Abstract
Most algorithms for type 2 diabetes mellitus (T2DM) do not recommend treatment escalation until glycated haemoglobin (HbA1c) fails to reach the recommended target of 7% (53 mmol/mol) within approximately 3 months on any treatment regimen (“treat to failure”). Clinical inertia and/or poor adherence to therapy contribute to patients not reaching glycaemic targets when managed according to this paradigm. Clinical inertia exists across the entire spectrum of anti‐diabetes therapies, although it is most pronounced when initiating and optimizing insulin therapy. Possible reasons include needle aversion, fear of hypoglycaemia, excessive weight gain and/or the need for increased self‐monitoring of blood glucose. Studies have suggested, however, that early intensive insulin therapy in newly diagnosed, symptomatic patients with T2DM with HbA1c >9% (75 mmol/mol) can preserve beta‐cell function, thereby modulating the disease process. Furthermore, postprandial plasma glucose is a key component of residual dysglycaemia, evident especially when HbA1c remains above target despite fasting normoglycaemia. Therefore, to achieve near normoglycaemia, additional treatment with prandial insulin or a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) is often required. Long‐ or short‐acting GLP‐1 RAs offer effective alternatives to basal or prandial insulin in patients inadequately controlled with other therapies or basal insulin alone, respectively. This review highlights the limitations of current algorithms, and proposes an alternative based on the early introduction of insulin therapy and the rationale for the sequential or fixed combination of GLP‐1 RAs with insulin (“treat‐to‐success” paradigm).
Keywords: GLP‐1, insulin therapy, treatment algorithm, type 2 diabetes mellitus
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a complex, metabolic and progressive disease commonly associated with diverse pathophysiology, which is characterized by relative insulin insufficiency and insulin resistance leading to excessive hepatic glucose production, and fasting and postprandial hyperglycaemia.1, 2 In clinical practice, this manifests as a broad phenotypic expression due to patients presenting at different stages in the natural history of the disease. Pancreatic beta‐cell dysfunction is critical to disease development,2 and insulin resistance in the liver and peripheral target tissues also has a central role in the development of cardiometabolic changes.3 Genetic susceptibility to T2DM is also an important factor as many genes interact with environmental factors, which, in turn, affect downstream pathways, resulting in the heterogeneous nature of the disease.4
The currently available glucose‐lowering agents are reviewed, and guidance for clinicians on treatment choices based on an individual patient‐centred approach is provided in guidelines published by the European Association for the Study of Diabetes (EASD), American Diabetes Association (ADA), American Association of Clinical Endocrinology (AACE), American College of Endocrinology (ACE), and International Diabetes Federation.5, 6, 7 The advised treatment target is generally glycated haemoglobin (HbA1c) ≤7% (53 mmol/mol), seen as indicative of good control. Among patients in the USA diagnosed with T2DM, only 52% have HbA1c <7% (53 mmol/mol), while 13% have values >9% (75 mmol/mol). Indeed, only 14% of individuals achieve recognized targets for glucose, blood pressure and lipids (alongside non‐smoking status).8 Of considerable concern, in Eastern Europe, Latin America and Asia, 36% of patients with T2DM have never had their HbA1c measured; among those with measurements, 36% achieved HbA1c <7% (53 mmol/mol), and only 3.6% achieved all three recommended targets for glucose, blood pressure and low‐density lipoprotein cholesterol.9 Possible reasons for these poor results include poor understanding of the disease and its potential consequences, as well as the necessary lifestyle changes and available treatment options, all of which contribute to non‐compliance. In addition, the lack of agreement among healthcare providers regarding the value, and hence the proper application, of self‐monitoring of blood glucose (SMBG) can be a compounding factor.
Initial pharmacotherapy is generally metformin introduced at, or soon after, diagnosis in conjunction with diet and lifestyle changes. Alternative therapies are recommended if the patient is intolerant to metformin or its use is contraindicated.7, 10 Where monotherapy does not achieve or sustain the mutually agreed HbA1c target after approximately 3 months, treatment will need to be advanced, with a number of options advocated as second‐line therapies. These include agents within the following categories: sulphonylurea, thiazolidinedione, alpha‐glucosidase inhibitor, dipeptidyl peptidase‐4 (DPP‐4) inhibitor, glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA), sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitor and basal insulin.7 These options can be tailored according to a patient's treatment needs and disease characteristics. In patient populations with elevated cardiovascular (CV) risk, the CV outcome trials LEADER (liraglutide), EMPA‐REG (empagliflozin) and SUSTAIN 6 (semaglutide) all showed a reduction in CV events (according to the primary 3‐point MACE).11, 12, 13 In contrast to the GLP‐1 RAs, treatment with empagliflozin was associated with a reduction in CV death from early in the trial, suggesting a haemodynamic mechanism. This is supported by the significant reduction in hospitalization due to heart failure in this study.13 The National Institute for Health and Care Excellence advocates continuation of current therapy until the patient no longer meets the desired target [HbA1c 7.5% (58 mmol/mol)], and only then should further options be considered, i.e. a “treat‐to‐failure” approach.10
For patients who do not reach target HbA1c after 3 months, clinicians are encouraged to discuss the aforementioned second‐line treatment classes, usually in combination with metformin, with the choice of treatment being dependent on patient preference and disease status.7 Basal insulin or premixed insulin formulations are often not considered until advanced disease when patients do not achieve target HbA1c despite intensive treatment with a single or a combination of anti‐diabetes therapy. There is, however, evidence demonstrating that the early introduction of intensive insulin therapy (first‐line), especially in those with an HbA1c >9% (75 mmol/mol; usually symptomatic with a short disease history), positively influences glycaemic control, maintenance of beta‐cell function and rates of remission (many patients maintaining optimum glycaemic control for at least 12 months).2 Basal insulin alone has also obtained similar responses to continuous insulin infusion (CSII) in an equivalent setting.14 In cases where basal insulin has been up‐titrated to achieve a near‐normal fasting plasma glucose (FPG) yet HbA1c remains above target, a short‐acting GLP‐1 RA or prandial insulin can be added to reduce postprandial plasma glucose (PPG) excursions.7 In patients with HbA1c >9% (75 mmol/mol; with/without symptoms), EASD/ADA and AACE/ACE guidelines advocate first‐line combination therapy over sequential treatment.5, 7 However, there remains a considerable delay in progressing treatment in clinical practice.15
Most patients with T2DM require multiple treatments to maintain adequate glycaemic control. For example, in the UK Prospective Diabetes Study, about 50% of patients with newly diagnosed T2DM maintained HbA1c <7% (53 mmol/mol) with monotherapy for 3 years, although only about 25% achieved this glycaemic target with monotherapy after 9 years.16 Even with basal insulin treatment, a significant proportion of patients continue to have an HbA1c above target.17
This review considers the limitations of the current treatment algorithms and proposes an alternative paradigm based around the early introduction of insulin therapy (especially, based on available evidence, in those with high HbA1c) and combination therapy with a GLP‐1 RA.
