this addition. Immunofluorescence assay (×200 magnification) (n=5) was used to detect the inhibition of high glucose-induced p65 nuclear translocation with EOFAZ or pyrrolidine dithiocarbamate (PDTC). Images were obtained using a fluorescent microscope. Nuclei were stained with DAPI (blue). NF-κB p65 was stained with Cy3 (red). NF-κB p65 was localized in the cytoplasm of control cells. HUVECs exposed to high glucose levels exhibited significant translocation of p65 to the nucleus. After pretreatment with different concentrations of EOFAZ or PDTC (20 μM) for 1 h, p65 was significantly retained in the cytoplasm.
Effects of EOFAZ on the DNA binding activity of NF-κB p65 induced by high glucose in HUVECs
High glucose stimulation was found to increase significantly the NF-κB p65 DNA binding activity. Following pretreatment of HUVECs with different doses of EOFAZ for 1 h, NF-κB p65 DNA binding activity was found to decrease in a dose-dependent manner (Figure 5).
Figure 5.
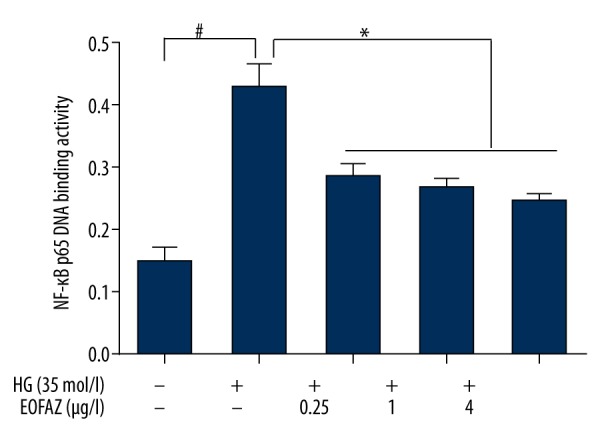
Effects of the essential oil of the fruit, Fructus Alpiniae zerumbet (EOFAZ) on the DNA binding activity of nuclear transcription factor-kappa B (NF-κB) p65 induced by high glucose levels in human umbilical vein endothelial cells (HUVECs). The nuclear proteins were extracted using a commercial nuclear extraction kit. NF-κB p65 subunit DNA-binding activity was then studied in the nuclear extract obtained using the NF-κB p65 transcription factor assay kit (n=5). Data are presented as the mean ±SD. * P<0.01.
Discussion
Diabetic vascular disease provides a key pathological basis for vascular endothelial dysfunction, for which high concentrations of glucose is one of the important risk factors for vascular endothelial dysfunction. A high glucose concentration is also a primary factor involved in the development of vascular inflammation [24]. Also, high levels of glucose cause activation of the innate immune system and the persistence of a microinflammatory state. Therefore, endothelial inflammation and dysfunction may provide potential targets for intervention as an effective strategy to prevent and delay complications associated with diabetes mellitus [25]. Recently, our laboratory has reported that the Chinese herbal medicine, essential oil of Fructus Alpiniae zerumbet (EOFAZ), may protect against cardiovascular disease via its anti-inflammatory and anti-hypertensive effects [26]. In this in vitro study, human umbilical vein endothelial cells (HUVECs) treated with high levels of glucose to determine whether EOFAZ could protect endothelial cells from high glucose-induced injury.
To the best of our knowledge, this is the first report to demonstrate the effects of EOFAZ on endothelial cell injury induced by high levels of glucose. The MTT and lactate dehydrogenase (LDH) leakage assay showed that pre-incubation with EOFAZ significantly reduced high glucose-induced cell injury. Also, EOFAZ significantly decreased the secretion of the proinflammatory cytokines, tumor necrosis factor (TNF)-α, and interleukin (IL)-8, thereby reducing the HUVEC inflammatory phenotype. These findings indicate that EOFAZ is a cytokine inhibitor.
Adhesion molecules are a class of glycoprotein molecules that interact on the cell surface and mediate cell-cell interactions and interactions between cells and the extracellular matrix (ECM). The cell adhesion molecules, ICAM-1and VCAM-1, mediate leukocyte and vascular endothelial cell adhesion, as well as leukocyte activation, thereby triggering a proinflammatory response [27]. Previous studies have shown that ICAM-1 and VCAM-1 expression is closely related to the development of diabetic vascular disease [28]. Also, NF-κB signaling manifests as a deep involvement in the inflammatory process, particularly regarding the regulation of ICAM-1and VCAM-1 expression [29]. Moreover, the expression of these adhesion molecules is regulated by nuclear transcription factor-kappa B (NF-κB) and other transcription factors. The results of this study show that EOFAZ can inhibit the elevated expression of ICAM-1and VCAM-1 induced by high glucose concentrations, suggesting that EOFAZ inhibits the inflammation associated with diabetic vascular endothelium. This anti-inflammatory effect may be achieved by inhibiting the activation of transcription factors. NF-κB translocation into the nucleus initiates a variety of proinflammatory cytokines, adhesion molecules, chemokines, and growth factors and thus, participates in host defense and inflammatory responses.
