Abstract
Importance
Current prehospital traumatic brain injury guidelines utilize a systolic blood pressure threshold of <90mmHg for treating hypotension (age≥10) based on studies showing higher mortality when blood pressure drops below this level. However, the guidelines also acknowledge the weakness of the supporting evidence.
Objective
In a statewide, multisystem study of traumatic brain injury, to evaluate whether any statistically supportable systolic pressure-versus-mortality threshold emerges from the data, a priori, without assuming that a cut-point exists.
Design
Observational evaluation of a large prehospital database established as a part of the Excellence in Prehospital Injury Care (EPIC) Traumatic Brain Injury Study (NIH/NINDS-1R01NS071049). The generalized additive model and logistic regression were utilized to determine the relationship between systolic pressure and probability of death, adjusting for significant/important confounders.
Setting
The pre-implementation cohort (1/1/2007–3/31/2014) of the EPIC Study.
Participants
Patients (age≥10) with moderate/severe traumatic brain injury (Barell Matrix-Type 1 and/or International Classification of Disease-9 head region severity ≥3 and/or Abbreviated Injury Scale head-region severity ≥3) and lowest prehospital systolic pressure between 40 and 119mmHg were included.
Main Outcome Measure
The main outcome measure was in-hospital mortality.
Results
Among the 3,844 included cases, the model revealed a monotonically-decreasing relationship between systolic pressure and adjusted probability of death across the entire range (40–119mmHg). Each ten-point increase of systolic pressure was associated with a decrease in the adjusted odds of death of 18.8% (aOR=0.812; 95% confidence interval: 0.748–0.883). Thus, the adjusted odds of mortality increase as much for a drop from, say, 110 to 100mmHg as for 90 to 80mmHg, and so on, throughout the range.
Conclusions and Relevance
We found a linear relationship between lowest prehospital systolic blood pressure and severity-adjusted probability of mortality across an exceptionally wide range. There is no identifiable threshold or inflection point between 40 and 119mmHg. Thus, in traumatic brain injury, the concept that 90mmHg represents a unique or important physiological “cut-point” may be wrong. Furthermore, clinically-meaningful “hypotension” may not be as low as current guidelines suggest. Randomized trials evaluating treatment levels significantly above 90mmHg are needed.
INTRODUCTION
The societal burden of Traumatic Brain Injury (TBI) is enormous—each year leading to 2.2 million emergency department (ED) visits, 280,000 hospitalizations, 52,000 deaths, and over 60 billion dollars in economic costs in the U.S.1,2 In addition, over five million Americans have major long-term disabilities as a result of TBI.1 Fortunately, there is growing evidence that proper and aggressive management of TBI in the minutes immediately following injury may improve patient outcomes by preventing or lessening secondary brain injury. This has led to the promulgation of evidence-based prehospital and in-hospital TBI treatment guidelines for both children and adults.3–6
One major focus of these guidelines is the prevention and treatment of hypotension.4,5 This is because it has been firmly established that even a single episode of hypotension during the prehospital or early hospital phases of TBI management is associated with dramatic increases mortality.3,7–26 Many studies have shown that “low” blood pressure (variously defined) increases the risk of death. However, the nearly universal assumption that a specific, clinically-relevant “threshold” actually exists is entirely without support. In other words, the design of essentially every relevant study presumes, a priori, that there is a “cut point” below which outcome significantly worsens. However, simply dichotomizing small populations and then showing that it is worse to have “low” blood pressure than higher blood pressure is not the same as identifying a true threshold. A clinically meaningful cut-point would be one that correlates with a marked change in physiological response and patient outcome if blood pressure drops below a particular level. This requires study populations that are large enough to allow evaluation of blood pressure as a continuous variable rather than merely as a “low vs. not low” categorical variable.
Objective
Given the absence of prehospital studies evaluating this specific issue, we analyzed the association between the lowest SBP (obtained prior to hospital arrival) and mortality among older children (age≥10 years) and adults in the Excellence in Prehospital Injury Care (EPIC) TBI Study (ClinicalTrials.gov-NCT01339702; NIH/NINDS R01NS071049).27 Specifically, we tested the null hypothesis that no supportable SBP-versus-mortality inflection point (“threshold”) would emerge from the data when evaluated without reference to any given definition for “hypotension.”
METHODS
Study Design, Setting, and Oversight
The parent study, EPIC, is evaluating the impact of implementing the prehospital TBI guidelines3–6 in patients with moderate or severe (“major”) TBI throughout Arizona. This is being done utilizing a before-after, multisystem, observational design. The study is expected to be completed in 2017 and has been described in detail elsewhere.27 Rather than reiterating the details of the parent study, here we limit the description to the design attributes relevant to this specific secondary analysis. The patients in this evaluation are in the pre-implementation cohort of EPIC. Post-interventional cases were excluded since one of the emphases of guideline implementation is the prevention and aggressive treatment of hypotension. Thus, including these cases might introduce significant bias into this evaluation since there was no intentional guideline implementation prior to EPIC.
The necessary regulatory approvals for EPIC have been obtained from the Arizona Department of Health Services (ADHS) and the State Attorney General. The University of Arizona Institutional Review Board and the ADHS Human Subjects Review Board have approved the project and publication of de-identified data.