2. CURRENT TREATMENT LANDSCAPE
Type 2 diabetes mellitus is often inadequately controlled despite the availability of numerous injectable and oral anti‐diabetic drugs (OADs) supported by a variety of treatment guidelines. Between 24% and 54% of patients treated with basal insulin do not achieve HbA1c ≤7% (53 mmol/mol), despite meeting target FPG levels.18
Clinical inactivity, “inertia,” among healthcare professionals, and poor patient adherence to therapy are among the major reasons why many patients do not reach their glycaemic target when adopting current treatment paradigms, i.e. therapy is not intensified despite poor glycaemic control on an existing regimen.19, 20 Furthermore, numerous studies (in the USA and Europe) suggest that clinical inertia is unacceptably common in the management of T2DM.20, 21, 22 Brown and colleagues reported that, on average, patients accumulated approximately 10 years of excess glycaemic burden with HbA1c >7% (53 mmol/mol) from diagnosis until beginning insulin treatment.15 This may have a significant impact on disease severity and risk of micro‐ and macrovascular complications.23
Several factors can potentially contribute to clinical inertia and poor patient adherence to therapy: the introduction of newer, more complicated and expensive therapies compounded by inadequate educational support, which will undoubtedly influence the time taken for clinicians to become acquainted with these treatments. To date, there are few head‐to‐head cost–benefit comparisons between the ever‐increasing number of newer treatments available to assist clinicians in making informed decisions. Tolerability is also a major factor, for example, insulin and sulphonylureas are associated with hypoglycaemia and weight gain; thiazolidinediones with increased risk of weight gain, oedema, heart failure and bone fractures; SGLT‐2 inhibitors with genito‐urinary infections, polyuria, volume depletion, hypotension, dizziness, increased low‐density lipoprotein cholesterol, transient increases in creatinine, increased risk of bone fractures24 and non‐hyperglycaemic ketoacidosis.25 GLP‐1 RAs induce transient gastrointestinal (GI) intolerance in the majority of patients, although <10% of individuals discontinue treatment due to any adverse event.26 It is also becoming increasingly recognized that there is considerable variation in patients’ response to anti‐diabetes therapy, which can be explained by genetic differences (e.g. for metformin, sulphonylureas and incretin‐based therapies).27, 28
Despite a growing number of treatments, 5 to 10 years after diagnosis, most patients will eventually require insulin therapy to achieve their glycaemic target.29 Clinical inertia is most pronounced when introducing insulin,30 and may relate to a number of factors, including fear of hypoglycaemia and excessive weight gain,31, 32 together with the need for structured education including SMBG.33 In the START study of patients with stable but poorly controlled T2DM, a 1 unit/d self‐titration bolus insulin algorithm to reach a target 2‐hour PPG of 5.0 to 8.0 mmol/L was seen to be as effective as a physician‐managed algorithm.34 Similar results were reported in a study of 4961 patients with poorly controlled T2DM who initiated and titrated insulin according to either an investigator‐ or self‐led algorithm.35 In this study, patients using the self‐led algorithm experienced greater decreases in HbA1c vs patients whose insulin treatment was investigator led (P < .001), and there was no significant difference in the occurrence of severe hypoglycaemia between the 2 groups.35 Of note, even though there is some unity and agreement between the current treatment algorithms,36 algorithm‐driven insulin use in advanced T2DM is often associated with a greater incidence of hypoglycaemia and weight gain than when initiated earlier in the course of disease.30 In a pooled analysis of 11 prospective trials, early use of insulin in combination with metformin resulted in less weight gain and symptomatic hypoglycaemia compared with insulin added to 2 OADs (reflective of treatment later in the disease course).37
The issues discussed above highlight the need to re‐consider the current treatment algorithms for the management of patients with T2DM. Alternative approaches include the early initiation of insulin therapy with the addition of a GLP‐1 RA when appropriate. This option raises the prospect of improved success in achieving glycaemic targets sooner and maintaining them for longer than with the conventional approach, while reducing the risk of hypoglycaemia and mitigating weight gain due to lower doses of the individual components.
3. THE CASE FOR THE EARLY INTRODUCTION OF INSULIN THERAPY
Studies have suggested that the introduction of intensive insulin treatment in patients presenting with even moderately high HbA1c (median of 7.2%) can alter the progressive nature of T2DM by reversing the adverse impact of glucotoxicity and lipotoxicity on beta‐cell function and insulin sensitivity.38 In patients with newly diagnosed T2DM [mean baseline HbA1c ≥9.5% (80 mmol/mol)], both CSII and multiple daily insulin (MDI) injections increased target glycaemia attainment in less time than with OADs.2 Markers of beta‐cell secretion also improved more dramatically following early and intensive insulin therapy.2 Furthermore, rates of remission were increased with CSII and MDI injections vs OADs. In a separate study,39 a greater proportion of patients newly diagnosed with T2DM [HbA1c ≥9% (75 mmol/mol)] achieved glycaemic targets with a combination of an OAD plus insulin in less time than with OADs alone. Following 1 year of treatment, more patients maintained target glycaemia with an OAD plus insulin than with OADs alone, and maintained beta‐cell function more effectively than when OADs were employed solely.39
A recent 21‐year update to a study that treated patients with T2DM and microalbuminuria (mean disease duration ~6 years) over 7.8 years with an intensive, multifactorial step‐wise approach (including dietary changes followed by OADs stepping up to Neutral Protamine Hagedorn insulin and multiple insulin injections in patients failing to achieve stringent blood pressure, HbA1c and cholesterol targets as well as behavioural approaches) vs a conventional treatment approach reported a marked legacy effect on CV outcomes with intensive early treatment of these risk factors.40 Significantly fewer patients receiving the early intensive intervention died during the follow‐up period compared with those treated conventionally (P = .005); the former group also had 8.1 years longer before experiencing a CV event (P = .001) and lived 7.9 years longer than the conventional cohort.40 A post hoc analysis of the LIBRA (LIraglutide and Beta cell RepAir) trial identified that the most important factor in improving outcomes is the duration of disease at the initiation of intensive treatment.41 In LIBRA, 25 patients with T2DM for ≤7 years received 4 weeks of intensive insulin therapy to achieve fasting glucose <6.94 mmol/L followed by placebo for 48 weeks. Rates of remission [HbA1c <6.5% (48 mmol/mol) and no medication during the 48‐week placebo period] were 78%, 71% and 58% in patients with T2DM of <1, 2 and 3 years duration, respectively, and loss of remission was slower in patients with disease duration of <2 years vs those with a more established disease.41
In a population‐based study, the best HbA1c reductions were observed when basal insulin was introduced directly after failure of metformin monotherapy,37 while the improvement of HbA1c was less marked when basal insulin was added only after combinations of OADs had failed. This demonstrates that a better response to basal insulin can be expected in the earlier stages of T2DM.37 Aside from glycaemic control, the additional anti‐inflammatory and anti‐oxidant properties of insulin may also help to protect against endothelial dysfunction and consequent vascular disease.23, 42 Nevertheless, even in cases where early insulin treatment has been shown to be beneficial (symptomatic, markedly hyperglycaemic, newly diagnosed T2DM), such treatment may not be applied due to the perceived increased work load for the patient as well as healthcare professionals. However, studies show that the improvements in glycaemic control, beta‐cell function and plasma lipid profiles associated with basal insulin monotherapy are comparable with those seen with CSII. Therefore, there is the option of a less complex basal insulin regimen in patients newly diagnosed with T2DM.14
When OADs fail to control glycaemia, adding basal insulin predominantly targets FPG, which reflects the reduction of overnight hepatic glucose production and suppression of free fatty acid release by adipose tissue.29 Several randomized studies have shown that the decrements in HbA1c are linearly related to insulin dose (approximately 0.5% decrease in percentage HbA1c for each increase in insulin dose equivalent to 0.1 U/kg/d up to 0.5 U/kg).43 Above this dosing threshold, the improvement in HbA1c is halved (0.5% decrease in percentage HbA1c for each increase in insulin dose of 0.2 U/kg/d). Weight gain, however, increases linearly throughout the dose range.44
Patients with T2DM typically exhibit substantial PPG excursions early in the evolution of the disease, which is why basal insulin monotherapy, despite achieving near‐normal FPG levels, fails to normalize HbA1c in approximately 30% of patients45; as T2DM progresses, defects in PPG are quickly followed by fasting hyperglycaemia due to the dawn phenomenon.46, 47 Therefore, as PPG can be a significant contributor to overall hyperglycaemia at lower levels of HbA1c,46 reducing postprandial excursions is particularly important in patients with relatively modest HbA1c elevations or when no further improvement in FPG is feasible with basal insulin. Despite adequate basal insulin dose titration, a substantial proportion of patients on basal insulin therapy will require additional treatment with either a prandial insulin or a GLP‐1 RA to address PPG and further improve HbA1c.7 While prandial insulin is routinely added to basal insulin in a three‐times‐daily basal‐bolus approach, it has been found that a once‐ or twice‐daily regimen can achieve similar HbA1c reductions, with a lower risk of hypoglycaemia.48 The hypoglycaemia risk associated with increments in insulin doses highlights the need for treatments that augment glycaemic control, but which carry limited additional risk of hypoglycaemia.
3.1. Rationale for GLP‐1 RA therapy
Native GLP‐1 is secreted to augment insulin secretion and suppress glucagon release primarily in response to a nutrient challenge. In the circulation, it is quickly degraded (within 2‐3 minutes).49 GLP‐1 RAs have been developed that are capable of resisting degradation, therefore providing longer in vivo actions with the added possibility of reduced insulin doses when used in combination while preventing weight gain and lowering the risk of hypoglycaemia compared with insulin monotherapy.49 Numerous studies confirm the potency of GLP‐1 RAs,26, 50 including a systematic literature review and meta‐analysis of 15 randomized controlled studies that compared GLP‐1 RAs plus basal insulin with other anti‐diabetes treatments.51 GLP‐1 RAs, therefore, offer an effective, alternative treatment for T2DM, introduced either before or after the initiation of basal insulin or in combination with basal insulin.52, 53, 54, 55, 56 The combination improves glycaemic control irrespective of whether a GLP‐1 RA is added to basal insulin or vice versa.
