Abstract
Aims
To assess the effects of 16 weeks of tofogliflozin (sodium‐glucose co‐transporter‐2 [SGLT2] inhibitor) treatment vs placebo on glycated haemoglobin (HbA1c) levels in Japanese patients with type 2 diabetes mellitus (T2DM) inadequately controlled with insulin monotherapy or insulin plus a dipeptidyl peptidase‐4 (DPP‐4) inhibitor.
Methods
The study comprised a 16‐week, multicentre, double‐blind, placebo‐controlled period and a 36‐week extension (NCT02201004). Men and women (aged ≥20 and ≤75 years) with T2DM (HbA1c ≥7.5% and ≤10.5%) were randomized 2:1 to tofogliflozin 20 mg once/day or placebo. The primary endpoint was change in HbA1c from baseline. Insulin reduction was not permitted during this study.
Results
A total of 211 patients were randomized (141 tofogliflozin, 70 placebo). Addition of tofogliflozin to insulin therapy was significantly superior to placebo for lowering HbA1c (−0.59 vs +0.48%; P < .0001), fasting plasma glucose (−27.2 vs +5.3 mg/dL; P < .0001), postprandial plasma glucose (−65.0 vs +3.2 mg/dL; P < 0.0001), serum uric acid (−0.18 vs +0.07 mg/dL; P = .0062), body weight (−1.34 vs +0.03 kg; P < .0001) and daily insulin dose (−1.3 vs −0.2 U, P = .0152). Hypoglycaemia occurred in 30.7% of patients receiving tofogliflozin vs 21.4% for placebo. Two patients treated with tofogliflozin each had a genital or urinary tract infection.
Conclusions
This 16‐week double‐blind study indicated that, in patients with T2DM whose HbA1c levels were poorly controlled with insulin monotherapy or insulin plus a DPP‐4 inhibitor, addition of tofogliflozin was an effective treatment option with an acceptable safety profile.
Keywords: basal insulin (or insulin therapy), glycaemic control, hypoglycaemia, randomized trial, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Many patients with type 2 diabetes mellitus (T2DM) have progressive β‐cell failure that ultimately requires the use of insulin as part of their treatment regimen.1, 2, 3 Current treatment guidelines recommend continuation of oral antidiabetic drugs in patients being transitioned to insulin,4 and nearly all oral antidiabetic drugs, including metformin, sulphonylurea and dipeptidyl peptidase‐4 (DPP‐4) inhibitors, have been combined effectively with insulin.5, 6, 7
Sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors are the newest class of oral antidiabetic drug and have several important characteristics that make them attractive for use in combination with insulin.8 These drugs lower plasma glucose levels by increasing urinary excretion.8, 9 Their mechanism of action is independent of insulin and their glucose‐lowering effects should be expected to complement those of insulin therapy.8 SGLT2 inhibitors also promote weight loss and reduce blood pressure and are associated with a relatively low risk of hypoglycaemia.8, 9 The American Diabetes Association guidelines state that adjunctive use of SGLT2 inhibitors may improve glycaemic control and reduce the amount of insulin needed in patients with T2DM4; however, the combination of SGLT2 inhibitors with insulin or sulphonylurea treatment may lead to increases in hypoglycaemia.10, 11
Tofogliflozin, a highly selective SGLT2 inhibitor, has been approved in Japan for the treatment of T2DM.12 Clinical trials have shown that tofogliflozin, when used as monotherapy or in combination with oral antidiabetic agents, significantly decreases glycated haemoglobin (HbA1c), fasting plasma glucose (FPG), body weight and systolic and diastolic blood pressure (BP) in patients with T2DM, while maintaining a low risk of hypoglycaemia.13, 14
At present, there is no information about the efficacy and safety of tofogliflozin when added to insulin therapy in patients with T2DM. The aim of the present study was to assess the effects of tofogliflozin vs placebo on glycaemic control when added to insulin treatment in terms of HbA1c reduction in patients with T2DM.
2. MATERIALS AND METHODS
2.1. Study design
This was a 16‐week, randomized, double‐blind, placebo‐controlled multicentre trial that also included a 36‐week extension period (NCT02201004). The study was conducted in 30 diabetes outpatient clinics in Japan. During the 16‐week double‐blind treatment period, patients with T2DM inadequately controlled on insulin were assigned randomly 2:1 to tofogliflozin 20 mg or placebo once daily (before or after breakfast). Randomization was stratified based on a minimization method including HbA1c level (<8.0% and ≥8.0%), insulin regimen (basal‐bolus, bolus, premix, basal) and estimated glomerular filtration rate (eGFR; ≥90, ≥60 to <90, ≥30 to <60 mL/min/1.73 m2) and was conducted via an interactive web response system (IWRS).
The 16‐week double‐blind treatment period was followed by a 36‐week open‐label extension in which all patients received 20 mg/d tofogliflozin. The primary endpoints during the double‐blind treatment period were efficacy and safety and that for the open‐label extension was safety. Patients were dispensed their allotted medication at each study visit according to the treatment numbers provided by the IWRS. Tofogliflozin and placebo containers were visually indistinguishable.