Data Collection
The Arizona State Trauma Registry (ASTR) contains extensive trauma center data on all patients taken to the eight designated Level I trauma centers in the state. From the ASTR, all cases meeting study criteria (described below) are entered into the EPIC Database. Each participating Emergency Medical Services (EMS) agency then receives a list of the EPIC patients that were cared for in their system. The cases are matched by incident date, name, and other patient identifiers. Either scanned copies [paper-based patient care records (PCRs)] or electronic data files (electronic PCRs) are then sent to the Study Data Center for entry of the EMS data into the database. This provides an extensive, linked dataset for study patients that includes both prehospital and trauma center data. The entire process of case identification, EMS/trauma center linkage, accessing EMS PCRs, data entry, and the structure of the EPIC Database have been reported.27 Over 20,000 cases have been enrolled in EPIC and over 31,000 EMS PCRs have been entered into the database (multi-agency cases have more than one PCR). The successful linkage rate is exceptionally high (e.g., throughout the study, cases with missing SBP has been consistently <5%).
Participants
EPIC Inclusion criteria
Patients with physical trauma who have trauma center diagnosis(es) consistent with TBI (either isolated or multisystem trauma that includes TBI) and meet at least one of the following definitions for moderate/severe TBI: a) Centers for Disease Control (CDC) Barell Matrix-Type 1, b) head region severity score [International Classification of Diseases, Version 9 (ICD-9)] ≥3, and/or c) Abbreviated Injury Scale (AIS)-head region severity score ≥3.27
Exclusions for this subgroup analysis
age <10, SBP<40mmHg or ≥120mmHg, transfers, death before ED arrival. In addition, cases that were missing data for age, SBP, or trauma type (penetrating vs. blunt) were excluded. The 120mmHg upper limit was chosen as this represents the highest reported “threshold” in the previous literature7–9,11,14,15,17–22,26,28–36 and because including a large number of patients with near-normal or normal perfusion in the mortality model would dilute the effects of the patients who are actually at risk for hypoperfusion.
Interventions
This is a secondary analysis of the pre-implementation cohort and entails no interventions.
Main Outcome
The outcome is in-hospital mortality.27
Statistical Analysis
Continuous variables were summarized by median and range and were compared between the two cohorts (survived/died) using the Wilcoxon rank-sum test. Categorical variables were summarized by frequency and proportion [with 95% Clopper-Pearson confidence intervals (CI)] when appropriate and were compared between the two groups by Fisher’s exact test.
The overall trend in crude (unadjusted) mortality rates over the range of lowest prehospital SBP was explored using moving average plots. To plot the moving average, the crude death rate and corresponding 95%CI were calculated for subjects with lowest SBP in each interval spanning 10 consecutive values (i.e., 40–49mmHg, 41–50, 42–51, and so on, through 110–119mmHg). Then the estimated death rate and corresponding 95%CI were plotted against the midpoint of the interval (i.e., the range of plotting is 44.5mmHg for 40–49, and so on, through 114.5 for the 110–119mmHg interval). The moving window of 10mmHg was selected to prevent any false cut-points being created by data anomalies in the frequency of the last digit of lowest recorded SBP (e.g., in the dataset, even numbers were preferred to odd numbers and the digit 0 was the most popular, followed by 8 and 6). Thus, utilizing a window length of 10 prevents abnormalities arising from the uneven recording distribution of the last SBP digit.
The risk-adjusted associations between mortality and SBP were examined by logistic regression, which modeled the log odds of death, adjusting for important risk factors and potential confounders [age, sex, race, ethnicity, payment source, trauma type (blunt/penetrating), prehospital hypoxia, prehospital intubation, and treating trauma center]. The linkage of EMS data to the ASTR allowed the use of actual diagnostic/anatomic injury scoring to adjust for overall injury severity (Injury Severity Score)37 and TBI severity [head region severity score [ICD-9 matched to AIS scale]38–44 rather than having to rely on far less reliable prehospital physiological injury indicators (e.g., GCS). The effects of continuous variables (SBP, age) in the logistic regression were fitted non-parametrically using penalized thin plate regression splines through the generalized additive model.45 The model was penalized to avoid overfitting (excessive “wiggliness” in the transformation function due to random noise) and the smoothing parameters were chosen to optimize the Akaike Information Criterion (AIC), a measure of the predictive power of the model.45 Thus, the functional forms of these variables were determined by the data.
The software environment R was used for the analysis46 and the R package mgcv45,47 was used for the generalized additive model. P-values were calculated from a Wald-type test using the Bayesian covariance matrix.48 All tests were two-sided with α=0.05.
RESULTS
Enrollment
There were 17,105 subjects in the pre-intervention group from 1/1/2007–3/31/2014. The following were excluded: children <10 years (n = 1,162; 6.8%), inter-facility transfers (4,823; 28.2%), lowest prehospital SBP <40mmHg or ≥120mmHg (6,352; 37.1%), missing data [SBP (300; 1.8%), transfer status (623; 3.6%), and trauma type (1; 0.006%). This left 3,844 patients (study cohort).