Glucagon‐like peptide‐1 receptor agonists, based on their differential pharmacokinetic/pharmacodynamic characteristics, can be classified as short acting/“prandial” (e.g. exenatide, lixisenatide) or long‐acting/“non‐prandial” [e.g. liraglutide, exenatide (long‐acting release), albiglutide, dulaglutide, semaglutide].49, 57 The short‐acting GLP‐1 RAs predominantly lower PPG by slowing gastric emptying and inhibiting glucagon secretion, whereas the long‐acting GLP‐1 RAs predominantly act on FPG by stimulating insulin secretion.49 In contrast to the short‐acting GLP‐1 RAs, which sustain the delay in gastric emptying over time, continuous infusion of endogenous GLP‐1 or long‐acting GLP‐1 RAs induces tachyphylaxis.49
The currently available GLP‐1 RAs allow different patterns of administration, ranging from twice daily to once weekly, providing a flexibility in patient treatment options around their differing pharmacological properties (Table 1). For example, short‐acting/prandial GLP‐1 RAs might be used once or twice daily58 before the main meal(s) in addition to insulin therapy.
Table 1.
Comparison of GLP‐1 RAs Phase III randomized controlled trials
| Short‐acting GLP‐1 RAs | Long‐acting GLP‐1 RAs | |
|---|---|---|
| Compounds | Exenatide | Albiglutide |
| Lixisenatide | Dulaglutide | |
| Exenatide LAR | ||
| Liraglutide | ||
| Half‐life | 2‐5 h | 12 h–several days |
| Frequency of administration | Once or twice daily58 | Once daily–once weekly58 |
| Fasting blood glucose levels | Modest reduction (12,59 1660 and 2456, 59, 61, 62, 63 weeks) | Strong reduction (26 and 52 weeks64) |
| Postprandial hyperglycaemia | Strong reduction (12,59 1660 and 2454, 55, 56, 59, 61, 62, 63 weeks) | Modest reduction (26 weeks63) |
| Fasting insulin secretion | Modest stimulation (24 weeks56) | Strong stimulation49 |
| Postprandial insulin secretion | Reduction (24 weeks56) | Modest stimulation (14 weeks65) |
| Glucagon secretion | Reduction | Reduction |
| Gastric emptying rate | Deceleration | No effect |
| Blood pressure | Reduction (2466 and 2667 weeks) | Reduction (4068 and 5269 weeks) |
| Heart rate | No effect or small increase (0‐2 bpm49) | Moderate increase (2‐5 bpm49) |
| Body weight reduction | 1‐5 kg (12,59 2454, 55, 56, 59, 62 and 3070 weeks) | 2‐5 kg (24,71 26,63 3072 and 5269 weeks) |
| Nausea | 20‐50%, attenuates slowly (from 1259 to 2454, 55, 56, 59, 62 weeks to many months) | 20‐40%, attenuates quickly (~4‐8 weeks49) |
Abbreviations: bpm, beats per minute; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; LAR, long‐acting release.
Adapted with permission from Macmillan Publishers Ltd (Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012; 8: 728–742), copyright 2012.
In addition to their effects on glycaemic control, hypoglycaemia and weight, it is important to consider the effects of the GLP‐1 RAs on the CV and other systems. The GLP‐1 receptor is widely expressed throughout the body, including the heart (predominantly in the sino‐atrial node),73 and GLP‐1 has been demonstrated to have neuroprotective properties, for example, crosses the blood–brain barrier, enhances neurogenesis and reduces the chronic inflammatory responses in the brain.74 Incretin‐based therapies, i.e. GLP‐1 RAs and DPP‐4 inhibitors, reportedly exert cardio‐protective effects, including positively influencing cardiac function (in vitro and animal data), and improving endothelial function (human data).75 In clinical trials, however, the CV effects of the individual GLP‐1 RAs appear to vary. For example, a meta‐analysis of 60 trials comparing GLP‐1 RAs with placebo, insulin and sulphonylureas has reported that GLP‐1 RAs decreased systolic blood pressure by 1.84 to 4.60 mmHg.76 However, of note, exenatide (2 mg once weekly) and liraglutide (1.2 and 1.8 mg once daily) increased the heart rate by 2.06 to 3.35 beats per min (bpm) vs placebo.76 After 8 weeks of treatment with lixisenatide or liraglutide, 24‐hour mean heart rate was measured using a 24‐hour ambulatory monitor. Treatment with liraglutide resulted in a clinically significant increase in mean 24‐hour heart rate by approximately 9 bpm (compared with 3 bpm with lixisenatide).77 The clinical significance of these changes in heart rate is unclear and needs further investigation78 in view of the findings from the ELIXA, LEADER and SUSTAIN‐6 studies.11, 12, 79
As mentioned above, CV outcomes and long‐term safety have been investigated in 3 large, recently published trials with liraglutide, lixisenatide and semaglutide.11, 12, 79 The effects of the short‐acting GLP‐1 RA lixisenatide on CV outcomes were investigated in the ELIXA trial, which included >6000 patients with T2DM who had recently experienced an acute coronary event and who were at the highest level of CV risk. Primary endpoint events (CV death, myocardial infarction, stroke or hospitalization for unstable angina) occurred in 13.4% and 13.2% of patients given lixisenatide and placebo, respectively, indicating that lixisenatide was non‐inferior to placebo for these outcomes [hazard ratio (HR): 1.02; 95% confidence interval (CI): 0.89‐1.17; P < .001]. Adding lixisenatide to usual care had no significant impact on the rate of major CV events over a median follow‐up period of 2.1 years.79 The long‐term CV safety of the long‐acting GLP‐1 RA liraglutide (vs placebo) was investigated in the LEADER study in >9000 adults with T2DM at high risk of major adverse CV events. The median follow‐up was 3.8 years. In the time‐to‐event analysis, the rate of the first occurrence of death from CV causes, non‐fatal myocardial infarction or non‐fatal stroke (primary outcome) was significantly lower with liraglutide compared with placebo (13% vs 14.9%; HR: 0.87; 95% CI: 0.78‐0.97; P < .001), and fewer patients died from CV causes with liraglutide compared with placebo (4.7% vs 6.0%, respectively; HR: 0.78; 95% CI: 0.66‐0.93; P = .007).11 In the SUSTAIN‐6 study of 3297 patients with T2DM given 0.5 or 1.0 mg semaglutide per week vs placebo (median follow‐up 2.1 years), the proportion of patients with first occurrence of CV death, non‐fatal myocardial infarction or non‐fatal stroke was 6.6% vs 8.9% with either semaglutide dose vs placebo, respectively (HR: 0.74; 95% CI: 0.58‐0.95; P < .001). The rate of CV death was comparable in both treatment groups in SUSTAIN‐6 (semaglutide 2.7%, placebo 2.8%; HR: 0.98; 95% CI: 0.65‐1.48; P = .92).12
Differences in the populations recruited and study designs make it difficult to compare the findings of ELIXA, LEADER and SUSTAIN‐6 directly. The LEADER study had greater statistical power and was extended over a longer period compared with ELIXA and SUSTAIN‐6, while the liraglutide and semaglutide CV outcomes studies included patients with higher baseline HbA1c compared with those in the lixisenatide CV trial [8.7% (LEADER and SUSTAIN‐6) vs 7.6% (ELIXA)]. Furthermore, HbA1c in the LEADER control group remained close to 8% (64 mmol/mol) throughout the study, which may have led to a between‐group difference in HbA1c that varied over time from >1% to 0.4% at 36 months. In addition, patients in the ELIXA study all had established CV disease (myocardial infarction or hospitalized for unstable angina within 180 days prior to enrolment), and were at a much higher risk of further CV events than those in the LEADER and SUSTAIN‐6 studies. Overall, these large CV outcomes studies clearly demonstrate that these particular GLP‐1 RAs do not exacerbate CV disease and some exhibit cardio‐protective properties. It will be interesting to see what findings will emerge with the remaining GLP‐1 RAs still under investigation (exenatide long‐acting release, dulaglutide, albiglutide).