The trial was conducted in accordance with the principles in the protocol and in Article 14.4.4, 14.4.7 of the Japanese Pharmaceutical Affairs Act, the Good Clinical Practice Standards for the Implementation of Clinical Trials (Ordinance of the Pharmaceutical Affairs Bureau, Ministry of Health and Welfare No. 28 of March 1997), the Good Post‐Marketing Study Practice (Ordinance of the Japanese Pharmaceutical Affairs Bureau, Ministry of Health, Labour and Welfare No.171 of 20 December 2004) and related notices. The study protocol was also approved by local institutional review boards. All patients provided informed written consent.
2.2. Participant inclusion and exclusion criteria
Patients presenting at the diabetes outpatient clinics of the participating investigators were eligible for participation. Participating investigators screened patients for eligibility and entered them into the randomization sequence.
The trial included men and women (aged ≥20 to ≤75 years) with T2DM inadequately controlled on insulin monotherapy or on combination of basal insulin and a DPP‐4 inhibitor. Patients were treated for ≥3 months prior to screening with stable insulin doses (±20%) in basal‐bolus, bolus, premix (low and high) or basal regimens. At screening, patients had HbA1c concentrations between 7.5% and 10.5% (inclusive), FPG levels ≤220 mg/dL and body mass index (BMI) between 18.5 and 35.0 kg/m2. No change in DPP‐4 inhibitor dose was permitted during the 3 months before screening.
Potential patients were excluded if they had: type 1 diabetes mellitus; unstable proliferative diabetic retinopathy or any other rapidly progressive diabetic retinopathy or macular oedema; history of metabolic acidosis ≤1 year prior to screening visit; severe uncontrolled hyperglycaemia; history of drug or alcohol abuse; transient ischaemic attacks; or acute cerebrovascular or cardiovascular events requiring hospitalization ≤6 months prior to screening. Severe or uncontrolled congestive heart failure, initiation of weight loss drugs ≤3 months prior to screening, use of systemic glucocorticoids for a total of 1 week or more within 3 months prior to screening visit, and use of any investigational drugs within 3 months prior to the screening visit were also grounds for exclusion. Potential patients were also excluded if they had haemoglobinopathy or haemolytic anaemia, had received blood or plasma products within 3 months prior to screening visit, or required dosing changes within 4 weeks before screening for lipid‐lowering, antihypertensive or uric acid‐lowering drugs, or for a thyroid hormone preparation. Blood pressure >180/110 mm Hg, alanine aminotransferase or alanine aminotransferase levels >3.0 times the upper limit of the reference range (× ULN), total bilirubin >1.5 × ULN, elevated serum creatinine (2.0 mg/dL for men, 1.5 mg/dL for women), evidence of hepatitis B or C virus infection, a positive serum pregnancy test result, severe renal impairment (eGFR <30 mL/min/1.73 m2 and/or on dialysis), serious infection, abnormal pituitary or adrenal function, and urinary tract or genital infections were also grounds for exclusion.
2.3. Insulin dosing
The insulin dose was not to be changed during the 16‐week double‐blind treatment period unless the patient experienced hypoglycaemia. The insulin dose could only be reduced if hypoglycaemia occurred or to prevent the occurrence of this adverse event (AE). The insulin dose changed if FPG was ≥240 mg/dL and did not decrease to below 240 mg/dL with appropriate treatment during the 16‐week period (defined as rescue therapy).
2.4. Study endpoints
2.4.1. Efficacy
The primary endpoint was the change from baseline to 16 weeks in HbA1c for tofogliflozin vs placebo. Secondary endpoints included comparison of the changes from baseline to week 16 for tofogliflozin vs placebo in FPG, postprandial plasma glucose (PPG; glucose level measured 2 hours after standardized breakfast at weeks 0 and 16), body weight, systolic and diastolic BP, uric acid, lipid profile (total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides) and insulin dose. The between‐group differences in the proportions of patients who achieved HbA1c <7.0% or ≤6.5% at week 16 were also determined.
2.4.2. Safety
Hypoglycaemic episodes were assessed by patient diary entries, which were reviewed at each study visit. Hypoglycaemia was classified as severe, documented symptomatic, asymptomatic, probable symptomatic or nocturnal; see File S1 for definitions of these classifications. AE data (treatment‐emergent AEs [TEAEs] and serious AEs [SAEs]) and vital signs were collected at each study visit. Urinary tract or genital infections were recorded based on diagnosis by the investigator. Clinical laboratory tests were performed at randomization and at the final visit in the double‐blind period (week 16).
2.5. Statistical analyses
Efficacy was assessed in the modified intention‐to‐treat (mITT) population, which included patients randomized and exposed to at least 1 dose of tofogliflozin or placebo who had both a baseline measurement and at least 1 post‐baseline measurement for any efficacy variable.
Efficacy variables compared with placebo including the primary endpoint were analysed using a mixed model with repeated measurements (MMRM), with treatment group, visit, treatment group‐by‐visit interaction, randomization strata of screening HbA1c, insulin regimen and eGFR as fixed effects, and baseline HbA1c and baseline HbA1c‐by‐visit interaction as covariates. The least squares (LS) mean changes in each variable from baseline to week 16 for each treatment group were provided in the framework of this model, as well as the difference between treatment groups and the 95% confidence interval (CI) for the LS mean. Statistical tests for efficacy variables had a 2‐sided 5% significance level.
For the primary efficacy endpoint, sensitivity analyses were performed with imputed value by method of last observation carried forward (LOCF) by means of analysis of covariance and including data collected after rescue therapy using the MMRM model. Subgroup analyses were also performed using the MMRM model.