Outcome and Analysis
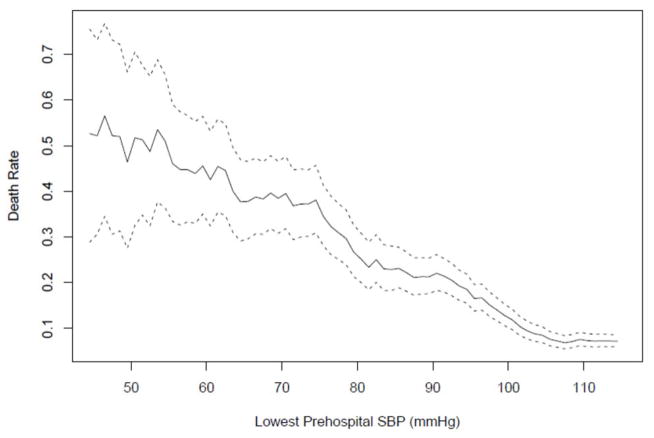
Among these cases, 528 (13.7%) died. Table 1 summarizes the demographics and patient characteristics by survival status. Figure 1 shows the crude (unadjusted) moving average of death rate by lowest EMS SBP. This plot reveals a relatively steady slope from 40mmHg to nearly 110mmHg. A logistic regression model was fitted that examined the effect of lowest prehospital SBP on mortality risk, controlling for risk adjusters and potential confounders. For continuous variables (SBP, age), the functional form of the covariate effect was obtained non-parametrically with the value of the smoothing parameter calculated to optimize the AIC. All other confounders were categorical (Table 1). Table 2 shows the effects and p-values of all covariates in the model [except for the continuous variables (SBP, age) and treating trauma center, which were all significant at p<0.0001]. As has been found by many previous studies,7,8,11,17,18,49,50 hypoxia was a highly significant risk factor and was included as a confounder in the model. The data by trauma center, while parametric, are not shown in Figure 2. Since absolute anonymity is required by state regulations and the IRB (for subjects, EMS agencies, and hospitals), we are not able to report specific trauma center-related data, even generically. Since trauma center volumes are a matter of public record, presentation of these data could conceivably lead to hospital-specific information being inferred or identified (e.g., due to comparisons of the sizes of the 95%CIs). However, because treating trauma center was a significant confounder, we adjusted for it in the model.
Table 1.
Patient Characteristics by Survival Status
| Alive* | Dead* | p-value# | ||
|---|---|---|---|---|
| Characteristic+ | N=3316 | N=528 | ||
| Age (year) | 34 (10, 99) | 42 (10, 95) | < 0.0001 | |
| Male | No | 1125 (33.9%) | 154 (29.2%) | 0.035 |
| Yes | 2191 (66.1%) | 374 (70.8%) | ||
| Race | Black | 101 (3%) | 15 (2.8%) | 0.527 |
| Asian | 38 (1.1%) | 5 (0.9%) | ||
| American Indian/Alaska Nat. | 239 (7.2%) | 27 (5.1%) | ||
| White | 2548 (76.8%) | 405 (76.7%) | ||
| Other | 360 (10.9%) | 61 (11.6%) | ||
| Unknown | 30 (0.9%) | 15 (2.8%) | ||
| Hispanic | No | 2443 (73.7%) | 376 (71.2%) | 0.997 |
| Yes | 785 (23.7%) | 120 (22.7%) | ||
| Unknown | 88 (2.7%) | 32 (6.1%) | ||
| Payer | Private Insurance | 1291 (38.9%) | 139 (26.3%) | < 0.0001 |
| AHCCCS^/Medicaid | 987 (29.8%) | 136 (25.8%) | ||
| Medicare | 356 (10.7%) | 85 (16.1%) | ||
| Self Pay | 497 (15%) | 115 (21.8%) | ||
| Other | 151 (4.6%) | 25 (4.7%) | ||
| Unknown | 34 (1%) | 28 (5.3%) | ||
| Trauma Type | Blunt | 3196 (96.4%) | 392 (74.2%) | < 0.0001 |
| Penetrating | 120 (3.6%) | 136 (25.8%) | ||
| Head Region Severity Score (ICD&) | 1–3 | 2060 (62.1%) | 40 (7.6%) | < 0.0001 |
| 4 | 883 (26.6%) | 53 (10%) | ||
| 5, 6 | 331 (10%) | 425 (80.5%) | ||
| Unknown | 42 (1.3%) | 10 (1.9%) | ||
| Injury Severity Score (ICD) | 1–14 | 1317 (39.7%) | 5 (0.9%) | < 0.0001 |
| 16–24 | 1038 (31.3%) | 19 (3.6%) | ||
| 25+ | 961 (29%) | 504 (95.5%) | ||
| Prehospital minimum SBP (mmHg) | 107 (40, 119) | 92 (40, 119) | < 0.0001 | |
| Prehospital hypoxia | No | 2886 (87%) | 274 (51.9%) | < 0.0001 |
| Yes | 282 (8.5%) | 162 (30.7%) | ||
| Unknown | 148 (4.5%) | 92 (17.4%) | ||
| Prehospital intubation | No | 2863 (86.3%) | 202 (38.3%) | < 0.0001 |
| Yes | 453 (13.7%) | 326 (61.7%) | ||
Median (min, max) for continuous variables and count (percentage) for categorical variables
Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables
International Classification of Diseases-Version 9
Trauma center was also highly significant (not shown; p < 0.0001)
Arizona Health Care Cost Containment System
Figure 1. Unadjusted Moving Average of Death Rate by Lowest SBP.
The solid curve represents the moving average of the estimated death rate for each interval spanning 10 consecutive values and the broken curves represent the pointwise 95% confidence bands. The details of these calculations are given in the Statistical Analysis section.
Table 2.