3.2. GLP‐1 RA therapy in combination with insulin therapy
Combining basal insulin with a short‐acting GLP‐1 RA that predominantly lowers PPG is a logical management approach when the former fails to achieve adequate glycaemic control even when optimally titrated.49 Long‐acting GLP‐1 RAs, used in combination with basal insulin, result in comparable HbA1c reductions with weight loss and lower hypoglycaemia risk when compared with basal insulin alone.80, 81 Recently, combining GLP‐1 RAs (short or long acting) and insulin in a single injection (lixisenatide plus insulin glargine, liraglutide plus insulin degludec) has been shown to result in a larger glycaemic reduction than when using basal insulin alone, reflecting their distinct complementary glucose‐lowering mechanisms.82, 83 This combination increases the proportion of patients who reach target HbA1c while mitigating some of the side‐effects observed with basal‐bolus insulin regimes.7
The short‐acting GLP‐1 RA lixisenatide has been investigated in a series of multinational, Phase III trials (GetGoal clinical trial programme) across a spectrum of patients with T2DM. This programme included the GetGoal Duo‐1 study, which enrolled patients with HbA1c 7–10% (53–86 mmol/mol) despite OADs55; the GetGoal‐L trial, with patients inadequately controlled on basal insulin,54 and the GetGoal‐L‐Asia trial, which included Asian patients with T2DM inadequately controlled with basal insulin with/without sulphonylureas.61 Figures 1 and 2 illustrate the findings for lixisenatide and liraglutide when added to basal insulin. A meta‐analysis of five studies encompassing 1184 patients compared lixisenatide and once‐daily, rapid‐acting insulin when added to basal insulin.86 Patients taking lixisenatide were approximately twice as likely to reach composite outcomes, i.e. HbA1c <7% (53 mmol/mol) and no symptomatic hypoglycaemia [odds ratio (OR): 1.90], HbA1c <7% (53 mmol/mol) and no severe hypoglycaemia (OR: 1.97), and were almost three times more likely to achieve the composite outcome of HbA1c <7% (53 mol/mol), no weight gain and no documented symptomatic hypoglycaemia (P = .0046). Large multinational, Phase III clinical development studies have also been carried out to investigate the safety and efficacy of the other GLP‐1 RAs, for example, the liraglutide LEAD, exenatide DURATION, dulaglutide AWARD, semaglutide SUSTAIN and albiglutide HARMONY programmes.80, 87, 88, 89, 90 These extensive programmes have helped establish the efficacy and safety/tolerability profile of GLP‐1 RAs in a range of people with T2DM, including those who are treatment naïve, failing oral agents and treated with GLP‐1 RAs as an adjunct to basal insulin.
Figure 1.
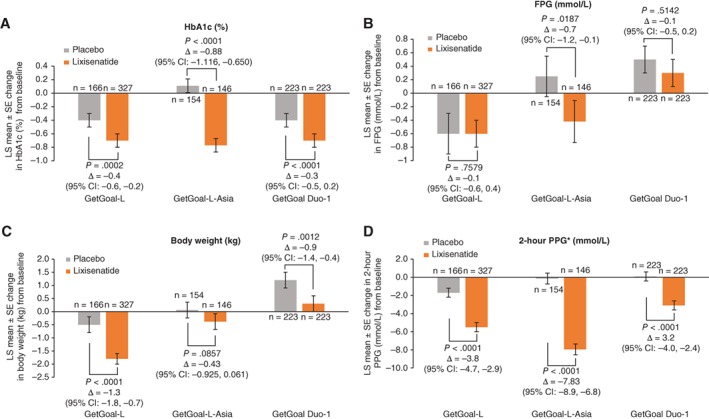
Composite of A, glycated haemoglobin (HbA1c); B, fasting plasma glucose (FPG); C, body weight and D, 2‐hour postprandial plasma glucose (PPG) changes with lixisenatide. Lixisenatide: modified intent‐to‐treat populations. Week 24 data are last observation carried forward (GetGoal‐L,54 GetGoal‐L‐Asia 61 and GetGoal Duo‐1 55). Patient populations enrolled in each lixisenatide trial: GetGoal‐L (patients inadequately controlled on basal insulin); GetGoal‐L‐Asia (Asian patients inadequately controlled with basal insulin with/without sulphonylurea); GetGoal Duo‐1 (insulin‐naïve patients inadequately controlled with oral anti‐diabetic drugs). CI, confidence interval; LS, least squares; SE, standard error. *After a standardized liquid breakfast meal test (Ensure Plus)
Figure 2.
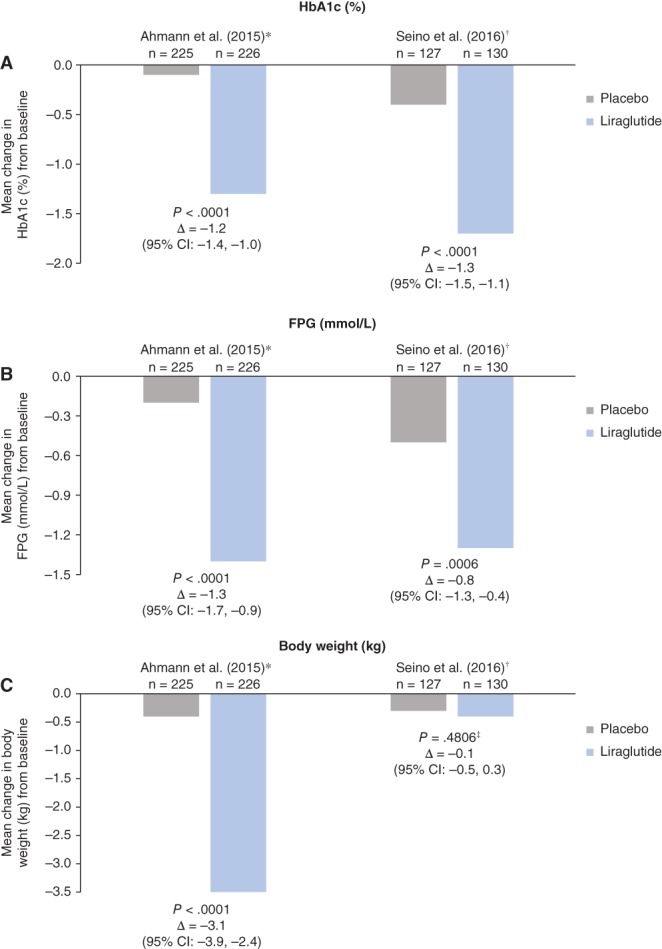
Composite of A, glycated haemoglobin (HbA1c); B, fasting plasma glucose (FPG) and C, body weight changes with liraglutide in combination with basal insulin.84, 85 Modified intent‐to‐treat populations: *26‐week study; †16‐week study; ‡study conducted in Japanese patients with low body mass index at enrolment. CI, confidence interval. Standard errors/standard deviations were not reported in the text of these studies, but were reported in corresponding line graphs
Adding GLP‐1 RAs to basal insulin achieves similar or greater reductions in HbA1c when compared with basal insulin and prandial insulin combinations.7 In the GetGoal Duo‐2 trial, involving patients with inadequate glycaemic control on basal insulin despite continued titration, the addition of lixisenatide resulted in similar HbA1c reductions but less hypoglycaemia (symptomatic and nocturnal) and less weight gain when compared with once‐ or thrice‐daily prandial insulin (glulisine).91 Furthermore, PPG was significantly reduced with lixisenatide compared with both glulisine regimens, although, as expected, transient nausea, vomiting and diarrhoea events were more common with lixisenatide.91 These findings suggest that a short‐acting GLP‐1 RA, such as lixisenatide, could be a preferred intensification treatment option compared with prandial insulin when a basal insulin regimen is found to be insufficient.
3.3. Fixed‐ratio combinations (FRCs) of basal insulin and GLP‐1 RAs
Combining insulin and incretin therapy is suggested as a treatment option in the EASD/ADA guidelines.7 Titratable FRCs of basal insulin and GLP‐1 RA are now available for single, daily administration. The FRC of insulin glargine 100 U and lixisenatide (iGlarLixi) delivers increments of insulin glargine up to a daily maximum dose of 60 U and lixisenatide up to 20 µg. Although delivery of FRCs is simplified by the use of a single pen injection, clear instructions and education are necessary in order to minimize potential medication errors in the clinical‐care setting.
Studies assessing FRCs of GLP‐1 RA and insulin currently in development are summarized in Table 2. In the DUAL‐I study (insulin‐naïve patients with T2DM inadequately controlled with metformin ± pioglitazone), IDegLira [insulin degludec (IDeg) and liraglutide (Lira)] was non‐inferior to basal insulin degludec in terms of HbA1c reduction [decreases in HbA1c of −1.9% vs −1.4% (insulin degludec), but with more patients achieving the glycaemic target [HbA1c <7% (53 mmol/mol)] at equivalent insulin doses (81% vs 65%)]. IDegLira was superior to the GLP‐1 RA component liraglutide when administered alone (decrease in HbA1c of −1.3% with 60% of patients achieving their glycaemic goal). Furthermore, a greater proportion of patients achieved glycaemic targets without weight gain or hypoglycaemia with IDegLira (36%) compared with insulin degludec alone (14%).52 In DUAL‐II, patients with T2DM previously treated with basal insulin plus metformin ± sulphonylureas/glinides, IDegLira achieved superior glycaemic control compared with insulin degludec alone (−1.9% vs −0.9%, respectively), with 60% of patients reaching glycaemic targets compared with 23% with insulin degludec.52, 53 Again, a greater proportion of patients achieved glycaemic targets without weight gain or hypoglycaemia with IDegLira (40%) compared with insulin degludec (8.5%). The additional Phase IIIb DUAL trials (DUAL III‐VI) have investigated IDegLira in increasingly difficult‐to‐treat T2DM patient populations, including patients who had failed basal insulin therapy despite unlimited titration [e.g. for the fully published trials to date, DUAL‐III reported decreases in HbA1c of −1.3% (IDegLira) vs −0.3% (liraglutide or exenatide); DUAL‐V reported HbA1c decreases of −1.8% (IDegLira) vs −1.1% (insulin glargine) with 39% vs 12% of patients, respectively, achieving target HbA1c without weight gain or hypoglycaemia; DUAL‐VI reported decreases in HbA1c from 8.2% to 6.1% (IDegLira once‐weekly titration) and from 8.1% to 6.0% (IDegLira twice‐weekly titration) with approximately 90% patients achieving target HbA1c, again without weight gain or hypoglycaemia].96
Table 2.