Also, nuclear transcription factor-kappa B (NF-κB) is expressed in endothelial cells and plays a key role in the regulation of vascular endothelial growth factor [30,31]. Therefore, the inhibition of NF-κB activity may be an important pathway for the control of vascular inflammation, as well as delaying the progression and improving vascular complications of diabetes mellitus. We used different concentrations of EOFAZ in the culture medium before stimulating the vascular endothelial cells with a high glucose concentration. Our results demonstrate that EOFAZ inhibited NF-κB activation in high glucose-induced vascular endothelial cells in a dose-dependent manner. The findings of this study have shown, for the first time, that EOFAZ as a natural product can protect endothelial cells cultured in vitro from high glucose-induced endothelial dysfunction by inhibiting the activation of the NF-κB signaling pathway.
Conclusions
The findings of this study have shown that the natural essential oil of the fruit, Fructus Alpiniae zerumbet (EOFAZ) might be a modulator of NF-κB signaling pathway and this finding may explain the protective effects of EOFAZ on endothelial dysfunction induced by high concentrations of glucose. The findings from this in vitro study require support from controlled clinical studies to determine the potential role of EOFAZ in the treatment of diabetes-related vascular disease.
Footnotes
Conflicts of interests
None.
Source of support: The National Natural Science Foundation of China (81360650, 81560811,81760725,31760294), the foundation of science and technology department of Guizhou Provincial (2016-1120), the foundation of Guiyang science and technology bereau(2017-5-14), the Modern Novel Drug Project of Guiyang City (20151001-07), the Fund of High-Level Innovation Talents (2015-4029), the Fund of the Innovation Team of Guizhou Province (2015-4025), the Science and Technology Fund of Guizhou Provincial Health Department (2014-1-031), and the Fund of Innovated Team of the Education Department of Guizhou Province (2014-31)
References
- 1.Sena CM, Pereira AM, Seiça R. Endothelial dysfunction – a major mediator of diabetic vascular disease. Biochimi Biophysica Acta. 2013;1832(12):2216–31. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Domingueti CP, Dusse LM, Carvalho Md, et al. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2015;30(4):738–45. doi: 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Hansen NW, Hansen AJ, Sams A. The endothelial border to health: Mechanistic evidence of the hyperglycemic culprit of inflammatory disease acceleration. IUBMB Life. 2017;69(3):148–61. doi: 10.1002/iub.1610. [DOI] [PubMed] [Google Scholar]
- 4.Shin ES, Sorenson CM, Sheibani N. Diabetes and retinal vascular dysfunction. J Ophthalmic Vis Res. 2014;9(3):362–73. doi: 10.4103/2008-322X.143378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iantorno M, Campia U, Di Daniele N, et al. Obesity, inflammation and endothelial dysfunction. J Biol Regul Homeost Agents. 2014;28(2):169–76. [PubMed] [Google Scholar]
- 6.Wu N, Shen H, Liu H, et al. Acute blood glucose fluctuation enhances rat aorta endothelial cell apoptosis, oxidative stress and pro-inflammatory cytokine expression in vivo. Cardiovasc Diabetol. 2016;15(1):109. doi: 10.1186/s12933-016-0427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt EP, Kuebler WM, Lee WL, Downey GP. Adhesion molecules: Master controllers of the circulatory system. Compr Physiol. 2015;6(2):945–73. doi: 10.1002/cphy.c150020. [DOI] [PubMed] [Google Scholar]
- 8.Derosa G, Maffioli P. A review about biomarkers for the investigation of vascular function and impairment in diabetes mellitus. Vasc Health Risk Manag. 2016;12:415–19. doi: 10.2147/VHRM.S64460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu YP, Shen T, Lin YJ, et al. Astragalus polysaccharides suppress ICAM-1 and VCAM-1 expression in TNF-α-treated human vascular endothelial cells by blocking NF-κB activation. Acta Pharmacol Sin. 2013;34(8):1036–42. doi: 10.1038/aps.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang CC, Chu CF, Wang CN, et al. The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-α-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine. 2014;21(3):207–16. doi: 10.1016/j.phymed.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277(19):16639–47. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 12.Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Liu YT, Xiao L, et al. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-κB pathway. Inflammation. 2014;37(6):2085–90. doi: 10.1007/s10753-014-9942-x. [DOI] [PubMed] [Google Scholar]
