Abstract
Aim
To confirm glycaemic control superiority of mealtime fast‐acting insulin aspart (faster aspart) in a basal–bolus (BB) regimen vs basal‐only insulin.
Materials and methods
In this open‐label, randomized, 18‐week trial (51 sites; 6 countries), adults (n = 236) with inadequately controlled type 2 diabetes (T2D; mean glycosylated haemoglobin [HbA1c] ± SD: 7.9% ± 0.7% [63.1 ± 7.5 mmol/mol]) receiving basal insulin and oral antidiabetic drugs underwent 8‐week optimization of prior once‐daily basal insulin followed by randomization 1:1 to either a BB regimen with faster aspart (n = 116) or continuation of once‐daily basal insulin (n = 120), both with metformin. Primary endpoint was HbA1c change from baseline after 18 weeks of treatment. Secondary endpoints included: postprandial plasma glucose (PPG) change and overall PPG increment (all meals); weight; treatment‐emergent adverse events; hypoglycaemic episodes.
Results
HbA1c decreased from 7.9% (63.2 mmol/mol) to 6.8% (50.7 mmol/mol; BB group) and from 7.9% (63.2 mmol/mol) to 7.7% (60.7 mmol/mol; basal‐only group); estimated treatment difference [95% confidence interval] −0.94% [−1.17; −0.72]; −10.3 mmol/mol [−12.8; −7.8]; P < .0001. Reductions from baseline in overall mean 2‐hour PPG and overall PPG increment for all meals (self‐measured plasma glucose profiles) were statistically significant in favour of BB treatment (P < .0001). Severe/blood glucose confirmed hypoglycaemia rate (12.8 vs 2.0 episodes per patient‐years of exposure), total daily insulin (1.2 vs 0.6 U/kg) and weight gain (1.8 vs 0.2 kg) were greater with BB than with basal‐only treatment.
Conclusions
In T2D, faster aspart in a BB regimen provided superior glycaemic control as compared with basal‐only insulin, but with an increase in the frequency of hypoglycaemia and modest weight gain.
Keywords: glycaemic control, hypoglycaemia, insulin therapy, phase 3 study, randomized trial, type 2 diabetes
1. INTRODUCTION
The progressive nature of type 2 diabetes (T2D)1 means that many individuals who commence oral antidiabetic drugs (OADs) will require treatment intensification. Addition of basal insulin therapy as part of an individualized, patient‐centred approach is recommended when OADs alone do not achieve, or no longer maintain, glycaemic control.2 Further treatment intensification may be required when target glycosylated haemoglobin (HbA1c) levels3, 4 are not reached after 3 to 6 months of basal titration.5 Postprandial plasma glucose (PPG) contributes substantially to glycaemic control6, 7 and, as HbA1c levels approach 7.0%, it becomes the dominant contributor to HbA1c.8 Thus, optimal PPG control is an important component of achieving target HbA1c.9, 10 In T2D, blunted and/or delayed postmeal insulin secretion is a major pathophysiological factor underlying postprandial hyperglycaemia.11
Current guidelines recommend a variety of injectable intensification therapies to reduce PPG excursions,2, 12 and addition of mealtime rapid‐acting insulin analogues (RAIAs) to basal insulin is a common approach to intensify treatment.13, 14 However, approved RAIA formulations do not adequately and fully approach the physiological mealtime insulin response. Additionally, evidence suggests some degree of clinical inertia with regard to intensifying therapy in individuals with T2D who are receiving basal insulin.13
Fast‐acting insulin aspart (faster aspart) is an ultra‐fast‐acting mealtime insulin that is insulin aspart in a new formulation, containing 2 additional excipients, niacinamide and L‐arginine. Non‐clinical data show that the addition of niacinamide promotes the formation of insulin aspart monomers after subcutaneous injection, facilitating a more rapid rate of insulin aspart absorption across the endothelium into the blood.15 Faster aspart has a twice‐as‐fast onset of appearance (4 vs 9 minutes) and, within the first 30 minutes, a two‐fold higher insulin concentration and 74% greater insulin action compared with conventional insulin aspart in individuals with type 1 diabetes (T1D).16
As part of a basal‐bolus (BB) regimen (and with no overall increase in the rate of hypoglycaemia), mealtime faster aspart has shown superior PPG control and statistically significant HbA1c reductions as compared with insulin aspart in T1D,17 and effective lowering of HbA1c with improved PPG control 1 hour after a meal test in inadequately controlled T2D.18
Here, we report findings from the 18‐week onset 3 trial, which evaluated the efficacy and safety of adding faster aspart to basal insulin therapy vs basal insulin alone, both in combination with metformin, in patients with T2D.
2. MATERIALS AND METHODS
2.1. Trial design
This was an 18‐week, multicentre, randomized, open‐label, parallel‐group trial comparing faster aspart in a BB regimen to basal insulin therapy alone, both in combination with metformin, in adults with T2D. Prior to randomization there was an 8‐week run‐in period. Participant follow‐up occurred at 7 and 30 days from the end of trial (EOT).