Summary of Phase III studies assessing FRCs of GLP‐1 RA and insulin
| Study and Phase | Treatment | Decrease in mean HbA1c (2nd value is at end of treatment) | Summary of efficacy | Change in body weight | Rates of nausea | Hypoglycaemic events |
|---|---|---|---|---|---|---|
| DUAL‐I | IDegLira (insulin degludec and liraglutide) | At 26 weeks | IDegLira: non‐inferior to degludec; superior to liraglutide | Confirmed hypoglycaemic events per patient‐year | ||
| Phase IIIa53 | IDegLira: 1.9% (SD 1.1) to 6.4% (SD 1.0) | IDegLira: −0.5 kg | IDegLira: 8.8% | IDegLira: 1.8 | ||
| Degludec: 1.4% (SD 1.0) to 6.9% (SD 1.1) | Degludec: +1.6 kg | Degludec: 3.6% | Degludec: 2.6 | |||
| Liraglutide: 1.3% (SD 1.1) to 7.0% (SD 1.2) | Liraglutide: −3.0 kg | Liraglutide: 19.7% | Liraglutide: 0.2 | |||
| DUAL‐II | IDegLira | At 26 weeks | IDegLira achieved glycaemic control superior to that of degludec at equivalent insulin doses | Incidence rate | ||
| Phase IIIa52 | IDegLira: 1.9% to 6.9% | IDegLira: −2.7 kg | IDegLira: 6.5% | IDegLira: 24% | ||
| Degludec: 0.9% to 8.0% | Degludec: 0 kg | Degludec: 3.5% | Degludec: 25% | |||
| DUAL‐III | IDegLira | At 26 weeks | IDegLira was superior to treatment with unchanged GLP‐1 RAs in terms of HbA1c reductions | Confirmed hypoglycaemic events per patient‐year | ||
| Phase IIIb92 | IDegLira: 1.3% to 6.4% | IDegLira: +2.0 kg | NR | IDegLira: 2.82 | ||
| Liraglutide/exenatide: 0.3% to 7.4% | Liraglutide/exenatide: −0.8 kg | Liraglutide/exenatide: 0.12 | ||||
| DUAL‐IV | IDegLira | At 26 weeks | IDegLira significantly improved glycaemic control in combination with sulphonylurea ± metformin compared with placebo | Incidence rate | ||
| Phase IIIb93 | IDegLira: 1.5% to 6.4% | IDegLira: +0.5 kg | NR | IDegLira: 41.7% | ||
| Placebo: 0.5% to 7.4% | Placebo: −1.0 kg | Liraglutide/exenatide: 17.1% | ||||
| Confirmed hypoglycaemic events per patient‐year | ||||||
| IDegLira: 3.5 | ||||||
| Placebo: 1.4 | ||||||
| DUAL‐V | IDegLira | At 26 weeks | IDegLira improved glycaemic control more than up‐titration of insulin glargine alone with fewer hypoglycaemic events, no weight gain and a lower insulin dose | Confirmed hypoglycaemic events per patient‐year | ||
| Phase IIIb94 | IDegLira: 1.8% to 6.6% | IDegLira: −1.4 kg | IDegLira: <4% | IDegLira: 2.23 | ||
| Insulin glargine: 1.1% to 7.1% | Insulin glargine: +1.8 kg | Insulin glargine: 5.05 | ||||
| DUAL‐VI | IDegLira | IDegLira 1 × weekly titration was non‐inferior to IDegLira 2 × weekly titration for HbA1c reduction | Severe or blood glucose confirmed | |||
| Phase IIIb95 | IDegLira: 2.0% to 6.1% (1 × weekly titration) and 2.0% to 6.0% (2 × weekly titration) | IDegLira: −1.0 kg (1 × weekly titration) and −2.0 kg (2 × weekly titration) | NR | IDegLira: 8.6% (1 × weekly titration) and 23.8% (2 × weekly titration) | ||
| Severe or blood glucose confirmed symptomatic | ||||||
| IDegLira: 5.7% (1 × weekly titration) and 16.2% (2 × weekly titration) | ||||||
| LixiLan‐O | iGlarLixi (insulin glargine and lixisenatide) | At 30 weeks | iGlarLixi: statistical superiority to lixisenatide; non‐inferiority to iGlar | Documented (≤3.9 mmol/L) symptomatic hypoglycaemia events per patient‐year | ||
| Phase III83 | iGlarLixi: 1.6% to 6.5% | iGlarLixi: −0.3 kg | iGlarLixi: 9.6% | iGlarLixi: 1.44 | ||
| iGlar: 1.3% to 6.8% | iGlar: +1.1 kga | iGlar: 3.6% | iGlar: 1.22 | |||
| Lixisenatide: 0.9% to 7.3% | Lixisenatide: −2.3 kg | Lixisenatide: 24% | Lixisenatide: 0.34 | |||
| LixiLan‐L | iGlarLixi | At 30 weeks | iGlarLixi: statistical superiority to iGlar | Documented (≤3.9 mmol/L) symptomatic hypoglycaemia events per patient‐year | ||
| Phase III82 | iGlarLixi: 1.1% to 6.9% | iGlarLixi: −0.7 kg | iGlarLixi: 10.4% | iGlarLixi: 3.03 | ||
| iGlar: 0.6% to 7.5% | iGlar: +0.7 kga | iGlar: 0.5% | iGlar: 4.22 |
Abbreviations: GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin; NR, not reported; SD, standard deviation.
Least‐squares mean data.
In a proof‐of‐concept study, iGlarLixi was shown to be non‐inferior to iGlar in patients continuing metformin treatment.97 This was followed by the LixiLan‐O trial (insulin‐naïve patients with T2DM inadequately controlled with metformin ± a second oral glucose‐lowering drug), in which a higher proportion of patients receiving iGlarLixi (74%) achieved HbA1c <7% (53 mmol/mol) compared with insulin glargine 100 U (59%) or lixisenatide (33%) alone; respective reductions in HbA1c were −1.6%, −1.3%, −0.9%. A total of 32%, 19% and 27% of patients, respectively, achieved target HbA1c, without increasing the risk of hypoglycaemia or weight gain. Furthermore, iGlarLixi demonstrated lower GI side‐effects compared with lixisenatide [9.6% (nausea) and 3.2% (vomiting) vs 24% (nausea) and 6.4% (vomiting), respectively].83 In LixiLan‐L, which comprised difficult‐to‐treat patients (uncontrolled glycaemia with basal insulin ± up to two oral glucose‐lowering agents), similar efficacy and safety results were observed to those of LixiLan‐O [target HbA1c was achieved in 55% (iGlarLixi) and 30% (iGlar) of patients; reductions in HbA1c were −1.1% and −0.6%; and 20% and 9% of patients reached the composite endpoint, respectively].82 Both iGlarLixi and IDegLira mitigate the weight gain seen with insulin, with rates of hypoglycaemia similar to those observed with the basal insulin component of the combination. Both IDegLira and iGlarLixi (60 U glargine and 20 µg lixisenatide) were recently approved by the US Food and Drug Administration98, 99; IDegLira is approved in Europe.
As expected, GI episodes (nausea and vomiting) were the most common adverse events reported by participants receiving FRCs, although the overall incidence was lower when compared with the GLP‐1 RA component alone.52, 53, 82, 83, 97 This improved GI tolerability may be due to the slower release of the GLP‐1 RA component in the FRC formulation in parallel with the up‐titration of the insulin dose guided by the FPG response.
Pen devices utilized for FRCs slow titration and facilitate individualization. For example, iGlarLixi's dual pen system delivers insulin glargine and lixisenatide in 2 different ratios and offers a wider range of insulin doses than would be possible with a single pen, while retaining lixisenatide at the maximum‐allowed daily dose; in the USA, where iGlarLixi is only indicated for patients already taking basal insulin, a single pen (range 15‐60 U) is available. Furthermore, a major decrease in insulin dose is not a limitation when switching to iGlarLixi, as patients on basal insulin have different iGlarLixi starting doses available to them depending on their previous insulin requirement. However, with IDegLira, which is indicated for patients uncontrolled on either basal insulin or liraglutide (dose: up to 50 U insulin degludec and 1.8 mg liraglutide), some patients switching from insulin alone may need to start with a significant insulin dose reduction that could result in a longer time needed to achieve optimal treatment. Therefore, for both FRCs, labelling and instructions need to be very clear.