- 14.Meyerovich K, Fukaya M, Terra LF, et al. The non-canonical NF-κB pathway is induced by cytokines in pancreatic beta cells and contributes to cell death and proinflammatory responses in vitro. Diabetologia. 2016;59(3):512–21. doi: 10.1007/s00125-015-3817-z. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Li Z, Meng Q, et al. Chronic calcium channel inhibitor verapamil antagonizes TNF-α-mediated inflammatory reaction and protects against inflammatory arthritis in mice. Inflammation. 2016;39(5):1624–34. doi: 10.1007/s10753-016-0396-1. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh S, Karin M. Missing Pieces in the NF-κB Puzzle. Cell. 2002;109(2):81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell S, Vargas J, Hoffmann A. Signaling via the NF κB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8(3):227–41. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song KH, Park JH, Jo I, et al. Telmisartan attenuates hyperglycemia-exacerbated VCAM-1 expression and monocytes adhesion in TNFα-stimulated endothelial cells by inhibiting IKKβ expression. Vascul Pharmacol. 2016;78(3):43–52. doi: 10.1016/j.vph.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Chuang CM, Wang HE, Peng CC, et al. Hypolipidemic effects of different angiocarp parts of Alpinia zerumbet. Pharm Biol. 2011;49(12):1257–64. doi: 10.3109/13880209.2011.589856. [DOI] [PubMed] [Google Scholar]
- 20.Shen XC, Tao L, Li WK, et al. Evidence-based antioxidant activity of the essential oil from Fructus A. zerumbet on cultured human umbilical vein endothelial cells’ injury induced by ox-LDL. BMC Complement Altern Med. 2012;12:174. doi: 10.1186/1472-6882-12-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Li D, Xu Y, et al. Essential oils from Fructus A. zerumbet protect human aortic endothelial cells from apoptosis induced by Ox-LDL in vitro. Evid Based Complement Alternat Med. 2014;11:956824. doi: 10.1155/2014/956824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao T, Zeng Y, Xu Y, et al. The endothelial protective properties of essential oil from Fructus Alpiniae zerumbet via the Akt/NOS-NO signaling pathway in vitro. Planta Med. 2014;80(17):1628–34. doi: 10.1055/s-0034-1383129. [DOI] [PubMed] [Google Scholar]
- 23.Ling T, Han SH, Xiang CS. Endothelium-dependent vasodilatation effects of the essential oil from Fructus alpiniae zerumbet (EOFAZ) on rat thoracic aortic rings in vitro. Phytomedicine. 2013;20(5):387–93. doi: 10.1016/j.phymed.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Sáez T, Sobrevia L, Faas M. Effect of high glucose exposure on endothelial function mediated by fetoplacental endothelial exosomes. Placenta. 2016;45:111–20. [Google Scholar]
- 25.Tousoulis D, Kampoli AM, Stefanadis C. Diabetes mellitus and vascular endothelial dysfunction: current perspectives. Curr Vasc Pharmacol. 2012;10(1):19–32. doi: 10.2174/157016112798829797. [DOI] [PubMed] [Google Scholar]
- 26.Linghu K, Dan L, Hong Y, et al. Ameliorating effects of 1,8-cineole on LPS-induced human umbilical vein endothelial cell injury by suppressing NF-κB signaling in vitro. Eur J Pharmacol. 2016;789:195–201. doi: 10.1016/j.ejphar.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 27.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7(6):467–77. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 28.Tang LQ, Ni WJ, Cai M, et al. The renoprotective effects of berberine and its potential impact on the expression of β-arrestins and ICAM-1/VCAM-1 in streptozocin induced-diabetic nephropathy rats. J Diabetes. 2015;8(5):248–55. doi: 10.1111/1753-0407.12349. [DOI] [PubMed] [Google Scholar]
- 29.Arnott C, Punnia-Moorthy G, Tan J, et al. The vascular endothelial growth factor inhibitors ranibizumab and aflibercept markedly increase expression of atherosclerosis-associated inflammatory mediators on vascular endothelial cells. PloS One. 2016;11(3):0150688. doi: 10.1371/journal.pone.0150688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panday A, Inda ME, Bagam P, et al. Transcription factor NF-κB: An update on intervention strategies. Arch Immunol Ther Exp. 2016;64(6):463–83. doi: 10.1007/s00005-016-0405-y. [DOI] [PubMed] [Google Scholar]
- 31.Won M, Byun HS, Park KA, Hur GM. Post-translational control of NF-κB signaling by ubiquitination. Arch Pharm Res. 2016;(8):1–10. doi: 10.1007/s12272-016-0772-2. [DOI] [PubMed] [Google Scholar]