Parametric Terms in the Multivariate Logistic Regression Model for Death
| Covariate* | OR# | 95% CI | p-value | |
|---|---|---|---|---|
| Male | No | --- | --- | 0.541 |
| Yes | 0.91 | (0.67, 1.23) | ||
| Race | Black | --- | --- | 0.750 |
| Asian | 1.09 | (0.22, 5.37) | ||
| American Indian/Alaska Native | 1.02 | (0.36, 2.88) | ||
| White | 1.29 | (0.53, 3.11) | ||
| Other | 1.19 | (0.42, 3.36) | ||
| Unknown | 2.89 | (0.66, 12.75) | ||
| Hispanic | No | --- | --- | 0.058 |
| Yes | 0.61 | (0.40, 0.92) | ||
| Unknown | 1.03 | (0.46, 2.34) | ||
| Payer | Private | --- | --- | < 0.0001 |
| AHCCCS+/Medicaid | 1.24 | (0.86, 1.78) | ||
| Medicare | 1.72 | (1.00, 2.97) | ||
| Self Pay | 3.65 | (2.36, 5.65) | ||
| Other | 1.76 | (0.89, 3.48) | ||
| Unknown | 9.56 | (3.78, 24.16) | ||
| Trauma Type | Blunt | --- | --- | < 0.0001 |
| Penetrating | 3.89 | (2.53, 5.98) | ||
| Head Region Severity Score (ICD&) | 1–3 | --- | --- | < 0.0001 |
| 4 | 1.34 | (0.82, 2.20) | ||
| 5–6 | 13.2 | (8.41, 20.72) | ||
| Unknown | 6.31 | (2.36, 16.86) | ||
| Injury Severity Score (ICD) | 1–14 | --- | --- | < 0.0001 |
| 16–24 | 2.63 | (0.91, 7.60) | ||
| 25+ | 15.96 | (6.00, 42.50) | ||
| Prehospital hypoxia | No | --- | --- | < 0.0001 |
| Yes | 1.89 | (1.35, 2.65) | ||
| Unknown | 4.3 | (2.71, 6.83) | ||
| Prehospital intubation | No | --- | --- | < 0.0001 |
| Yes | 2.81 | (2.08, 3.78) |
Also adjusted for trauma centers (details not shown; p <0.0001)
Odds ratio for death compared to the referent category
International Classification of Diseases-Version 9
Arizona Health Care Cost Containment System
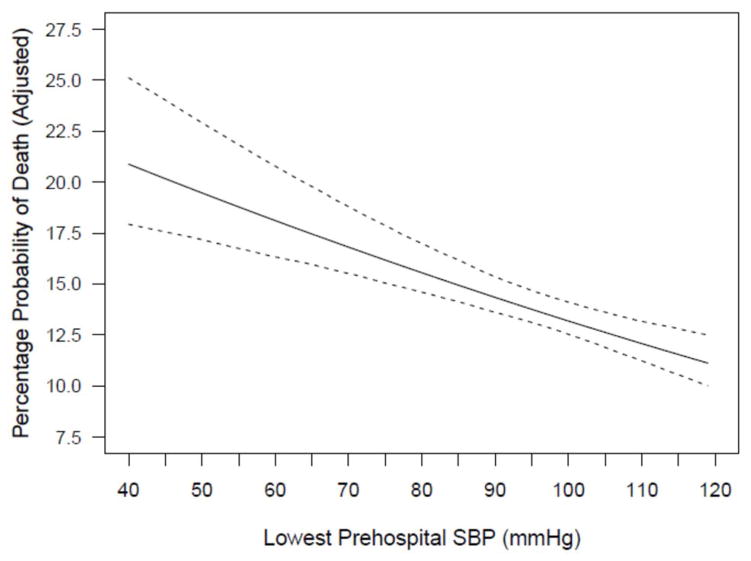
Figure 2. Adjusted Probability of Death by Lowest SBP.
Adjusted probability of death shown graphically over the range of 40 to 120 mmHg. The rate shown is the marginal rate in the sense that, at any fixed value of SBP, the rate is the average of the predicted death rates for all subjects in the dataset with the SBP value changed to the fixed value and with values of all other covariates unchanged from the actual observed values.
In the optimal model (based on AIC), the adjusted effect of lowest SBP on log odds of death was nearly perfectly linear, with an adjusted odds ratio (aOR) of 0.812 (95%CI: 0.748, 0.883; p<0.0001) associated with a 10mmHg increase in SBP at any level between 40 and 120mmHg (e.g., a patient with SBP=110mmHg has an 18.8% lower adjusted odds of death than one with SBP=100mmHg, and so on, throughout the entire range). Figure 2 shows graphically the adjusted probability of death over the range of 40–120mmHg. As can be seen, the rate of change in estimated probability of death is essentially constant. In other words, there is a striking absence of any identifiable SBP-versus-mortality threshold and major reductions in both crude and adjusted mortality continue far to the right of the “classical” 90mmHg hypotension level. Additional evidence comes from the receiver operating characteristic curve plot of the data. The area-under-the-curve is 0.705 and there is no cut-point that gives satisfactory levels of both sensitivity and specificity to indicate a threshold.