The labelling for iGlarLixi states that in those patients inadequately controlled on <30 U basal insulin or on 20 µg lixisenatide, the recommended initial dose is 15 U (15 U iGlar/5 µg Lixi) once daily. The starting dose of iGlarLixi increases to 30 U (30 U iGlar/10 µg Lixi) once daily in patients inadequately controlled on 30 to 60 U basal insulin. IDegLira may be added to existing OADs with a recommended starting dose of 10 U insulin degludec and 0.36 mg liraglutide as part of a 10‐dose step schedule, as required, with a reduction in sulphonylurea therapy to be considered to lower the risk of hypoglycaemia. When transferring from basal insulin only, the recommended initial dose is 16 U insulin degludec and 0.6 mg liraglutide with the option of 16 incremental dose steps.
4. IMPLICATIONS
Initiation of basal insulin early in T2DM, and using an FRC with a GLP‐1 RA, provides a new therapeutic approach for second‐ or third‐line therapy in the management algorithm of patients with T2DM, as FRCs have been shown to retain similar efficacy to free combinations.52, 53, 82, 83, 97
It is well recognized that clinical inertia in T2DM leads to suboptimal glycaemic control with a delay in the escalation of therapy and the introduction of insulin due to fear of injections, the risk of hypoglycaemia, weight gain as well as the need for more SMBG.100 The more recent availability of GLP‐1 RAs offers an alternative to insulin with equivalent glycaemic control, with the added advantage of weight loss and a low risk of hypoglycaemia in the absence of a sulphonylurea but with increased GI side‐effects, which, fortunately, are transient in the majority of patients. If increasing the dose of basal insulin is not an option and the earlier introduction of a GLP‐1 RA fails to achieve the required glycaemic target, then the combination of the two therapies becomes the next logical step. The recently available FRCs, iGlarLixi and IDegLira, provide a proven alternative to the free combinations52, 53, 82, 83 while minimizing the adverse effect profiles of the 2 components. While sequential treatment is common practice, this alternative approach using an FRC has several advantages, including the likelihood of improved adherence by reducing administration burden with once‐ vs multiple‐daily injections. However, it must be acknowledged that there are currently no studies directly comparing sequential treatment with earlier FRC use.
Failure to attain glycaemic control has implications for patients, healthcare professionals and healthcare systems due to the increased risk of micro‐ and macrovascular complications associated with diabetes. Currently, only a third of people with T2DM achieve and maintain their target HbA1c level, and it is expected that the number of T2DM cases will dramatically increase over the coming decades, with the potential for a substantial negative impact on medical costs and healthcare resources.101 Achieving good glycaemic control in an attempt to avoid complications is, therefore, paramount but the hurdles are not insignificant. Prompt treatment with insulin and GLP‐1 RA offers the opportunity of achieving glycaemic targets at an early stage of T2DM, which could potentially modulate the disease process and lower the rate of progression, thereby reducing the risk of complications and improving the individual's quality of life.102 It is important to note that when comparing second‐line treatment strategies in patients with T2DM and inadequate control on metformin alone, GLP‐1 RAs were associated with a discounted incremental benefit of 0.10 and 0.25 quality‐adjusted life years (QALYs) compared with DPP‐4 inhibitors and Neutral Protamine Hagedorn insulin, respectively.102 Liraglutide has been shown to be cost effective compared with the DPP‐4 inhibitor, sitagliptin, added to metformin, with an incremental cost‐effectiveness ratio below $50 000 per QALY gained.103 Using a medical economic model, lixisenatide was also considered a cost‐efficient therapy when compared with bolus insulin, with benefits in terms of QALYs gained and a reduction in lifetime healthcare costs.104
The rationale for developing FRCs of basal insulin and GLP‐1 RAs is scientifically valid, allowing the introduction of complementary therapies for improved outcomes with a lower risk of adverse events at any stage in the treatment algorithm.51 Importantly, the FRCs offer a real opportunity of introducing these therapies at an earlier stage in the disease process when, arguably, the greatest benefit will accrue.37, 45 FRC delivery devices that allow slow titration according to clinical response and tolerance can substantially reduce the GI side‐effects of GLP‐1 RAs as well as the risk of hypoglycaemia. This change in approach to treatment should reduce the impact on patients and healthcare resources alike. However, further studies are needed to support this proposed change to the T2DM treatment paradigm, and clear instructions will be required as to when and how to initiate and optimize FRCs in future guidelines. This shift reflects the aspiration of tailoring treatment to patients’ needs to maximize glycaemic control safely and effectively alongside improved quality of life.
To be successful, this proposed change to the current treatment paradigm will require adequate education in order to realize the full benefits of the early introduction of insulin therapy and GLP‐1 RAs in the form of FRCs.30, 31, 32, 100, 105 The ability to self‐titrate FRCs in a similar manner to basal insulin, but with no additional risk of hypoglycaemia and/or weight gain, is a clear benefit. Also, FRCs could offer the potential to reduce the time taken to achieve good glycaemic control and modulate the disease process, which may reduce the burden on associated healthcare resources and result in cost benefits.
5. CONCLUSIONS
Poor glycaemic control underscores the limitations of current treatment options and algorithms advocated in management guidelines. An unacceptably high proportion of people with T2DM, despite the many therapeutic options available, fail to reach their glycaemic targets due to a combination of factors, including poor adherence to therapy and clinical inertia from healthcare professionals. This review suggests a new treatment paradigm based around the early introduction of insulin and the rationale for a combination therapy of insulin plus a GLP‐1 RA in the form of an FRC. This may facilitate the likelihood of achieving and sustaining good glycaemic control with fewer side‐effects and better adherence. Improving clinical outcomes and controlling resource use in the management of people with T2DM is imperative especially as the demand for treatment continues to grow. Further studies are required to explore the clinical advantages, limitations and best ways to utilize this proposed new paradigm in the management of T2DM.
ACKNOWLEDGEMENTS
Editorial assistance was provided by Mark Greener and Debby Moss for Caudex, Oxford, UK, funded by Sanofi.
Conflicts of interest
D. R. O. has received honoraria for lectures and advisory board meetings with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Roche Diagnostics, Sanofi and Takeda. L. M. has no competing interest with the content of this review. A. H. B. has received honoraria for lectures and advisory work from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, MSD, Novartis, Novo Nordisk and Sanofi‐Aventis.
Author contributions
All authors confirm that they meet the International Committee of Medical Journal Editors uniform requirements for authorship and that they have: provided help with writing and reviewing the manuscript at all stages of development (D. R. O.); participated in the critical revision of the manuscript (L. M.); helped write the manuscript; and provided critical comments and recommendations (A. H. B.). All authors have read, reviewed and agreed to the final version.
Owens DR, Monnier L and Barnett AH. Future challenges and therapeutic opportunities in type 2 diabetes: Changing the paradigm of current therapy. Diabetes Obes Metab. 2017;19:1339–1352. https://doi.org/10.1111/dom.12977
Funding Information Editorial assistance was funded by Sanofi.
REFERENCES
- 1. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32:S151‐S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta‐cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel‐group trial. Lancet. 2008;371:1753‐1760. [DOI] [PubMed] [Google Scholar]
- 3. Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90‐year perspective. Postgrad Med J. 2016;92:63‐69. [DOI] [PubMed] [Google Scholar]
- 4. Abbas S, Raza ST, Ahmed F, Ahmad A, Rizvi S, Mahdi F. Association of genetic polymorphism of PPARgamma‐2, ACE, MTHFR, FABP‐2 and FTO genes in risk prediction of type 2 diabetes mellitus. J Biomed Sci. 2013;20:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary. Endocr Pract. 2016;22:84‐113. [DOI] [PubMed] [Google Scholar]
- 6. International Diabetes Federation . IDF global guideline for type 2 diabetes. 2012. http://www.idf.org/sites/default/files/IDF‐Guideline‐for‐Type‐2‐Diabetes.pdf. Accessed September 26, 2016.
- 7. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429‐442. [DOI] [PubMed] [Google Scholar]
- 8. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;368:1613‐1624. [DOI] [PubMed] [Google Scholar]
- 9. Chan JC, Gagliardino JJ, Baik SH, et al. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care. 2009;32:227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. NICE . Type 2 diabetes in adults: management. NICE guidelines (NG28). 2015. https://www.nice.org.uk/guidance/ng28/resources/type‐2‐diabetes‐in‐adults‐management‐1837338615493. Accessed June 17, 2016.