Responder rates for HbA1c <7.0% and ≤6.5% at week 16 in the two treatment groups were compared using a Cochran–Mantel–Haenszel test, stratified as described above, and both P values and 95% CIs were determined.
All safety analyses were performed on the safety population, which was defined as all patients randomized and exposed to at least one dose of study treatment.
2.5.1. Sample size determination
A sample size of 128 patients for the tofogliflozin group and 64 for the placebo group with a 2:1 allocation rate was calculated as sufficient to detect a between‐group difference of 0.5% in the absolute change in HbA1c from baseline to week 16 with a power of 90%. This assumed a common standard deviation of 1.0% at a two‐sided 5% significance level. Considering dropout, a total of 210 randomized patients (140 tofogliflozin, 70 placebo) was determined to be necessary.
3. RESULTS
3.1. Patients
A total of 320 patients were screened and 211 were randomized (141 tofogliflozin, 70 placebo). One patient in the tofogliflozin group underwent randomization but was not treated. The other 210 treated patients received treatment as per randomization and were included in the mITT population (Figure 1). The first patient enrolled in the study on June 30, 2014 and the final patient completed the 16‐week phase on January 27, 2016. Three patients discontinued tofogliflozin (2 because of AEs and 1 because of the patient's decision), whereas 2 discontinued placebo (1 because of the patient's decision and 1 because of failure to meet the entry criteria).
Figure 1.
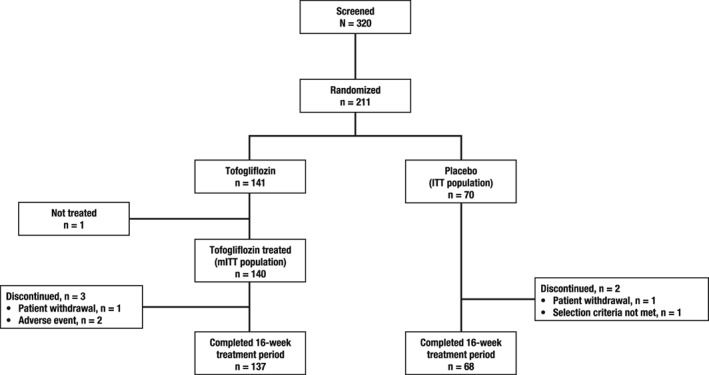
Patient disposition
There were minor differences between treatment groups with respect to several characteristics. Most notably, patients in the tofogliflozin group were older, were more likely to have existing cardiac disorders and had a longer mean duration of disease compared with those who received placebo (Table 1).
Table 1.
Baseline demographic and clinical characteristics
| Characteristics | Tofogliflozin (n = 141) | Placebo (n = 70) |
|---|---|---|
| Age, years | 59.1 ± 10.8 | 56.4 ± 10.0 |
| ≥65 years, n (%) | 59 (41.8) | 17 (24.3) |
| Male, n (%) | 90 (63.8) | 48 (68.6) |
| HbA1c, % | 8.53 ± 0.75 | 8.40 ± 0.65 |
| HbA1c ≥8.0%, n (%) | 107 (75.9) | 50 (71.4) |
| eGFR, mL/min/1.73 m2 | 79.7 ± 19.8 | 79.5 ± 17.0 |
| eGFR ≥30 to <60 mL/min/1.73 m2, n (%) | 14 (9.9) | 9 (12.9) |
| Body weight, kg | 68.87 ± 13.20 | 72.24 ± 11.12 |
| BMI, kg/m2 | 25.8 ± 3.5 | 26.9 ± 3.9 |
| BMI, n (%) | ||
| <25 kg/m2 | 61 (43.3) | 24 (34.3) |
| ≥25 to <30 kg/m2 | 62 (44.0) | 29 (41.4) |
| ≥30 kg/m2 | 18 (12.8) | 17 (24.3) |
| FPG, mg/dL | 163.4 ± 47.5 | 162.4 ± 43.2 |
| PPG, mg/dL | 299.9 ± 68.5 | 302.6 ± 65.6 |
| Duration of diabetes, years | 15.02 ± 9.36 | 12.39 ± 7.34 |
| Insulin treatment, n (%) | ||
| Basal‐bolus | 34 (24.1) | 17 (24.3) |
| Bolus | 16 (11.3) | 8 (11.4) |
| Premix | 23 (16.3) | 11 (15.7) |
| Basal | 68 (48.2) | 34 (48.6) |
| Basal only | 34 (24.1) | 9 (12.9) |
| Basal combined with DPP‐4 inhibitors | 34 (24.1) | 25 (35.7) |
| Comorbidity | ||
| Diabetic retinopathy | 67 (47.5) | 34 (48.6) |
| Diabetic sensory or motor neuropathya | 41 (29.1) | 13 (18.6) |
| Diabetic autonomic neuropathya | 9 (6.4) | 0 |
| Diabetic nephropathya | 63 (44.7) | 27 (38.6) |
| Cardiac disordersb | 21 (15.0) | 7 (10.0) |
| Concomitant cardiovascular medicationb | ||
| β‐blocking agents | 11 (7.9) | 10 (14.3) |
| Diuretics | 10 (7.1) | 4 (5.7) |
| Agents acting on the renin‐angiotensin system | 5 (3.6) | 8 (11.4) |
| Lipid‐modifying agents | 5 (3.6) | 5 (7.1) |
| Peripheral vasodilators | 1 (0.7) | 1 (1.4) |
| Vasoprotectives | 1 (0.7) | 1 (1.4) |
Data are mean ± standard deviation, unless indicated otherwise.