COMMENT
The previous literature related to this investigation consists of studies that were small,7,8,11,14–21,23,24,26,29,30,34,50 had limited or no prehospital data,7,11,14–17,20,21,24,26,28,29,34,36,50 or evaluated general trauma populations (were not TBI-specific).35,51–55 The current study is unique in both its size and its access to detailed prehospital data. A key reason for evaluating the impact of blood pressure measured before hospital arrival is because the injured brain is so highly sensitive to changes in perfusion and the timeframe during which neuronal damage begins is so short. It is well established that secondary brain injury is initiated by even brief periods of compromised blood flow.4,5,11–13,17,20,27 Thus, decreased perfusion occurring during the prehospital time interval may have a profound impact on outcome. Indeed, our results reveal a strong, independent association between mortality and blood pressure measured in the field. This is remarkable given the large number of factors that potentially impact survival in TBI patients. It appears that the effectiveness of subsequent interventions may be highly dependent upon patients being delivered to the trauma center who are neurologically viable so they have the potential to benefit from subsequent specialized care.
One of the most striking aspects of the literature evaluating the association between blood pressure and TBI mortality is the underlying assumption that there is a clinically-relevant “threshold.” Some might argue that this is merely an “operational” reality inherent to the studies (that some level of hypotension must be chosen as a treatment threshold). However, even if the threshold concept isn’t always explicitly affirmed, its use is so ubiquitous that, functionally, it is treated as a given in the literature. In other words, there is a nearly universal concept of the existence of a level of SBP that represents a cut-point, below which it is highly deleterious to drop. However, the results of the current investigation seem to provide a significant contrast to current thinking about the implications of hypotension in the early care of TBI. Visually evaluating the plot of adjusted mortality risk versus SBP (Figure 2) reveals a surprising finding—the absence of even a hint of a cut-point at any level between 40 and 120mmHg. In addition, the mathematical expression of the data verifies this visual impression in that the association between SBP and the adjusted log odds of death is linear, with an aOR of 0.812 for mortality associated with a 10mmHg increase regardless of the level being assessed. Thus, any two patients with an SBP difference of 10mmHg (say, 115 vs. 105, 90 vs. 80, or 75 vs. 65) differ in their adjusted odds of death by 18.8% and this is true across the entire SBP range. These results raise the possibility that, perhaps, no threshold exists in the sense that the concept is typically used. It appears that the threshold concept may have been artificially generated by investigations that, because of their small size, basically had no alternative but to deal with prehospital blood pressure dichotomously (i.e., “low” versus “not low”). However, as this literature grew, the concept gained momentum and was incorporated into guidelines.
Another notable finding revealed by Figure 2 is the lack of a change in the slope even as the plot moves far to the right of the commonly-applied definition for hypotension. This raises the possibility that, for the injured brain, clinically-meaningful hypotension may not be as low as is currently thought. Indeed, despite the specifically-recommended threshold, the guidelines also state that it is unclear what the threshold ought to be. Hence the explicit statement in the section on resuscitation endpoints: “The value of 90mmHg as a threshold for hypotension has been defined by blood pressure distributions for normal adults (emphasis added). Thus, this is more a statistical than physiological finding.”5 Furthermore, the document goes on to forthrightly admit ambivalence about the recommended threshold: “Given the influence of cerebral perfusion pressure on outcome, it is possible that SBP higher than 90mmHg would be desirable during the prehospital and resuscitation phase, but no studies have been performed to corroborate this.”5 The lack of clarity surrounding this issue led the guideline authors to give it high priority in the section on “Key Issues for Future Investigation.” In the listing of recommended future research, topic number one is the identification of “the level of hypotension that correlates with poor outcome.”5
A careful reading of the extant studies reflects the complexity of defining hypotension in the setting of TBI. In fact, the literature varies widely and contains reports that have utilized cut-points as low as 79mmHg and as high as 120mmHg in adults.7–9,11,14,15,17–22,26,28–36 Furthermore, the size and design of these studies preclude them from identifying “the” threshold, even if one actually exists. If previous prehospital studies had been larger, they would have been able to identify significant differences in outcome using a wide range of potential thresholds, thereby revealing the arbitrary nature of choosing any one particular level. To highlight this limitation of the current literature, we analyzed a broader cohort of EPIC patients (40–200mmHg) and dichotomized “low” versus “not low” using various cut-points in increments of 5mmHg. This yields the remarkable result that there is a statistically significant difference in the adjusted probability of death for thresholds as low as 60mmHg and as high as 135mmHg (Figure 3). In other words, one can pick any cut-point throughout this entire range and obtain “significant” findings. So, despite decades of assuming otherwise, it appears that the interaction between prehospital blood pressure and outcome may be physiologically continuous, rather than dichotomous, across a remarkably wide range. While it is hard to conceive of an approach to managing TBI that doesn’t include some level of blood pressure that “requires treatment,” it appears that the science that forms the basis for the current guidelines may require an entirely new way of thinking.
Figure 3. Wide-Ranged SBP “Thresholds” and Adjusted Odds Ratios of Death.
The cohort of EPIC patients whose lowest prehospital SBP was between 40 and 200 mmHg was dichotomized into “low” versus “not low” groups using various cut-points in increments of 5 mmHg. Then logistic regression was used to estimate the odds ratio of death between the two groups, adjusting for factors shown in Table 2. Squares represent estimated adjusted odds ratios and line segments represent 95% confidence intervals.