The trial was conducted in accordance with the Declaration of Helsinki19 and the International Conference on Harmonization Good Clinical Practice.20 Prior to trial initiation, all documentation was reviewed and approved according to local regulations by appropriate health authorities, and by Institutional Review Boards. The trial protocol is available online (https://www.clinicaltrialsregister.eu/ctr‐search/trial/2012‐005583‐10/SI).
2.2. Participants
Participants were ≥18 years old with a body mass index ≤40.0 kg/m2, diagnosed with T2D ≥6 months and had been treated for ≥3 months prior to screening with basal insulin (once‐daily insulin detemir, insulin glargine U100 or neutral protamine Hagedorn [NPH]) and metformin ≥1000 mg, with or without other OADs (Appendix S1). Participants had laboratory‐measured HbA1c of 7.5% to 9.5% (58.5‐80.3 mmol/mol) if taking metformin, or 7.5% to 9.0% (58.5–74.9 mmol/mol) if taking metformin plus another OAD at the screening visit (Table S1, Appendix S1). Participants were requested to eat ≥3 main meals every day during the trial. Exclusion criteria included any use of bolus insulin, except short‐term use because of intermittent illness (≤14 days of consecutive treatment and not within 3 months prior to the screening visit); and glucagon‐like peptide 1 agonists and/or thiazolidinediones within 3 months prior to screening (Appendix S1).
2.3. Interventions
2.3.1. Basal titration
At the start of the 8‐week run‐in, participants continued once‐daily basal insulin and metformin at pre‐trial doses; all other OADs were discontinued. During run‐in, the basal insulin dose was optimized using a treat‐to‐target approach, with weekly adjustments to a pre‐breakfast target self‐measured plasma glucose (SMPG) of 4.0 to 6.0 mmol/L (71‐108 mg/dL) (Table S2, Appendix S1). After run‐in, the basal insulin dose was adjusted at the investigator's discretion. Basal insulin (100 U/mL) was injected subcutaneously once‐daily at approximately the same time every evening (insulin detemir and NPH using a 3 mL FlexPen; insulin glargine U100 using a 3 mL SoloStar pen).
2.3.2. Bolus titration
Participants requiring further intensification (ie, those meeting the randomization criterion of HbA1c 7.0% to 9.0% [53.0‐74.9 mmol/mol] following optimization of basal insulin during the run‐in period) were randomized (baseline; Week 0 [Visit 10]) 1:1 to receive a BB regimen with mealtime faster aspart or to continue once‐daily basal insulin, both in combination with metformin (pre‐screening dose). Randomization was stratified based on the type of basal insulin used. All participants randomized to the BB group commenced 4 U of faster aspart before each meal, which was self‐adjusted daily by 1 U increments (a ‘+1/0/–1’ titration algorithm, with no specified maximum value), aiming for a pre‐prandial or bedtime target of 4.0 to 6.0 mmol/L (71‐108 mg/dL) (Table S3, Appendix S1). Faster aspart (100 U/mL; 3 mL PDS290 pre‐filled pen injector) was injected subcutaneously into the abdomen 0 to 2 minutes before each main meal.
2.4. SMPG
At the run‐in visit, participants were given blood glucose (BG) meters (Abbott Freestyle Lite or Optium, calibrated to display plasma glucose [PG] values) and were instructed to perform 7‐point profiles (PG values taken before and 2 hours after each main meal, and at bedtime) on 3 consecutive days before the scheduled visit (Weeks 0, 6, 12 and 18). For titration, participants randomized to receive faster aspart + basal insulin recorded daily 4‐point profiles (pre‐prandial and at bedtime), while it was at the investigator's discretion to provide PG monitoring recommendations to participants randomized to receive basal insulin only.
2.5. Data conversions
HbA1c values were transformed from % to mmol/mol by multiplying by 10.929 and subtracting 23.49735. The conversion factor used for glucose between mmol/L and mg/dL was 0.0555.
2.6. Assessments
2.6.1. Primary endpoint
The primary endpoint was change in HbA1c from baseline (Week 0; Visit 10) after 18 weeks of randomized treatment.
2.6.2. Supportive secondary efficacy endpoints
Secondary efficacy endpoints after 18 weeks included achievement of HbA1c targets of <7.0% (53.0 mmol/mol; American Diabetes Association [ADA])3 and ≤6.5% (47.5 mmol/mol; International Diabetes Federation),4 with or without severe hypoglycaemia.
Supportive efficacy endpoints derived from SMPG values included overall mean 2‐hour PPG (for all meals), overall PPG increment (for all meals) and mean 8‐point SMPG profiles (from the second 7‐point SMPG profile and the before‐breakfast measurement from the following day). Other endpoints included the proportion of participants achieving an overall 2‐hour PPG target ≤7.8 mmol/L (140 mg/dL) and the same targets without severe hypoglycaemia,21 daily insulin dose and change from baseline to Week 18 in fasting plasma glucose (FPG), 1,5‐anhydroglucitol (1,5‐AG; a marker for postprandial hyperglycaemia)22 and body weight.