As reported by the attending physician.
n = 140.
None of the patients who received tofogliflozin and 2 (2.9%) who were treated with placebo required rescue therapy.
3.2. Efficacy
3.2.1. Primary endpoint
Treatment with tofogliflozin resulted in a statistically significant decrease in HbA1c from baseline to week 16 compared with placebo (LS mean difference of −1.07%; P < .0001; Figure 2 and Table 2). The changes from baseline were −0.59% for tofogliflozin and +0.48% for placebo.
Figure 2.
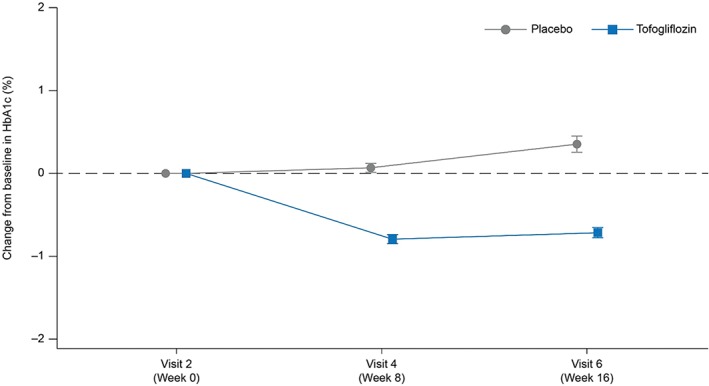
Change in HbA1c values for tofogliflozin and placebo from baseline to week 16. Values shown are mean ± standard error
Table 2.
Results for primary and secondary efficacy endpoints
| Tofogliflozin | Placebo | Estimated placebo vs tofogliflozin difference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | s.d. | n | Mean | s.d. | LS mean difference (s.e.) | 95% CI | P | ||
| HbA1c, % | Baseline | 140 | 8.53 | 0.76 | 70 | 8.40 | 0.65 | – | – | – |
| Week 16 | 135 | 7.80 | 0.85 | 66 | 8.74 | 0.91 | ||||
| Change from baseline to week 16 | 135 | −0.72 | 0.71 | 66 | 0.35 | 0.74 | ||||
| LS mean | 135 | −0.59 | 0.069 | 135 | 0.48 | 0.089 | −1.07 (0.090) | −1.246, −0.890 | <.0001 | |
| FPG, mg/dL | Baseline | 140 | 164.0 | 47.2 | 70 | 162.4 | 43.2 | −32.1 (4.37) | −40.69, −23.44 | <.0001 |
| Week 16 | 135 | 134.7 | 30.0 | 66 | 165.8 | 46.4 | ||||
| Change from baseline to week 16 | 135 | −27.2 | 38.4 | 66 | 5.3 | 41.9 | ||||
| PPG, mg/dL | Baseline | 140 | 300.3 | 68.6 | 70 | 302.6 | 65.6 | −67.7 (7.80) | −83.04, −52.27 | <.0001 |
| Week 16 | 134 | 236.4 | 61.9 | 66 | 302.1 | 74.6 | ||||
| Change from baseline to week 16 | 134 | −65.0 | 55.1 | 66 | 3.2 | 64.3 | ||||
| Body weight, kg | Baseline | 140 | 68.84 | 13.24 | 70 | 72.24 | 11.12 | −1.40 (0.229) | −1.847, −0.943 | <.0001 |
| Week 16 | 135 | 67.41 | 13.02 | 66 | 72.42 | 11.65 | ||||
| Change from baseline to week 16 | 135 | −1.34 | 1.62 | 66 | 0.03 | 1.36 | ||||
| Systolic BP, mm Hg | Baseline | 140 | 134.8 | 16.4 | 70 | 134.7 | 15.9 | −2.2 (1.93) | −5.99, 1.63 | .2605 |
| Week 16 | 135 | 130.1 | 17.2 | 66 | 132.2 | 16.6 | ||||
| Change from baseline to week 16 | 135 | −4.8 | 15.0 | 66 | −2.7 | 11.8 | ||||
| Diastolic BP, mm Hg | Baseline | 140 | 76.4 | 10.5 | 70 | 77.9 | 11.0 | −2.9 (1.26) | −5.38, −0.43 | .0218 |
| Week 16 | 135 | 74.5 | 11.7 | 66 | 78.6 | 10.4 | ||||
| Change from baseline to week 16 | 135 | −1.8 | 9.4 | 66 | 0.4 | 7.7 | ||||
| Uric acid, mg/dL | Baseline | 140 | 5.05 | 1.25 | 70 | 5.23 | 1.42 | −0.29 (0.105) | −0.500, −0.084 | .0062 |
| Week 16 | 135 | 4.87 | 1.24 | 66 | 5.27 | 1.44 | ||||
| Change from baseline to week 16 | 135 | −0.18 | 0.74 | 66 | 0.07 | 0.73 | ||||
| Total cholesterol, mg/dL | Baseline | 140 | 203.3 | 40.5 | 70 | 204.9 | 32.4 | 1.80 (3.51) | −5.10, 8.73 | .6056 |
| Week 16 | 135 | 206.7 | 35.8 | 66 | 204.7 | 31.4 | ||||
| Change from baseline to week 16 | 135 | 3.6 | 27.4 | 66 | 2.2 | 24.4 | ||||
| HDL cholesterol, mg/dL | Baseline | 140 | 57.0 | 17.0 | 70 | 55.6 | 13.9 | 2.7 (1.38) | −0.07, 5.37 | .0559 |
| Week 16 | 135 | 59.7 | 18.8 | 66 | 56.3 | 14.2 | ||||
| Change from baseline to week 16 | 135 | 2.9 | 9.0 | 66 | 0.3 | 9.6 | ||||
| LDL cholesterol, mg/dL | Baseline | 140 | 121.4 | 33.0 | 70 | 130.2 | 26.9 | 1.6 (2.99) | −4.25, 7.54 | .5822 |
| Week 16 | 135 | 125.6 | 32.8 | 66 | 129.5 | 26.8 | ||||