This study has limitations. First, the design is observational. Thus, we cannot establish cause-and-effect relationships related to the treatment of hypotension. For instance, these data do not prove that the therapeutic target for blood pressure should be higher than the current recommendations. However, they do highlight the great importance of perfusing the injured brain and that blood pressure is powerfully linked to outcome.16,25,28 Furthermore, these results do appear to support the statements in the TBI guidelines cautioning that the current recommendations may allow blood pressure to drop too low before intervening. A related concern is that we have not accounted for treatment of hypotension in the model. The parent study is designed specifically to identify the influence of treatment on outcomes using a controlled, before/after system design and the Analysis Plan27 includes only an interim analysis (completed) and a final analysis (scheduled) and does not allow for multiple “looks” at the interventional data. Thus, to prevent any encroachment on the main study hypotheses, we are deferring all evaluations of treatment effects until the final analysis. Second, this evaluation does not inform questions related to blood pressure management after the early resuscitative phase of care. This is true for several reasons: 1) ongoing pressure monitoring in neurocritical care utilizes mean arterial pressure and cerebral perfusion pressure rather than SBP and 2) the prehospital management of blood pressure focuses solely on treating hypotension.4 Thus, the implications of our study cannot be used to inform issues related to ongoing ICU management or controversies such as enhancing/optimizing perfusion.56,57 Third, there were some missing data. However, for a prehospital study, the rate of missing data is extremely low (e.g., 1.8% missing rate for SBP; no missing mortality data). Fourth, the database contains only those SBPs that were documented by EMS. Thus, we cannot know for sure that the reported measurements reflected the actual lowest SBP. Finally, there is no way to independently verify the accuracy of BP measurements. However, this is true of essentially all EMS investigations.58 One great advantage of the EPIC study is that the data team abstracts the PCRs directly and comprehensively. This level of scrutiny and consistency of data access is rare in prehospital research.58
CONCLUSION
In a statewide, multisystem analysis of major TBI, we found a linear relationship between lowest prehospital SBP and the severity-adjusted probability of death across an exceptionally wide range. This suggests that there may not be a clinically-meaningful “threshold.” Furthermore, for the injured brain, physiologically detrimental hypotension may occur at significantly higher levels than current guidelines suggest. These findings highlight the need for specific trials comparing various blood pressure treatment thresholds well above the “classic” 90mmHg.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R01NS071049. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding/Support and Role of Funder: The EPIC Study is funded by a grant from the National Institutes of Health (NIH/NINDS Grant # 1R01NS071049)
Role of the funding agency: The NIH had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest: The following authors have received support from the NIH grant via their university/academic appointments: DWS, BJB, VC, DS, BB, JBG, KRD, CV, PDA.
Study Registration: This is an observational, non-interventional analysis of a subset of the data in the EPIC study. The parent study, while not a randomized clinical trial, is registered at ClinicalTrials.gov: #NCT01339702
Author Contributions: Each author meets authorship criteria and specific criteria are identified in the individual authorship forms
-
Data Access, Responsibility, and Analysis:
DWS and CH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Chengcheng Hu (UA College of Public Health and Arizona Emergency Medicine Research Center) and Daniel Spaite (UA Department of Emergency Medicine and Arizona Emergency Medicine Research Center) conducted and are responsible for the data analysis.
Presentations: Presented, in part, to the National Association of EMS Physicians, January, 2014, Tucson, Arizona and to the International Brain Injury Association, San Francisco, California, March, 2014.
Footnotes
Registration: The parent study (EPIC), while not a randomized clinical trial, is registered at ClinicalTrials.gov: #NCT01339702
Study Registration: This is an observational, non-interventional analysis of a subset of the data in the EPIC study. The parent study, while not a randomized clinical trial, is registered at ClinicalTrials.gov: #NCT01339702
References
- 1.Bell JMBM, Jenkins EL, Haarbauer-Krupa J. Traumatic Brain Injury In the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control, Division of Unintentional Injury Prevention, Centers for Disease Control; 2014. [Google Scholar]
- 2.Finkelstein E, Corso PS, Miller TR. The incidence and economic burden of injuries in the United States. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- 3.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. 2003;3(S3):S2–S81. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- 4.Badjatia N, Carney N, Crocco TJ, et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care. 2008;12(Suppl 1):S1–52. doi: 10.1080/10903120701732052. [DOI] [PubMed] [Google Scholar]