- 11. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 13. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 14. Zeng L, Lu H, Deng H, Mu P, Li X, Wang M. Noninferiority effects on glycemic control and beta‐cell function improvement in newly diagnosed type 2 diabetes patients: basal insulin monotherapy versus continuous subcutaneous insulin infusion treatment. Diabetes Technol Ther. 2012;14:35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535‐1540. [DOI] [PubMed] [Google Scholar]
- 16. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005‐2012. [DOI] [PubMed] [Google Scholar]
- 17. Riddle MC, Rosenstock J, Gerich J. The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080‐3086. [DOI] [PubMed] [Google Scholar]
- 18. Raccah D, Chou E, Colagiuri S, et al. A global study of the unmet need for glycemic control and predictor factors among patients with type 2 diabetes mellitus who have achieved optimal fasting plasma glucose control on basal insulin. Diabetes Metab Res Rev. 2017;33(3):e2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yam FK, Adams AG, Divine H, Steinke D, Jones MD. Clinical inertia in type 2 diabetes: a retrospective analysis of pharmacist‐managed diabetes care vs. usual medical care. Pharm Pract (Granada). 2013;11:203‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract. 2007;57:455‐460. [PMC free article] [PubMed] [Google Scholar]
- 22. Mata‐Cases M, Benito‐Badorrey B, Roura‐Olmeda P, et al. Clinical inertia in the treatment of hyperglycemia in type 2 diabetes patients in primary care. Curr Med Res Opin. 2013;29:1495‐1502. [DOI] [PubMed] [Google Scholar]
- 23. Dandona P, Chaudhuri A, Mohanty P, Ghanim H. Anti‐inflammatory effects of insulin. Curr Opin Clin Nutr Metab Care. 2007;10:511‐517. [DOI] [PubMed] [Google Scholar]
- 24. Food and Drug Administration . FDA drug safety communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. 2015. http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm. Accessed July 11, 2016.
- 25. Food and Drug Administration . FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. 2015. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed August 20, 2015.
- 26. Trujillo JM, Nuffer W, Ellis SL. GLP‐1 receptor agonists: a review of head‐to‐head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goswami S, Yee SW, Stocker S, et al. Genetic variants in transcription factors are associated with the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther. 2014;96:370‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pawlyk AC, Giacomini KM, McKeon C, Shuldiner AR, Florez JC. Metformin pharmacogenomics: current status and future directions. Diabetes. 2014;63:2590‐2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough – what next? Diabetes Metab Res Rev. 2007;23:257‐264. [DOI] [PubMed] [Google Scholar]
- 30. Meneghini LF. Early insulin treatment in type 2 diabetes: what are the pros? Diabetes Care. 2009;32(suppl 2):S266‐S269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hermanns N, Mahr M, Kulzer B, Skovlund SE, Haak T. Barriers towards insulin therapy in type 2 diabetic patients: results of an observational longitudinal study. Health Qual Life Outcomes. 2010;8:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Polonsky WH, Hajos TR, Dain MP, Snoek FJ. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large, international population. Curr Med Res Opin. 2011;27:1169‐1174. [DOI] [PubMed] [Google Scholar]
- 33. Kabadi UM. Starting insulin in type 2 diabetes: overcoming barriers to insulin therapy. Int J Diabetes Dev Ctries. 2008;28:65‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris SB, Yale JF, Berard L, et al. Does a patient‐managed insulin intensification strategy with insulin glargine and insulin glulisine provide similar glycemic control as a physician‐managed strategy? Results of the START (Self‐Titration with Apidra to Reach Target) study: a randomized noninferiority trial. Diabetes Care. 2014;37:604‐610. [DOI] [PubMed] [Google Scholar]
- 35. Davies M, Storms F, Shutler S, Bianchi‐Biscay M, Gomis R. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282‐1288. [DOI] [PubMed] [Google Scholar]
- 36. Bailey T. Options for combination therapy in type 2 diabetes: comparison of the ADA/EASD position statement and AACE/ACE algorithm. Am J Med. 2013;126:S10‐S20. [DOI] [PubMed] [Google Scholar]
- 37. Fonseca V, Gill J, Zhou R, Leahy J. An analysis of early insulin glargine added to metformin with or without sulfonylurea: impact on glycaemic control and hypoglycaemia. Diabetes Obes Metab. 2011;13:814‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kramer CK, Choi H, Zinman B, Retnakaran R. Determinants of reversibility of beta‐cell dysfunction in response to short‐term intensive insulin therapy in patients with early type 2 diabetes. Am J Physiol Endocrinol Metab. 2013;305:E1398‐E1407. [DOI] [PubMed] [Google Scholar]
- 39. Mu PW, Chen YM, Lu HY, et al. Effects of a combination of oral anti‐diabetes drugs with basal insulin therapy on beta‐cell function and glycaemic control in patients with newly diagnosed type 2 diabetes. Diabetes Metab Res Rev. 2012;28:236‐240. [DOI] [PubMed] [Google Scholar]
- 40. Gaede P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow‐up on the Steno‐2 randomised trial. Diabetologia. 2016;59:2298‐2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kramer CK, Zinman B, Choi H, Retnakaran R. Predictors of sustained drug‐free diabetes remission over 48 weeks following short‐term intensive insulin therapy in early type 2 diabetes. BMJ Open Diabetes Res Care. 2016;4:e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Proinflammatory effects of glucose and anti‐inflammatory effect of insulin: relevance to cardiovascular disease. Am J Cardiol. 2007;99:15B‐26B. [DOI] [PubMed] [Google Scholar]
- 43. Reid T, Gao L, Gill J, et al. How much is too much? Outcomes in patients using high‐dose insulin glargine. Int J Clin Pract. 2016;70:56‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Monnier L, Colette C. Addition of rapid‐acting insulin to basal insulin therapy in type 2 diabetes: indications and modalities. Diabetes Metab. 2006;32:7‐13. [DOI] [PubMed] [Google Scholar]
- 45. Aschner P, Chan J, Owens DR, et al. Insulin glargine versus sitagliptin in insulin‐naive patients with type 2 diabetes mellitus uncontrolled on metformin (EASIE): a multicentre, randomised open‐label trial. Lancet. 2012;379:2262‐2269. [DOI] [PubMed] [Google Scholar]
- 46. Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30:263‐269. [DOI] [PubMed] [Google Scholar]
- 47. Monnier L, Colette C, Dejager S, Owens D. Magnitude of the dawn phenomenon and its impact on the overall glucose exposure in type 2 diabetes: is this of concern? Diabetes Care. 2013;36:4057‐4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395‐403. [DOI] [PubMed] [Google Scholar]
- 49. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728‐742. [DOI] [PubMed] [Google Scholar]
- 50. Esposito K, Mosca C, Brancario C, Chiodini P, Ceriello A, Giugliano D. GLP‐1 receptor agonists and HBA1c target of <7% in type 2 diabetes: meta‐analysis of randomized controlled trials. Curr Med Res Opin. 2011;27:1519‐1528. [DOI] [PubMed] [Google Scholar]
- 51. Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon‐like peptide‐1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta‐analysis. Lancet. 2014;384:2228‐2234. [DOI] [PubMed] [Google Scholar]
- 52. Buse JB, Vilsbøll T, Thurman J, et al. Contribution of liraglutide in the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37:2926‐2933. [DOI] [PubMed] [Google Scholar]
- 53. Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open‐label, randomised, 26‐week, treat‐to‐target trial in insulin‐naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885‐893. [DOI] [PubMed] [Google Scholar]
- 54. Riddle MC, Aronson R, Home P, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care. 2013;36:2489‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riddle MC, Forst T, Aronson R, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24‐week, randomized, placebo‐controlled study (GetGoal‐Duo 1). Diabetes Care. 2013;36:2497‐2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rosenstock J, Hanefeld M, Shamanna P, et al. Beneficial effects of once‐daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal‐S). J Diabetes Complications. 2014;28:386‐392. [DOI] [PubMed] [Google Scholar]
- 57. Owens DR, Monnier L, Bolli GB. Differential effects of GLP‐1 receptor agonists on components of dysglycaemia in individuals with type 2 diabetes mellitus. Diabetes Metab. 2013;39:485‐496. [DOI] [PubMed] [Google Scholar]