| Change from baseline to week 16 | 135 | 4.1 | 22.2 | 66 | 1.1 | 17.7 | ||||
| Triglycerides, mg/dL | Baseline | 140 | 177.0 | 218.9 | 70 | 128.6 | 65.6 | 10.1 (14.78) | −19.02, 39.29 | .4938 |
| Week 16 | 135 | 145.6 | 111.9 | 66 | 124.5 | 78.1 | ||||
| Change from baseline to week 16 | 135 | −31.1 | 214.2 | 66 | 3.9 | 60.6 | ||||
| Insulin dose, U | Baseline | 140 | 26.7 | 17.7 | 70 | 28.5 | 19.4 | −1.3 (0.52) | −2.31, −0.25 | .0152 |
| Week 16 | 128 | 25.5 | 15.9 | 64 | 28.7 | 19.1 | ||||
| Change from baseline to Week 16 | 128 | −1.3 | 4.8 | 64 | −0.2 | 0.8 | ||||
Abbreviations: s.d., standard deviation; s.e., standard error.
The sensitivity analysis using LOCF and excluding measurements after administration of rescue medication also indicated significant superiority of tofogliflozin over placebo for reduction of HbA1c (LS mean difference of −1.05; P < .0001). This was also the case for analysis of results after receipt of rescue medication (LS mean difference of −1.09; P < .0001).
Subgroup analyses indicated that addition of tofogliflozin to insulin was significantly superior to placebo in patients aged <65 or ≥65 years (LS mean differences of −1.14 and −0.90, respectively, both P < .0001); men and women (LS mean differences of −1.10 and −1.01, respectively, both P < .0001); patients with baseline BMI <25 kg/m2, ≥25 to <30 kg/m2 and ≥30 kg/m2 (LS mean differences of −1.01, −0.89, and −1.50, respectively; all P < .0001); and those with baseline HbA1c <8.0% or ≥8.0% (LS mean differences of −1.10 and −1.05, respectively; both P < .0001). Addition of tofogliflozin was also significantly superior to placebo in decreasing HbA1c in patients receiving basal‐bolus insulin (LS mean difference of −1.35; P < .0001), bolus insulin (LS mean difference of −1.29; P = .0002), premix (LS mean difference of −1.00, respectively; P = .0005) or basal insulin (LS mean difference of −1.06; P < .0001), and in patients with eGFR ≥90 mL/min/1.73 m2 (LS mean difference of −1.25; P < .0001), ≥60 to <90 mL/min/1.73 m2 (LS mean difference of −0.93; P < .0001) and ≥30 to <60 mL/min/1.73 m2 (LS mean difference of −1.39; P = .0014).
3.2.2. Secondary endpoints
Small percentages of patients in each group achieved HbA1c goals at the end of 16 weeks. For tofogliflozin, 17 patients (12.1%) achieved HbA1c <7.0% and 6 (4.3%) achieved HbA1c ≤6.5%. The respective values for placebo were 1 (1.4%; P = .0075) and 0 (0%; P = .0091).
Tofogliflozin was also significantly superior to placebo for most other secondary endpoints, including body weight (LS mean difference −1.40 kg; P < .0001), FPG (LS mean difference −32.1 mg/dL; P < .0001), PPG (LS mean difference −67.7 mg/dL; P < .0001), diastolic BP (LS mean difference −2.9 mm Hg; P = .0218) and uric acid (LS mean difference −0.29 mg/dL; P = .0062).
3.3. Safety
3.3.1. AEs
Most patients in both treatment groups experienced ≥1 TEAE (Table 3). There were no deaths in either treatment group and 1 patient in each group had an SAE considered to be possibly related to study treatment. The most common TEAEs were hypoglycaemia (43 patients [30.7%] for tofogliflozin and 15 patients [21.4%] for placebo) and nasopharyngitis (13 patients [9.3%] for tofogliflozin and 9 patients [12.9%] for placebo). Two patients treated with tofogliflozin had genital infections (one of which led to discontinuation) and one patient who received placebo had a urinary tract infection (Table 3).
Table 3.