- 5.Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 6.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012;13(Jan) Suppl 1:S1–S82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 7.Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Fearnside MR, Cook RJ, McDougall P, McNeil RJ. The Westmead Head Injury Project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg. 1993;7(3):267–279. doi: 10.3109/02688699309023809. [DOI] [PubMed] [Google Scholar]
- 9.Shutter LA, Narayan RK. Blood Pressure Management in Traumatic Brain Injury. Annals of Emergency Medicine. 2008;51(3, Supplement 1):S37–S38. doi: 10.1016/j.annemergmed.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Jankowitz BT, Adelson PD. Pediatric traumatic brain injury: past, present and future. Dev Neurosci. 2006;28(4–5):264–275. doi: 10.1159/000094153. [DOI] [PubMed] [Google Scholar]
- 11.Gentleman D. Causes and effects of systemic complications among severely head injured patients transferred to a neurosurgical unit. Int Surg. 1992;77(4):297–302. [PubMed] [Google Scholar]
- 12.Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993;28(3):310–314. doi: 10.1016/0022-3468(93)90223-8. discussion 315–316. [DOI] [PubMed] [Google Scholar]
- 13.Kokoska ER, Smith GS, Pittman T, Weber TR. Early hypotension worsens neurological outcome in pediatric patients with moderately severe head trauma. J Pediatr Surg. 1998;33(2):333–338. doi: 10.1016/s0022-3468(98)90457-2. [DOI] [PubMed] [Google Scholar]
- 14.Miller JD, Becker DP. Secondary insults to the injured brain. J R Coll Surg Edinb. 1982;27(5):292–298. [PubMed] [Google Scholar]
- 15.Barton CW, Hemphill JC, Morabito D, Manley G. A novel method of evaluating the impact of secondary brain insults on functional outcomes in traumatic brain-injured patients. Acad Emerg Med. 2005;12(1):1–6. doi: 10.1197/j.aem.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 16.Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L. Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Arch Surg. 2001;136(10):1118–1123. doi: 10.1001/archsurg.136.10.1118. [DOI] [PubMed] [Google Scholar]
- 17.Price DJ, Murray A. The influence of hypoxia and hypotension on recovery from head injury. Injury. 1972;3(4):218–224. doi: 10.1016/0020-1383(72)90104-0. [DOI] [PubMed] [Google Scholar]
- 18.Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma. 1996;40(5):764–767. doi: 10.1097/00005373-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Carrel M, Moeschler O, Ravussin P, Favre JB, Boulard G. Prehospital air ambulance and systemic secondary cerebral damage in severe craniocerebral injuries. Ann Fr Anesth Reanim. 1994;13(3):326–335. doi: 10.1016/s0750-7658(94)80041-3. [DOI] [PubMed] [Google Scholar]
- 20.Kohi YM, Mendelow AD, Teasdale GM, Allardice GM. Extracranial insults and outcome in patients with acute head injury--relationship to the Glasgow Coma Scale. Injury. 1984;16(1):25–29. doi: 10.1016/0020-1383(84)90110-4. [DOI] [PubMed] [Google Scholar]
- 21.Rose J, Valtonen S, Jennett B. Avoidable factors contributing to death after head injury. Br Med J. 1977;2(6087):615–618. doi: 10.1136/bmj.2.6087.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chesnut RM, Ghajar J, Maas AIR, et al. Part 2: Early indicators of prognosis in severe traumatic brain injury. J Neurotrauma. 2000;17(6–7):555. + [Google Scholar]
- 23.Hill DA, Abraham KJ, West RH. Factors affecting outcome in the resuscitation of severely injured patients. Aust N Z J Surg. 1993;63(8):604–609. doi: 10.1111/j.1445-2197.1993.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 24.Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien) 1993;59:121–125. doi: 10.1007/978-3-7091-9302-0_21. [DOI] [PubMed] [Google Scholar]
- 25.McHugh GS, Engel DC, Butcher I, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):287–293. doi: 10.1089/neu.2006.0031. [DOI] [PubMed] [Google Scholar]
- 26.Miller JD, Sweet RC, Narayan R, Becker DP. Early insults to the injured brain. Jama. 1978;240(5):439–442. [PubMed] [Google Scholar]
- 27.Spaite DW, Bobrow BJ, Stolz U, et al. Evaluation of the impact of implementing the emergency medical services traumatic brain injury guidelines in Arizona: the Excellence in Prehospital Injury Care (EPIC) study methodology. Acad Emerg Med. 2014;21(7):818–830. doi: 10.1111/acem.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry C, Ley EJ, Bukur M, et al. Redefining hypotension in traumatic brain injury. Injury. 2012;43(11):1833–1837. doi: 10.1016/j.injury.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Marmarou A, Anderson RL, Ward JD, et al. Impact of Icp Instability and Hypotension on Outcome in Patients with Severe Head Trauma. J Neurosurg. 1991;75:S59–S66. [Google Scholar]
- 30.Chi JH, Knudson MM, Vassar MJ, et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J Trauma. 2006;61(5):1134–1141. doi: 10.1097/01.ta.0000196644.64653.d8. [DOI] [PubMed] [Google Scholar]
- 31.Bernard SA, Nguyen V, Cameron P, et al. Prehospital rapid sequence intubation improves functional outcome for patients with severe traumatic brain injury: a randomized controlled trial. Ann Surg. 2010;252(6):959–965. doi: 10.1097/SLA.0b013e3181efc15f. [DOI] [PubMed] [Google Scholar]
- 32.Walia S, Sutcliffe AJ. The relationship between blood glucose, mean arterial pressure and outcome after severe head injury: an observational study. Injury. 2002;33(4):339–344. doi: 10.1016/s0020-1383(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 33.Cooke RS, McNicholl BP, Byrnes DP. Use of the Injury Severity Score in Head-Injury. Injury-International Journal of the Care of the Injured. 1995;26(6):399–400. doi: 10.1016/0020-1383(95)00064-g. [DOI] [PubMed] [Google Scholar]
- 34.Graham DI, Adams JH. Ischaemic brain damage in fatal head injuries. Lancet. 1971;1(7693):265–266. doi: 10.1016/s0140-6736(71)91003-8. [DOI] [PubMed] [Google Scholar]