2.6.3. Supportive secondary safety endpoints
Supportive secondary safety endpoints included the number of treatment‐emergent adverse events (TEAEs), hypoglycaemic episodes and injection‐site or allergic reactions related to insulin. An adverse event (AE) was defined as treatment‐emergent if the event onset occurred on or after the first day of exposure to, and no later than 7 days after the last day of, randomized treatment. Hypoglycaemia was categorized as “severe” according to the ADA classification of an event requiring assistance of another person to actively administer carbohydrates or glucagon or take other corrective actions,21 or as BG confirmed by a PG value <3.1 mmol/L (56 mg/dL), with or without symptoms (an additional cut‐off point, below which normal physiological symptoms of hypoglycaemia occur). A hypoglycaemic event was categorized as treatment‐emergent if onset occurred on or after the first day of exposure to, and no later than 1 day after the last day of, randomized treatment. Information on cardiovascular (CV) events and deaths occurring after randomization (baseline) was sent for evaluation by an external adjudication committee.
2.7. Statistical methods
Analyses of all efficacy endpoints were based on the full analysis set (FAS; all randomized participants). Safety endpoints were summarized based on the safety analysis set and statistical analyses for hypoglycaemic episodes were based on the FAS.
The primary endpoint was analyzed using a mixed‐effect model for repeated measurements (MMRM). All calculated changes in HbA1c from baseline at Visits 16, 22 and 28 were included in the analysis (Appendix S1). Several sensitivity analyses of the primary endpoint were performed (Appendix S1).
For the supportive secondary efficacy endpoints, HbA1c and PPG target endpoints were analyzed separately based on a logistic regression model using treatment, strata and region as factors, and baseline HbA1c or baseline mean 2‐hour PPG as covariate. The mean of the 8‐point profile (SMPG) was defined as the area under the profile divided by the measurement time and was calculated using the trapezoidal method. The overall mean 2‐hour PPG and PPG increments for all meals were derived from the individual mealtime mean 2‐hour PPG and PPG increment values. The change from baseline in overall mean 2‐hour PPG (for all meals), overall PPG increment (for all meals), mean 8‐point SMPG profiles, body weight, FPG and 1,5‐AG after 18 weeks of randomized treatment were analyzed using an MMRM similar to that used for analysis of the primary endpoint and the corresponding baseline value as covariate. Insulin doses were summarized descriptively in units or units/kg.
Safety endpoints, including TEAEs, injection‐site and allergic reactions and major adverse CV events (MACE) were summarized descriptively. The number of treatment‐emergent severe or BG confirmed hypoglycaemic episodes was analyzed using a negative binomial regression model (Appendix S1).
2.8. Role of the funding source
The sponsor of the trial was Novo Nordisk A/S (Bagsvård, Denmark). All authors had access to the study data and take responsibility for the accuracy of the analysis, and had authority in the decision to submit the manuscript for publication, in collaboration with Novo Nordisk A/S.
3. RESULTS
3.1. Baseline characteristics
In total, 236 participants were randomized to receive faster aspart + basal (n = 116) or basal insulin (n = 120) and 94.1% completed the trial (Figure 1). Baseline characteristics were similar between treatment groups (Table 1).
Figure 1.
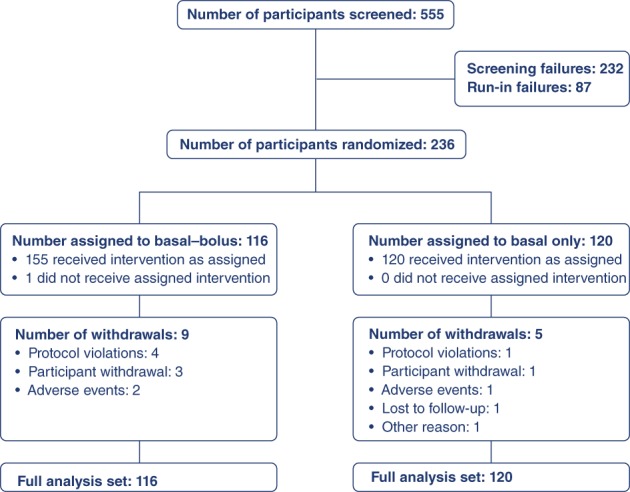
Participant disposition. A total of 323 participants entered the run‐in period of the trial; the most common reason for failure during the run‐in period was failure to meet randomization criteria (HbA1c 7.0%‐9.0% [53‐75 mmol/mol]) at Visit 9 (Week –1). The pattern of withdrawal was low and comparable between groups, with 94.1% of participants completing the trial. HbA1c, glycosylated haemoglobin
Table 1.