Safety results
| Tofogliflozin (N = 140) n (%) | Placebo (N = 70) n (%) | |
|---|---|---|
| Overall summary | ||
| Any TEAE | 95 (67.9) | 49 (70.0) |
| Any TEAE possibly related to study treatment | 58 (41.4) | 17 (24.3) |
| TEAE leading to death | 0 | 0 |
| Any serious TEAE | 1 (0.7) | 4 (5.7) |
| Any serious TEAE possibly related to study treatment | 1 (0.7) | 1 (1.4) |
| TEAE leading to permanent treatment discontinuation | 3 (2.1) | 0 |
| Individual TEAEs occurring in >3% of patients in either group | ||
| Hypoglycaemia | 43 (30.7) | 15 (21.4) |
| Nasopharyngitis | 13 (9.3) | 9 (12.9) |
| Thirst | 9 (6.4) | 0 |
| Blood ketones increased | 5 (3.6) | 2 (2.9) |
| Urine output increased | 5 (3.6) | 0 |
| Pharyngitis | 3 (2.1) | 3 (4.3) |
| Influenza | 1 (0.7) | 6 (8.6) |
| TEAEs of special interest | ||
| Urinary tract infection | 0 | 1 (1.4) |
| Cystitis | 0 | 1 (1.4) |
| Genital infection | 1 (0.7) | 0 |
| Vaginal infection | 1 (0.7) | 0 |
| Skin and subcutaneous tissue disorders | 8 (5.7) | 2 (2.9) |
| Asteatotic eczema | 3 (2.1) | 0 |
| Rash | 2 (1.4) | 0 |
| Cold urticaria | 1 (0.7) | 0 |
| Dyshidrotic eczema | 1 (0.7) | 0 |
| Eczema | 1 (0.7) | 0 |
| Prurigo | 1 (0.7) | 0 |
| Dermatitis contact | 0 | 2 (2.9) |
| Excessive urination | 10 (7.1) | 0 |
| Urine output increased | 5 (3.6) | 0 |
| Pollakiuria | 3 (2.1) | 0 |
| Nocturia | 1 (0.7) | 0 |
| Polyuria | 1 (0.7) | 0 |
| AEs related to volume depletion | 11 (7.9) | 2 (2.9) |
| Thirst | 9 (6.4) | 0 |
| Constipation | 3 (2.1) | 0 |
| Cerebellar infarction | 0 | 1 (1.4) |
| Lacunar infarction | 0 | 1 (1.4) |
| Hypoglycaemia | ||
| Any hypoglycaemia (per protocol) | 44 (31.4) | 15 (21.4) |
| Severe hypoglycaemia | 2 (1.4) | 0 |
| Hypoglycaemic unconsciousness | 1 (0.7) | 0 |
| Documented symptomatic hypoglycaemia | 26 (18.6) | 9 (12.9) |
| Plasma glucose <3.0 mmol/L (54 mg/dL) | 10 (7.1) | 6 (8.6) |
| Asymptomatic hypoglycaemia | 13 (9.3) | 4 (5.7) |
| Plasma glucose <3.0 mmol/L (54 mg/dL) | 3 (2.1) | 1 (1.4) |
| Probable symptomatic hypoglycaemia | 11 (7.9) | 2 (2.9) |
| Nocturnal hypoglycaemia | 12 (8.6) | 3 (4.3) |
| Daytime hypoglycaemia | 41 (29.3) | 14 (20.0) |
3.3.2. Hypoglycaemia
The overall frequencies of any hypoglycaemia (44 patients [31.4%] for tofogliflozin and 15 patients [21.4%] for placebo), documented symptomatic hypoglycaemia (26 patients [18.6%] for tofogliflozin and 9 patients [12.9%] for placebo), asymptomatic hypoglycaemia (13 patients [9.3%] for tofogliflozin and 4 patients [5.7%] for placebo) and both nocturnal (12 patients [8.6%] for tofogliflozin and 3 patients [4.3%] for placebo) and daytime hypoglycaemia (41 patients [29.3%] for tofogliflozin and 14 patients [20.0%] for placebo) were all numerically higher in patients who received tofogliflozin vs placebo (Table 3).
3.3.3. TEAEs of special interest
The overall frequencies of patients experiencing TEAEs of special interest was 62 patients (44.3%) for tofogliflozin and 10 patients (14.3%) for placebo (Table 3). Skin and subcutaneous tissue disorders (8 patients [5.7%] for tofogliflozin and 2 patients [2.9%] for placebo), asteatotic eczema (3 patients [2.1%] for tofogliflozin and 0 patients [0.0%] for placebo), excessive urination (10 patients [7.1%] for tofogliflozin and 0 patients [0.0%] for placebo), increased urine output (5 patients [3.6%] for tofogliflozin and 0 patients [0.0%] for placebo), pollakiuria (3 patients [2.1%] for tofogliflozin and 0 patients [0.0%] for placebo), AEs related to volume depletion (11 patients [7.9%] for tofogliflozin and 2 patients [2.9%] for placebo), thirst (9 patients [6.4%] for tofogliflozin and 0 patients [0.0%] for placebo) and constipation (3 patients [2.1%] for tofogliflozin and 0 patients [0.0%] for placebo) were all numerically higher in patients who received tofogliflozin vs placebo. Urinary tract infection was experienced by 1 patient in the placebo group (vs 0 patients in the tofogliflozin group) and genital infection was experienced by 1 patient in the tofogliflozin group (vs 0 patients in the placebo group).