- 35.Edwards M, Ley E, Mirocha J, Hadjibashi AA, Margulies DR, Salim A. Defining hypotension in moderate to severely injured trauma patients: raising the bar for the elderly. Am Surg. 2010;76(10):1035–1038. [PubMed] [Google Scholar]
- 36.Butcher I, Maas AI, Lu J, et al. Prognostic value of admission blood pressure in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):294–302. doi: 10.1089/neu.2006.0032. [DOI] [PubMed] [Google Scholar]
- 37.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 38.Rutledge R, Osler T, Emery S, Kromhout-Schiro S. The end of the Injury Severity Score (ISS) and the Trauma and Injury Severity Score (TRISS): ICISS, an International Classification of Diseases, ninth revision-based prediction tool, outperforms both ISS and TRISS as predictors of trauma patient survival, hospital charges, and hospital length of stay. J Trauma. 1998;44(1):41–49. doi: 10.1097/00005373-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Osler TM, Cohen M, Rogers FB, Camp L, Rutledge R, Shackford SR. Trauma registry injury coding is superfluous: a comparison of outcome prediction based on trauma registry International Classification of Diseases-Ninth Revision (ICD-9) and hospital information system ICD-9 codes. J Trauma. 1997;43(2):253–256. doi: 10.1097/00005373-199708000-00008. discussion 256–257. [DOI] [PubMed] [Google Scholar]
- 40.Hannan EL, Farrell LS, Gorthy SF, et al. Predictors of mortality in adult patients with blunt injuries in New York State: a comparison of the Trauma and Injury Severity Score (TRISS) and the International Classification of Disease, Ninth Revision-based Injury Severity Score (ICISS) J Trauma. 1999;47(1):8–14. doi: 10.1097/00005373-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Rutledge R, Hoyt DB, Eastman AB, et al. Comparison of the Injury Severity Score and ICD-9 diagnosis codes as predictors of outcome in injury: analysis of 44,032 patients. J Trauma. 1997;42(3):477–487. doi: 10.1097/00005373-199703000-00016. discussion 487–479. [DOI] [PubMed] [Google Scholar]
- 42.MacKenzie EJ, Steinwachs DM, Shankar B. Classifying trauma severity based on hospital discharge diagnoses. Validation of an ICD-9CM to AIS-85 conversion table. Med Care. 1989;27(4):412–422. doi: 10.1097/00005650-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 43.MacKenzie EJ, Garthe E. Compatibility of the ICD-9-CM and AIS-80: An Update. Association for the Advancement of Automotive Medicine (AAAM), Quarterly Journal. 1983;5:25–27. [Google Scholar]
- 44.Glance LG, Osler TM, Mukamel DB, Meredith W, Wagner J, Dick AW. TMPM-ICD9: a trauma mortality prediction model based on ICD-9-CM codes. Ann Surg. 2009;249(6):1032–1039. doi: 10.1097/SLA.0b013e3181a38f28. [DOI] [PubMed] [Google Scholar]
- 45.Wood SN. Generalized additive models : an introduction with R. Boca Raton, FL: Chapman & Hall/CRC; 2006. [Google Scholar]
- 46.Team RC. [Accessed October 5, 2015];R: A language and environment for statistical computing. 2015 http://www.R-project.org/
- 47.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc B. 2011;73:3–36. [Google Scholar]
- 48.Wood SN. On p-values for smooth components of an extended generalized additive model. Biometrika. 2013;100(1):221–228. [Google Scholar]
- 49.Silverston P. Pulse oximetry at the roadside: a study of pulse oximetry in immediate care. Bmj. 1989;298(6675):711–713. doi: 10.1136/bmj.298.6675.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooke RS, McNicholl BP, Byrnes DP. Early Management of Severe Head-Injury in Northern-Ireland. Injury-International Journal of the Care of the Injured. 1995;26(6):395–397. doi: 10.1016/0020-1383(95)00003-r. [DOI] [PubMed] [Google Scholar]
- 51.Bruns B, Gentilello L, Elliott A, Shafi S. Prehospital hypotension redefined. J Trauma. 2008;65(6):1217–1221. doi: 10.1097/TA.0b013e318184ee63. [DOI] [PubMed] [Google Scholar]
- 52.Hasler RM, Nuesch E, Juni P, Bouamra O, Exadaktylos AK, Lecky F. Systolic blood pressure below 110 mm Hg is associated with increased mortality in blunt major trauma patients: multicentre cohort study. Resuscitation. 2011;82(9):1202–1207. doi: 10.1016/j.resuscitation.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 53.Hasler RM, Nuesch E, Juni P, Bouamra O, Exadaktylos AK, Lecky F. Systolic blood pressure below 110 mmHg is associated with increased mortality in penetrating major trauma patients: Multicentre cohort study. Resuscitation. 2012;83(4):476–481. doi: 10.1016/j.resuscitation.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Eastridge BJ, Salinas J, McManus JG, et al. Hypotension begins at 110 mm Hg: redefining “hypotension” with data. J Trauma. 2007;63(2):291–297. doi: 10.1097/TA.0b013e31809ed924. discussion 297–299. [DOI] [PubMed] [Google Scholar]
- 55.Edelman DA, White MT, Tyburski JG, Wilson RF. Post-traumatic hypotension: should systolic blood pressure of 90–109 mmHg be included? Shock. 2007;27(2):134–138. doi: 10.1097/01.shk.0000239772.18151.18. [DOI] [PubMed] [Google Scholar]
- 56.Robertson CS, Valadka AB, Hannay HJ, et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med. 1999;27(10):2086–2095. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Howells T, Elf K, Jones PA, et al. Pressure reactivity as a guide in the treatment of cerebral perfusion pressure in patients with brain trauma. J Neurosurg. 2005;102(2):311–317. doi: 10.3171/jns.2005.102.2.0311. [DOI] [PubMed] [Google Scholar]
- 58.Spaite DW, Valenzuela TD, Meislin HW. Barriers to EMS System Evaluation: Problems Associated with Field Data Collection. Prehospital Disaster Med. 1993;8(S1):S35–S40. doi: 10.1017/s1049023x00040541. [DOI] [PubMed] [Google Scholar]