- 58. Uccellatore A, Genovese S, Dicembrini I, Mannucci E, Ceriello A. Comparison review of short‐acting and long‐acting glucagon‐like peptide‐1 receptor agonists. Diabetes Ther. 2015;6:239‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fonseca VA, Alvarado‐Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE. Efficacy and safety of the once‐daily GLP‐1 receptor agonist lixisenatide in monotherapy: a randomized, double‐blind, placebo‐controlled trial in patients with type 2 diabetes (GetGoal‐Mono). Diabetes Care. 2012;35:1225‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zinman B, Hoogwerf BJ, Duran GS, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477‐485. [DOI] [PubMed] [Google Scholar]
- 61. Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double‐blind, placebo‐controlled trial of the once‐daily GLP‐1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal‐L‐Asia). Diabetes Obes Metab. 2012;14:910‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ahrén B, Leguizamo DA, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once‐daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal‐M). Diabetes Care. 2013;36:2543‐2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet. 2009;374:39‐47. [DOI] [PubMed] [Google Scholar]
- 64. Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B. Liraglutide, a once‐daily human glucagon‐like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:348‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vilsboll T, Brock B, Perrild H, et al. Liraglutide, a once‐daily human GLP‐1 analogue, improves pancreatic B‐cell function and arginine‐stimulated insulin secretion during hyperglycaemia in patients with Type 2 diabetes mellitus. Diabet Med. 2008;25:152‐156. [DOI] [PubMed] [Google Scholar]
- 66. Yu Pan C, Han P, Liu X, et al. Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: a randomized, double‐blind, placebo‐controlled, 24‐week trial (GetGoal‐M‐Asia). Diabetes Metab Res Rev. 2014;30:726‐735. [DOI] [PubMed] [Google Scholar]
- 67. Paul S, Best J, Klein K, Han J, Maggs D. Effects of HbA1c and weight reduction on blood pressure in patients with type 2 diabetes mellitus treated with exenatide. Diabetes Obes Metab. 2012;14:826‐834. [DOI] [PubMed] [Google Scholar]
- 68. Buse JB, Sesti G, Schmidt WE, et al. Switching to once‐daily liraglutide from twice‐daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet. 2009;373:473‐481. [DOI] [PubMed] [Google Scholar]
- 70. Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083‐1091. [DOI] [PubMed] [Google Scholar]
- 71. Blevins T, Pullman J, Malloy J, et al. DURATION‐5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301‐1310. [DOI] [PubMed] [Google Scholar]
- 72. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet. 2008;372:1240‐1250. [DOI] [PubMed] [Google Scholar]
- 73. Drucker DJ. The cardiovascular biology of glucagon‐like peptide‐1. Cell Metab. 2016;24:15‐30. [DOI] [PubMed] [Google Scholar]
- 74. Holscher C. Potential role of glucagon‐like peptide‐1 (GLP‐1) in neuroprotection. CNS Drugs. 2012;26:871‐882. [DOI] [PubMed] [Google Scholar]
- 75. Avogaro A, Vigili de Kreutzenberg S, Fadini GP. Cardiovascular actions of GLP‐1 and incretin‐based pharmacotherapy. Curr Diab Rep. 2014;14:483. [DOI] [PubMed] [Google Scholar]
- 76. Sun F, Wu S, Guo S, et al. Impact of GLP‐1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetes Res Clin Pract. 2015;110:26‐37. [DOI] [PubMed] [Google Scholar]
- 77. Meier JJ, Rosenstock J, Hincelin‐Mery A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care. 2015;38:1263‐1273. [DOI] [PubMed] [Google Scholar]
- 78. Lonn EM, Rambihar S, Gao P, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all‐cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol. 2014;103:149‐159. [DOI] [PubMed] [Google Scholar]
- 79. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247‐2257. [DOI] [PubMed] [Google Scholar]
- 80. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP‐1 receptor agonist, versus thrice‐daily prandial insulin lispro. Diabetes Care. 2014;37:2317‐2325. [DOI] [PubMed] [Google Scholar]
- 81. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high‐dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16:827‐832. [DOI] [PubMed] [Google Scholar]
- 82. Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan‐L randomized trial. Diabetes Care. 2016;39:1972‐1980. [DOI] [PubMed] [Google Scholar]
- 83. Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan‐O randomized trial. Diabetes Care. 2016;39:2026‐2035. [DOI] [PubMed] [Google Scholar]
- 84. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo‐controlled trial. Diabetes Obes Metab. 2015;17:1056‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36‐week, randomized, double‐blind, parallel‐group trial. J Diabetes Invest. 2016;7:565‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Raccah D, Lin J, Wang E, et al. Once‐daily prandial lixisenatide versus once‐daily rapid‐acting insulin in patients with type 2 diabetes mellitus insufficiently controlled with basal insulin: analysis of data from five randomized, controlled trials. J Diabetes Complications. 2014;28:40‐44. [DOI] [PubMed] [Google Scholar]
- 87. Jendle J, Grunberger G, Blevins T, Giorgino F, Hietpas RT, Botros FT. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev. 2016;32:776‐790. [DOI] [PubMed] [Google Scholar]
- 88. Murphy CE. Review of the safety and efficacy of exenatide once weekly for the treatment of type 2 diabetes mellitus. Ann Pharmacother. 2012;46:812‐821. [DOI] [PubMed] [Google Scholar]
- 89. Nauck MA. The design of the liraglutide clinical trial programme. Diabetes Obes Metab. 2012;14(suppl 2):4‐12. [DOI] [PubMed] [Google Scholar]
- 90. Rodbard H, Lingvay I, Reed J. Efficacy and safety of semaglutide once weekly vs placebo as add on to basal insulin alone or in combination metformin in subjects with type 2 diabetes (SUSTAIN‐5). Diabetes. 2016;65(suppl 1):A221‐A360, 981‐P. [Google Scholar]
- 91. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal‐plus or basal‐bolus in type 2 diabetes: the GetGoal Duo‐2 trial. Diabetes Care. 2016;39:1318‐1328. [DOI] [PubMed] [Google Scholar]
- 92. Linjawi S, Bode B, Chaykin L, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP‐1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther. 2017;8:101‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rodbard HW, Bode B, Harris S. IDegLira in insulin‐naive patients with type 2 diabetes (T2D) inadequately controlled on sulfonylureas (SU) alone or in combination with metformin: the DUAL IV study. Diabetes. 2015;64(suppl 1):A255‐A256. [Google Scholar]
- 94. Buse JB, Perez Manghi FC, Garcia‐Hernandez PA, et al. Insulin degludec/liraglutide (IDegLira) is superior to insulin glargine (IG) in A1c reduction, risk of hypoglycaemia and weight change: DUAL V study. Diabetes Care. 2015;64(suppl 1):A43‐A44. [Google Scholar]
- 95. Harris SB, Kocsis G, Prager R, et al. Safety and efficacy of IDegLira titrated once weekly versus twice weekly in patients with type 2 diabetes uncontrolled on oral antidiabetic drugs: DUAL VI randomized clinical trial. Diabetes Obes Metab. 2017;19:858‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lingvay I, Manghi FP, Garcia‐Hernandez P, et al. Effect of insulin glargine up‐titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315:898‐907. [DOI] [PubMed] [Google Scholar]
- 97. Rosenstock J, Diamant M, Aroda VR, et al. Efficacy and safety of LixiLan, a titratable fixed‐ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan proof‐of‐concept randomized trial. Diabetes Care. 2016;39:1579‐1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Novo Nordisk . Xultrophy®: summary of product characteristics. 2014. http://ec.europa.eu/health/documents/community‐register/2014/20140918129550/anx_129550_en.pdf. Accessed January 25, 2017.
- 99. Sanofi . Soliqua®: US prescribing information. 2016. http://products.sanofi.us/Soliqua100‐33/Soliqua100‐33.pdf. Accessed January 23, 2017.
- 100. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26:S18‐S24. [DOI] [PubMed] [Google Scholar]
- 101. Paneni F, Cosentino F. Epidemiology, definition, and diagnosis of diabetes mellitus In: Diabetes and Cardiovascular Disease. Cham, Switzerland: Springer; 2015:3‐12. [Google Scholar]
- 102. Kiadaliri AA, Gerdtham UG, Eliasson B, Carlsson KS. Cost‐utility analysis of glucagon‐like peptide‐1 agonists compared with dipeptidyl peptidase‐4 inhibitors or neutral protamine hagedorn basal insulin as add‐on to metformin in type 2 diabetes in Sweden. Diabetes Ther. 2014;5:591‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lee WC, Samyshkin Y, Langer J, Palmer JL. Long‐term clinical and economic outcomes associated with liraglutide versus sitagliptin therapy when added to metformin in the treatment of type 2 diabetes: a CORE Diabetes Model analysis. J Med Econ. 2012;15(suppl 2):28‐37. [DOI] [PubMed] [Google Scholar]
- 104. Huetson P, Palmer JL, Levorsen A, Fournier M, Germe M, McLeod E. Cost‐effectiveness of once daily GLP‐1 receptor agonist lixisenatide compared to bolus insulin both in combination with basal insulin for the treatment of patients with type 2 diabetes in Norway. J Med Econ. 2015;18:573‐585. [DOI] [PubMed] [Google Scholar]
- 105. Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009;164:6‐10. [DOI] [PubMed] [Google Scholar]