Baseline characteristics at randomization
| Characteristic N, FAS | Faster aspart + basal (n = 116) | Basal (n = 120) | Total (N = 236) |
|---|---|---|---|
| Age, years (SD) | 57.5 (9.9) | 57.4 (8.5) | 57.4 (9.2) |
| Gender, n (%) | |||
| Male | 55 (47.4) | 59 (49.2) | 114 (48.3) |
| Female | 61 (52.6) | 61 (50.8) | 122 (51.7) |
| BMI, kg/m2 (SD) | 30.4 (5.0) | 31.1 (4.7) | 30.8 (4.8) |
| Body weight, kg (SD) | 82.2 (16.2) | 85.1 (17.3) | 83.7 (16.8) |
| Duration of diabetes, years (SD) | 10.9* (6.1) | 11.8 (7.4) | 11.3 (6.3) |
| HbA1c | |||
| % (SD) | 7.9 (0.7) | 7.9 (0.7) | 7.9 (0.7) |
| mmol/mol (SD) | 63.2 (7.6) | 63.1 (7.4) | 63.1 (7.5) |
| FPG | |||
| mmol/L (SD) | 7.4 (2.4) | 7.7† (2.9) | 7.5 (2.6) |
| mg/dL (SD) | 132.5 (43.5) | 138.9 (51.4) | 135.7 (47.7) |
| Basal insulin at baseline | n (%) | n (%) | N (%) |
| Insulin glargine | 76 (65.5) | 77 (64.2) | 153 (64.8) |
| Insulin detemir | 16 (13.8) | 17 (14.2) | 33 (14.0) |
| NPH | 24 (20.7) | 26 (21.7) | 50 (21.2) |
Abbreviations: BMI, body mass index; FAS, full analysis set; faster aspart, fast‐acting insulin aspart; FPG, fasting plasma glucose; HbA1c, glycosylated haemoglobin; NPH, neutral protamine Hagedorn. The conversion factor used for glucose between mmol/L and mg/dL was 0.0555.
*n = 115; †n = 119. Values for baseline characteristics are arithmetic means, unless stated otherwise.
3.2. Efficacy
3.2.1. Glycaemic control
Following run‐in, mean HbA1c decreased further in both treatment groups from baseline to Week 18 (Figure 2A). The estimated change from baseline in HbA1c was −1.2% (−12.7 mmol/mol) in the faster aspart + basal group and −0.2% (−2.4 mmol/mol) in the basal group (estimated treatment difference, ETD [95% confidence interval; CI]: −0.94% [−1.17; −0.72]; −10.3 mmol/mol [−12.8; −7.8]; P < .0001), confirming the superiority of mealtime faster aspart in a full BB regimen vs basal insulin therapy.
Figure 2.
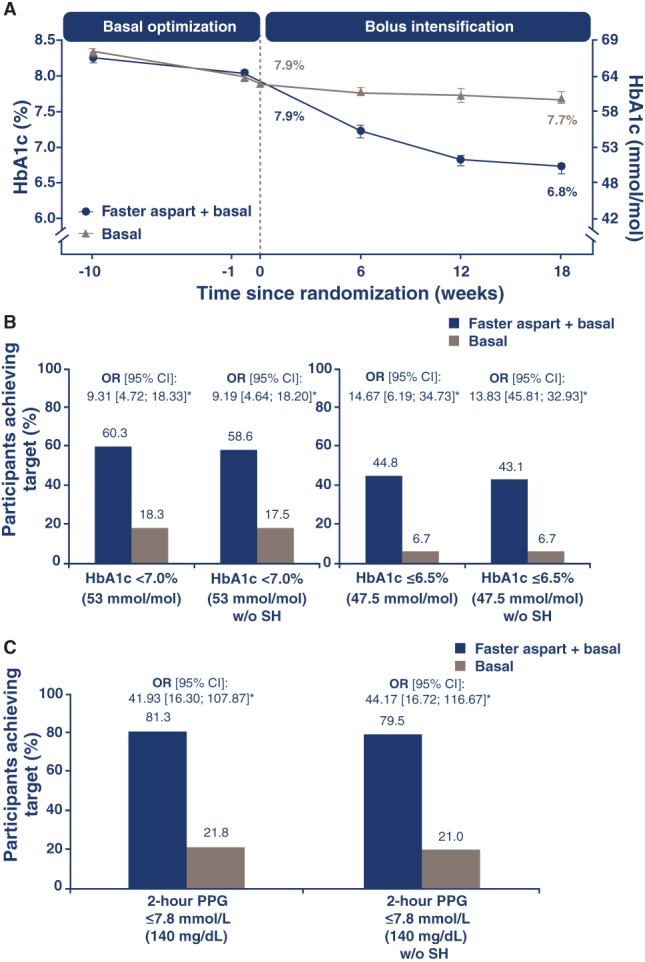
Observed mean HbA1c change from baseline to Week 18 (A), participants who achieved target HbA1c (B) and PPG levels (C) at Week 18. *P < .0001. Error bars: ± standard error of the mean. HbA1c targets: <7.0% (53.0 mmol/mol) and ≤6.5% (47.5 mmol/mol), and <7.0% (53.0 mmol/mol) and ≤6.5% (47.5 mmol/mol) without SH. PPG (based on SMPG) target: 2‐hour PPG ≤ 7.8 mmol/L (140 mg/dL) and 2‐hour PPG ≤7.8 mmol/L (140 mg/dL) without SH. Basal insulin: insulin detemir, insulin glargine U100 or NPH insulin. CI, confidence interval; faster aspart, fast‐acting insulin aspart; HbA1c, glycosylated haemoglobin; NPH, neutral protamine Hagedorn; OR, estimated odds ratio; PPG, postprandial plasma glucose; SH, severe hypoglycaemia during treatment period; SMPG, self‐measured plasma glucose. The conversion factor used for glucose between mmol/L and mg/dL was 0.0555
A greater number of participants in the faster aspart + basal group than in the basal group achieved HbA1c <7.0% (53.0 mmol/mol), HbA1c <7.0% (53.0 mmol/mol) without severe hypoglycaemia, ≤6.5% (47.5 mmol/mol) or ≤6.5% (47.5 mmol/mol) without severe hypoglycaemia (Figure 2B).