3.3.4. Clinical laboratory evaluations and vital signs
There were few potentially clinically significant abnormalities in clinical laboratory values for either treatment group and none for post‐baseline vital signs. Evaluation of lipid profiles indicated no significant differences between treatment groups for total cholesterol, LDL cholesterol, HDL cholesterol or triglycerides (Table 2). Compared with placebo, from baseline to week 16 tofogliflozin treatment increased total cholesterol (LS mean difference of 1.80 mg/dL), HDL cholesterol (LS mean difference 2.7 mg/dL), LDL cholesterol (LS mean difference of 1.6 mg/dL) and triglycerides (LS mean difference 10.1 mg/dL).
4. DISCUSSION
This randomized trial showed that administering tofogliflozin for 16 weeks in Japanese patients with T2DM who were receiving insulin therapy alone or basal insulin plus a DPP‐4 inhibitor but had poor glycaemic control, resulted in a significant reduction in HbA1c compared with placebo, along with significant reductions in FPG, PPG, diastolic BP, body weight and uric acid levels. These benefits were observed despite the fact that patients in the tofogliflozin group were older and had a longer mean duration of disease compared with those who received placebo. They were also less likely to be receiving a DPP‐4 inhibitor as part of their treatment regimen. It should also be noted that patients in both treatment groups had elevated LDL cholesterol concentrations at baseline and that those in the tofogliflozin group also had elevated triglyceride levels.14
Subgroup analyses indicated that addition of tofogliflozin was significantly superior to placebo regardless of age, gender, BMI, baseline HbA1c or differences in renal function at baseline. AEs observed with SGLT2 inhibitors,15, 16 including hypoglycaemia, increased urine output, thirst and urinary tract/genital infection events, all occurred more frequently with tofogliflozin than with placebo.
The improved glycaemic control (reflected by decreased HbA1c, FPG and PPG) observed with tofogliflozin over 16 weeks of treatment was similar to that observed in previous studies in which other SGLT2 inhibitors were added to treatment for patients with T2DM not adequately controlled on insulin treatment alone or on insulin plus other oral antidiabetic drugs.15, 17, 18, 19 Addition of tofogliflozin to treatment resulted in significant reductions in HbA1c and achievement of HbA1c <7% or ≤6.5% in some patients. The mean baseline HbA1c for patients treated with tofogliflozin was high (8.53%) and a tofogliflozin dose of 20 mg/d has been shown to lower HbA1c by −1.02% when administered as monotherapy in patients with T2DM not controlled on diet and exercise alone.13 These results would suggest that the addition of tofogliflozin to insulin or insulin plus a DPP‐4 inhibitor in the present study would not result in a large percentage of patients achieving aggressive HbA1c targets of <7%. The similarity between the HbA1c reductions with tofogliflozin monotherapy and when added to insulin is consistent with the independent mechanisms of action for these two antidiabetic treatments.20 The improved glyaemic control observed in the present study is similar to that observed in other studies investigating the addition of an SGLT2 inhibitor to insulin treatment in Japanese patients.21, 22, 23 Canagliflozin, ipragliflozin and dapagliflozin have all demonstrated greater reductions in HbA1c levels from baseline to 16 weeks when compared with placebo.21, 22, 23
It has been shown that SGLT2 inhibitors act in the kidney by increasing urinary excretion of glucose; therefore, patients with renal impairment may experience reduced efficacy in terms of Hb1Ac during treatment with SGLT2 inhibitors, particularly those with moderate to severe renal impairment.24 Dapogliflozin, empagliflozin and ipragliflozin each demonstrated reduced Hb1Ac efficacy in patients with moderate or severe chronic kidney disease.24
Addition of tofogliflozin to insulin treatment also significantly lowered both FPG and PPG compared with addition of placebo. The effects of tofogliflozin on FPG are consistent with those reported for other SGLT2 inhibitors when added to insulin.15, 18, 21, 22, 23 Other SGLT2 inhibitors have also been shown to reduce PPG.25, 26, 27 A study investigating the addition of dapagliflozin to insulin therapy in Japanese patients also demonstrated a significant reduction from baseline in PPG in the treatment group.22 The ability of tofogliflozin to lower PPG is important because PPG has been shown to be an independent risk factor for macrovascular disease in patients with diabetes.28
Consistent with other studies, the addition of tofogliflozin to insulin therapy significantly decreased body weight by −1.34 kg vs a slight (0.03 kg) increase with placebo.21, 22, 23 Weight change may be attributable to a combination of calorie loss in urine and osmotic diureseis. Weight control is an important consideration in the overall management of T2DM and it has been estimated that each −1% reduction in HbA1c achieved with insulin treatment is associated with an ~2 kg increase in body weight over 1 year of therapy.29 Weight gain may worsen insulin resistance,30 resulting in the need for higher insulin doses. Addition of other SGLT2 inhibitors to treatment has also been shown to decrease insulin dose requirements in patients with T2DM.15, 31