At EOT, observed overall mean 2‐hour PPG for all meals decreased from 10.0 mmol/L (180 mg/dL) at baseline to 7.2 mmol/L (130 mg/dL) in the faster aspart + basal group, and from 10.3 mmol/L (186 mg/dL) to 9.6 mmol/L (173 mg/dL) in the basal group; the estimated reduction from baseline in overall mean 2‐hour PPG for all meals between treatments was statistically significant in favour of faster aspart + basal (ETD [95% CI]: −2.48 mmol/L [−2.92; −2.03]; −44.6 mg/dL [−52.7; −36.6]; P < .0001). Overall mean PPG increment (for all meals) in the faster aspart + basal group decreased from 2.4 mmol/L (43 mg/dL) at baseline to 0.9 mmol/L (17 mg/dL) at EOT, whereas, in the basal group, it decreased from 2.5 mmol/L (46 mg/dL) to 2.0 mmol/L (37 mg/dL); the estimated reduction from baseline in overall PPG increment was statistically significant in favour of faster aspart + basal insulin (ETD [95% CI]: −1.14 mmol/L [−1.50; −0.77]; −20.5 mg/dL [−27.1; −13.8]; P < .0001).
A statistically significant difference in favour of faster aspart + basal vs basal only was observed in the decrease from baseline in mean 8‐point SMPG profile. Mean 8‐point SMPG profile decreased from 8.7 mmol/L (157 mg/dL) at baseline to 6.7 mmol/L (121 mg/dL) at EOT in the faster aspart + basal group and from 8.9 mmol/L (161 mg/dL) to 8.4 mmol/L (152 mg/dL) in the basal only group (ETD [95% CI]: −1.88 mmol/L [−2.21; −1.54]; −33.8 mg/dL [−39.9; −27.8]; P < .0001). The 8‐point SMPG profiles averaged for each time point at Week 18 are shown in Figure 3.
Figure 3.
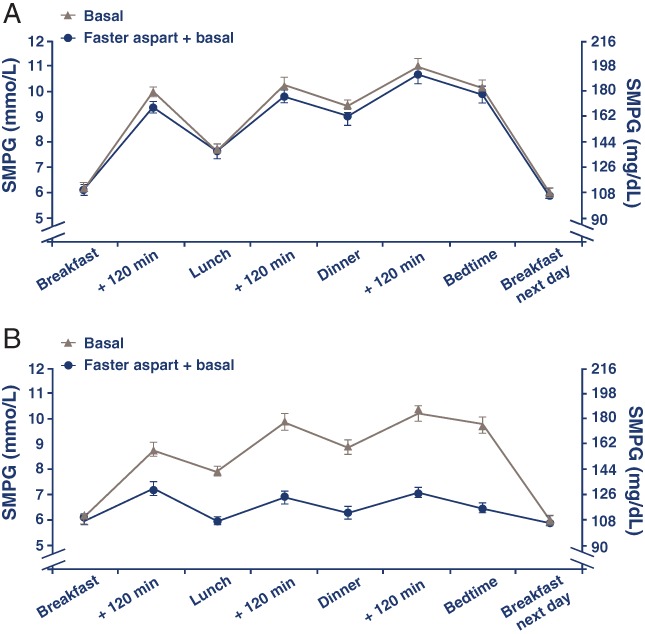
Eight‐point SMPG profiles at baseline (A) and Week 18 (B). Values are based on the full analysis set and averaged for each time point ± standard error of the mean. Basal insulin: insulin detemir, insulin glargine U100 or NPH insulin. Faster aspart, fast‐acting insulin aspart; NPH, neutral protamine Hagedorn; SMPG, self‐measured plasma glucose. The conversion factor used for glucose between mmol/L and mg/dL was 0.0555
At baseline, comparable numbers of participants in both groups achieved overall mean 2‐hour PPG values ≤7.8 mmol/L (140 mg/dL; 22.3% in the faster aspart + basal group vs 16.0% in the basal group). After 18 weeks of randomized treatment, approximately 4 times as many participants in the faster aspart + basal vs the basal group reached overall mean 2‐hour PPG values ≤7.8 mmol/L (140 mg/dL) or 2‐hour PPG ≤7.8 mmol/L (140 mg/dL) without severe hypoglycaemia (Figure 2C).
Estimated reductions from baseline to EOT in FPG were small and comparable in both groups (Table S4, Appendix S1). Estimated change from baseline in 1,5‐AG was greater in the faster aspart + basal group vs the basal group (ETD [95% CI]: 4.24 μg/mL [3.04; 5.44]; P < .0001) (Table S4, Appendix S1).
Mean body weight increased from baseline to EOT in the faster aspart + basal group. The ETD (faster aspart + basal – basal only) for change from baseline in body weight was 1.66 kg (95% CI, 0.89; 2.43), which was statistically significant (P < .0001) (Table S4, Appendix S1).
3.2.2. Daily insulin dosing
Total daily insulin dose increased in the faster aspart + basal group from baseline to Week 18 as a result of bolus intensification, and mean total insulin doses at EOT were 1.2 U/kg and 0.6 U/kg (faster aspart + basal vs basal only) (Table S5, Appendix S1). The proportion of total daily insulin delivered as a bolus, relative to basal insulin, was approximately 55% after 18 weeks of randomized treatment.
3.2.3. Safety endpoints
Overall, TEAEs were reported in 40.9% of participants (n = 47) in the faster aspart + basal group and 51.7% of participants (n = 62) in the basal‐only group (Table S6, Appendix S1). Most TEAEs in both groups were mild or moderate. Four injection‐site reactions were observed (1, faster aspart + basal; 3, basal). The most common allergic reaction was cough (4 [3.5%], faster aspart + basal; 1 [0.8%] basal).
Information on 2 non‐treatment‐emergent CV events was sent for adjudication (1 in the run‐in period, which was fatal, and 1 in the follow‐up period in the faster aspart + basal group); there were no MACE in this trial, and no deaths during the treatment period.
Overall, severe or BG confirmed hypoglycaemia was more frequent with faster aspart + basal (n = 67 [58.3%]) than with basal only treatment (n = 30 [25.0%]; overall hypoglycaemia rates, 12.8 vs 2.0 episodes per patient‐years of exposure; treatment rate ratio [95% CI]: 8.24 [4.93; 13.76]; P < .0001) (Table S7, Appendix S1). Severe hypoglycaemia was also more frequent in the faster aspart + basal group than in the basal group (0.18 vs 0.02 episodes per patient‐years of exposure; treatment rate ratio [95% CI]: 8.89 [0.27; 292.97]; P = .22) (Table S7, Appendix S1).
4. DISCUSSION
In this trial, addition and titration of mealtime faster aspart to basal insulin + metformin effectively improved glycaemic control in individuals with T2D, demonstrating the expected superiority to basal insulin + metformin alone for HbA1c and postprandial glycaemic control. There were no unexpected AEs, and treatments were well tolerated. As anticipated, hypoglycaemia rates, weight gain and daily insulin dose were higher in the BB group compared with the basal‐only group.
Participants had similar HbA1c at baseline and, importantly, similar FPG and pre‐breakfast SMPG levels at EOT; therefore, the PPG improvements may explain the improvement in HbA1c. The importance of targeting both FPG and PPG was highlighted in the GINGER and PREFER studies, which demonstrated statistically significant improvements in glycaemic control, in terms of both HbA1c reduction and PPG control, following administration of a BB regimen, as compared with twice‐daily premixed insulin in participants with T2D.23, 24
The averaged 8‐point SMPG profiles after 18 weeks of randomized treatment illustrate rising prandial PG and 2‐hour PPG levels in the basal‐only group (which appeared to accumulate) over a 24‐hour period, emphasizing the need for prandial glycaemic control in this population. The substantial improvements in PG increments with the addition of mealtime insulin were mirrored by the 1,5‐AG results.
BB regimens are known to increase the risk of weight gain compared with basal insulin regimens.25, 26 The expected increase in body weight in the BB group is modest and in line with the weight gain following bolus intensification observed previously.23, 24, 25, 26, 27
As anticipated in the context of a BB regimen and the lower mean HbA1c levels achieved, there were more severe or BG confirmed hypoglycaemic episodes in the faster aspart + basal group than in the basal‐only group. However, hypoglycaemia rates were in line with those observed in other trials when initiating a full BB regimen in T2D. Indeed, in a recent meta‐analysis of randomized controlled trials in T2D in which insulin therapy was intensified to a BB regimen, the event rate for overall hypoglycaemia averaged 12.1 episodes per patient‐year.28 Nevertheless, in the current study, a large proportion of participants achieved the more ambitious HbA1c target of ≤6.5% without experiencing severe hypoglycaemia, highlighting the feasibility of targeting PPG control to achieve glycaemic targets without severe hypoglycaemia, even for participants close to recommended targets.29 Participants were recommended to achieve uniform and near‐normal levels of fasting and postprandial glucose, whereas, in clinical practice, target levels would be adjusted for each individual. The tight titration targets and frequent SMPG measurements probably contributed to the rate of hypoglycaemia in the BB arm of this trial.
No conclusion can be drawn from this study regarding the benefit of faster aspart over other mealtime insulins; other trials have demonstrated non‐inferiority of faster aspart to insulin aspart in terms of HbA1c control, and superiority in terms of PPG control in individuals with T1D or T2D.17, 18 The current study shows that faster aspart can be added to a basal regimen using a simple patient‐driven titration algorithm and this quantifies the benefit:risk assessment in terms of improvement in PPG and HbA1c vs increased risk of hypoglycaemia and weight gain. This is valuable information for physicians who are considering different intensification options, including addition of a mealtime insulin, especially given the scarcity of trials wherein addition of bolus insulin is being compared with basal insulin + metformin. Although it is well‐known that addition of bolus insulin for patients with T2D who are inadequately controlled on basal insulin improves glycaemic control, in real‐world clinical practice there seems to be clinical inertia with regard to intensifying therapy, owing to several physician and patient barriers. As a result, patients eligible for intensification may remain for too long on basal insulin only. A recently published retrospective cohort study that investigated clinical inertia, involving 11 696 patients with T2D, identified a failure to intensify treatment regimens when required in patients receiving basal insulin.13 Indeed, treatment regimens were actually intensified in only 30.9% of patients who were clinically eligible for intensification (HbA1c ≥7.5%). Moreover, the median time to intensification with bolus or premix insulin or glucagon‐like peptide‐1 analogue after the first recording of HbA1c ≥ 7.5% (58 mmol/mol) was 3.7 years. The reasons for not intensifying treatment may be multiple and complex. In the current study, we demonstrate a simple method of treatment intensification, which could be applied by clinicians in routine practice.
The open‐label design of the trial may be a limitation; however, masking would have required a large number of placebo injections and may have led to unmasking as a result of the lack of effect of placebo on PPG levels. The protocol specified a full BB regimen for intensification rather than the step‐wise approach often used in clinical practice,12, 25, 30 and implementation of a step‐wise approach may have reduced hypoglycaemia rates. However, this trial was designed to show the maximum benefit of the addition and titration of mealtime insulin. Additionally, approximately 70% of individuals with T2D who commence treatment with a basal + one bolus insulin injection will require a full BB regimen within 1 year.12, 25, 26 The present trial was of relatively short duration (18 weeks). Initiating a new insulin treatment requires a period of dose adjustment characterized by successive titrations until PG levels and insulin dose stabilize. The use of a run‐in period ensured optimization of the basal insulin regimen in both groups prior to randomization, and the daily algorithm used to calculate bolus insulin doses mirrors popular clinical approaches.
A BB strategy remains an effective option to intensify glycaemic control following a basal + OAD regimen. The EOT HbA1c of 6.8% (50.7 mmol/mol) observed in the current study indicates the potential clinical benefit of this approach, using faster aspart as part of a BB regimen in T2D. The superior overall and PPG control that was observed following addition and titration of mealtime faster aspart to basal insulin + metformin, as compared with basal insulin + metformin alone, was accompanied by an expected increased rate of hypoglycaemia. Addition of bolus insulin is a valuable intensification approach for individuals with T2D who are inadequately controlled by treatment with basal insulin.
Supporting information
Appendix S1.
ACKNOWLEDGEMENTS
The authors are grateful to those who participated in this study, to Alexandru Lucian Dinita, MD and Anna Maria Louice Sandberg from Novo Nordisk A/S for their review of the manuscript and input, and to Stephanie Gibbons, PhD and Katharine A. Peregrin, PhD from AXON Communications for medical writing and editorial assistance.
The trial (NN1218‐4049) is registered at Clinicaltrials.gov (NCT01850615). It was initiated September 23, 2013 and was completed November 17, 2014. The data were presented previously at the 76th Scientific Sessions of the American Diabetes Association, June 10 to 14, 2016, New Orleans, Louisiana.
Conflict of interest
H. W. R. reports receipt of grants and personal fees from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Novo Nordisk, Sanofi and Regeneron. M. D. and S. T. are employees of Novo Nordisk. M. P. reports receipt of personal fees from Aventis, Lilly and Novo Nordisk. D. T. and M. V. V. have no conflicts of interest to disclose.
Author contributions
H. W. R. was the principal investigator of this study, the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M. D. was the responsible medical officer. S. C. T. was the responsible statistician. All authors had access to the study data, take responsibility for the accuracy of the analysis, and had authority in the decision to submit the manuscript for publication, in collaboration with Novo Nordisk. All authors meet the ICMJE criteria for authorship of this manuscript and approve the manuscript for publication.
Rodbard HW, Tripathy D, Vidrio Velázquez M, Demissie M, Tamer SC and Piletič M. Adding fast‐acting insulin aspart to basal insulin significantly improved glycaemic control in patients with type 2 diabetes: A randomized, 18‐week, open‐label, phase 3 trial (onset 3). Diabetes Obes Metab. 2017;19:1389–1396. https://doi.org/10.1111/dom.12955
Funding Information All contributors received compensation from, and the study was funded by, Novo Nordisk A/S. Novo Nordisk A/S provided the non‐investigational medicinal products used in this trial; metformin was not considered a trial product and was not supplied by Novo Nordisk A/S.
REFERENCES
- 1. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(suppl 2):S151‐S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140‐149. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association . Standards of medical care in diabetes—2016: summary of revisions. Diabetes Care. 2016;39(suppl 1):S4‐S5. [DOI] [PubMed] [Google Scholar]
- 4. International Diabetes Federation . Global Guideline for Type 2 Diabetes. Brussels, Belgium: International Diabetes Federation; 2012. http://www.idf.org/guideline‐type‐2‐diabetes. Accessed November 15, 2016. [Google Scholar]
- 5. Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough what next? Diabetes Metab Res Rev. 2007;23:257‐264. [DOI] [PubMed] [Google Scholar]
- 6. Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta‐analysis. Arch Public Health. 2015;73:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681‐1687. [DOI] [PubMed] [Google Scholar]
- 8. Peter R, Dunseath G, Luzio SD, Chudleigh R, Choudhury SR, Owens DR. Relative and absolute contributions of postprandial and fasting plasma glucose to daytime hyperglycaemia and HbA1c in subjects with type 2 diabetes. Diabet Med. 2009;26:974‐980. [DOI] [PubMed] [Google Scholar]
- 9. Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes: importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77:280‐285. [DOI] [PubMed] [Google Scholar]
- 10. Yanagisawa K, Ashihara J, Obara S, et al. Switching to multiple daily injection therapy with glulisine improves glycaemic control, vascular damage and treatment satisfaction in basal insulin glargine‐injected diabetic patients. Diabetes Metab Res Rev. 2014;30:693‐700. [DOI] [PubMed] [Google Scholar]
- 11. Rizza RA. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes. 2010;59:2697‐2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management‐‐exploring the concept of the basal‐plus approach in clinical practice. Diabet Med. 2013;30:276‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18:401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levin P, Fan T, Zhou S, et al. Clinical outcomes of T2D treatment intensification options vs. no intensification in patients on basal insulin treatment not meeting A1c goals. Diabetes. 2016;65(suppl 1):A247. [Google Scholar]
- 15. Buckley ST, Kildegaard J, Høiberg‐Nielsen R, et al. Mechanistic analysis into the mode of action of niacinamide in faster‐acting insulin aspart. Diabetes Technol Ther. 2016:A291. [Google Scholar]
- 16. Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast‐acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Russell‐Jones D, Bode BW, De Block C, et al. Fast‐acting insulin aspart improves glycemic control in basal‐bolus treatment for type 1 diabetes: results of a 26‐week multicenter, active‐controlled, treat‐to‐target, randomized, parallel‐group trial (Onset 1). Diabetes Care. 2017. Mar 29. doi: 10.2337/dc16‐1771 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Bowering K, Case C, Harvey J, et al. Faster‐acting insulin aspart vs insulin aspart as part of basal–bolus therapy improves postprandial glycemic control in uncontrolled T2D in the double‐blinded onset 2 trial. Diabetes. 2016;65(suppl 1):A63. [Google Scholar]
- 19. World Medical Association . WMA Declaration of Helsinki: ethical principles for medical research involving human subjects. Last amended by the 59th WMA General Assembly; 2008; Seoul, South Korea: [article online] 2008. https://www.wma.net/policies‐post/wma‐declaration‐of‐helsinki‐ethical‐principles‐for‐medical‐research‐involving‐human‐subjects/. Accessed April 11, 2017. [Google Scholar]
- 20. International Conference on Harmonisation . ICH harmonised tripartite guideline: guideline for good clinical practise [article online]. 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed November 15, 2016.
- 21. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dungan KM, Buse JB, Largay J, et al. 1,5‐anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29:1214‐1219. [DOI] [PubMed] [Google Scholar]
- 23. Fritsche A, Larbig M, Owens D, Haring HU, GINGER Study Group . Comparison between a basal‐bolus and a premixed insulin regimen in individuals with type 2 diabetes‐results of the GINGER study. Diabetes Obes Metab. 2010;12:115‐123. [DOI] [PubMed] [Google Scholar]
- 24. Liebl A, Prager R, Binz K, Kaiser M, Bergenstal R, Gallwitz B; PREFER Study Group. Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER Study: a randomized controlled trial. Diabetes Obes Metab. 2009;11:45‐52. [DOI] [PubMed] [Google Scholar]
- 25. Edelman SV, Liu R, Johnson J, Glass LC. AUTONOMY: the first randomized trial comparing two patient‐driven approaches to initiate and titrate prandial insulin lispro in type 2 diabetes. Diabetes Care. 2014;37:2132‐2140. [DOI] [PubMed] [Google Scholar]
- 26. Rodbard HW, Visco VE, Andersen H, Hiort LC, Shu DH. Treatment intensification with stepwise addition of prandial insulin aspart boluses compared with full basal‐bolus therapy (FullSTEP Study): a randomised, treat‐to‐target clinical trial. Lancet Diabetes Endocrinol. 2014;2:30‐37. [DOI] [PubMed] [Google Scholar]
- 27. Holman RR, Bethel MA, Chan JC, et al. Rationale for and design of the Acarbose Cardiovascular Evaluation (ACE) trial. Am Heart J. 2014;168:23‐29.e2. [DOI] [PubMed] [Google Scholar]
- 28. Guigliano D, Chiodini P, Maiorino MI, Bellastella G, Esposito K. Intensification of insulin therapy with basal‐bolus or premixed insulin regimens in type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Endocrine. 2016;51:417‐428. [DOI] [PubMed] [Google Scholar]
- 29. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care. 2003;26:881‐885. [DOI] [PubMed] [Google Scholar]
- 30. Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395‐403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