Tofogliflozin also significantly reduced diastolic BP by −2.9 mm Hg, as well as decreased systolic BP by −2.2 mm Hg, although this decrease was not statistically significant. Reductions in systolic and diastolic BP were also observed when tofogliflozin was administered as monotherapy to patients with T2DM13 and when other SGLT2 inhibitors were added to insulin therapy.15, 19 The reductions in BP observed with SGLT2 inhibitors are believed to be attributable to a combination of factors, including osmotic diuresis, natriuresis, weight loss and possible effects of improved endothelial nitric oxide release as a result of better glycaemic control.32 A recent meta‐analysis of clinical trial results for SGLT2 inhibitors has indicated that these agents significantly reduced the risks of all‐cause mortality, cardiovascular death, myocardial infarction and heart failure.33 Results from the EMPA‐REG OUTCOME study indicated that patients with T2DM at high risk of cardiovascular events who received empagliflozin had a lower rate of the primary composite cardiovascular outcome and of death from any cause compared with placebo when this SGLT2 inhibitor was added to standard care.34 It was suggested that multiple mechanisms may have contributed to this benefit of SGLT2 inhibition, including reduced arterial stiffness, improved cardiac function and lower myocardial oxygen demand, improvement in renal function, and lowering of uric acid, as well as improved glycaemic control, decreased adiposity and lower blood pressure.34
The addition of tofogliflozin to insulin therapy had no significant effects on the plasma lipid profile, which is consistent with long‐term safety data.13 In a 52‐week safety study investigating tofogliflozin 20 mg as an add‐on therapy to various antidiabetic drugs, triglycerides and LDL cholesterol were not significantly changed compared with baseline. Canagliflozin has been reported to increase LDL cholesterol in a dose‐dependent manner, so it may be prudent to closely monitor lipid profiles in clinical practice.13 Tofogliflozin significantly decreased uric acid levels. Elevated uric acid levels have been shown to be a significant risk factor for cardiovascular and renal disease, stroke and all‐cause mortality.35, 36
Hypoglycaemia was the most frequently reported TEAE in both treatment groups. In the present study, the frequency of hypoglycaemia associated with tofogliflozin was higher (~30.0%) than that when tofogliflozin was administered as monotherapy (0% for a 20‐mg dose).13 The increased risk of hypoglycaemia when tofogliflozin was administered with insulin is consistent with results for other SGLT2 inhibitors15, 18, 19, 37, 38, 39, 40 and with results for other SLGT2 inhibitors in Japanese patients.21, 22, 23 It is important to note that the insulin dose in the present study could only be changed if FPG was ≥240 mg/dL or patients experienced hypoglycaemia. It is reasonable to suggest that the frequency of hypoglycaemia should decline when patients are permitted to adjust insulin dosing after the addition of tofogliflozin. This suggestion is consistent with results from a previous study, which showed that addition of tofogliflozin to insulin therapy in patients with poor glycaemic control significantly decreased insulin therapy, permitting a reduction in the daily insulin dose.41 Safety results indicated that <2% of patients treated with tofogliflozin had genital or urinary tract infections and that 7.1% had excessive urination. Both of these AEs have been associated with administration of SGLT2 inhibitors in previous studies.8, 16, 41 There were no reports of volume depletion with tofogliflozin in the present study or when it was administered as monotherapy.13 Patients treated with tofogliflozin reported more AEs related to volume depletion than placebo (7.9% vs 2.9%); however, none led to study discontinuation. In previous studies investigating SGLT2 inhibitors, AEs related to volume depletion have been reported as being higher in the study group compared with the placebo or control group.8, 16, 41 Patients may be required to maintain adequate fluid intake in these instances.8 Previously, it has been speculated this difference may be attributable to patients receiving diuretics.8
It is important to note that the findings of the present study are limited by the short 16‐week duration. This period is not sufficient for assessment of the long‐term safety of tofogliflozin. The 36‐week extension phase will provide safety data to fill this important gap out to 52 weeks of treatment. There were differences between groups for age, duration of disease, neuropathy and whether or not a DPP‐4 inhibitor was part of the treatment regimen. Assessment of patients aged ≥65 years and <65 years as part of the subgroup analysis indicated that age did not have an impact on efficacy in terms of HbA1c reduction.
In conclusion, the results from this 16‐week double‐blind period indicate that addition of tofogliflozin is an effective treatment option for patients with T2DM whose blood glucose is poorly controlled with insulin therapy or with basal insulin combined with a DPP‐4 inhibitor. Overall, tofogliflozin demonstrated an acceptable safety profile; an increased risk of hypoglycaemia was observed, but insulin reduction was not permitted during the present study.
Supporting information
File S1.
ACKNOWLEDGEMENTS
The study was funded by Sanofi K.K. and Kowa Company, Ltd. The authors thank all physicians from the 30 institutions that participated in this study. Medical writing assistance was provided by Bob Rhoades, PhD and Jackie Marchington PhD, CMPP of Caudex, and publication support by Yuiko Kondo, MDS‐CMG Inc.; all their activities were funded by Sanofi K.K. and Kowa Company, Ltd.
Conflict of interest
Y. T. has received honoraria for speakers bureau from Astellas Pharma Inc., AstraZeneca K.K., Bayer Yakuhin, Ltd., Daiichi Sankyo Company Ltd, Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly Japan K.K., Kowa Pharmaceutical Company Ltd., Merck Sharp & Dohme K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Sanofi K.K., Shionogi & Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Company Ltd; and grants from Astellas Pharma Inc., AstraZeneca K.K., Bayer Yakuhin, Ltd., Daiichi Sankyo Company Limited, Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly Japan K.K., Kowa Pharmaceutical Company Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sanwa Kagaku Kenkyusho Co., Ltd., Sanofi K.K., Shionogi & Co., Ltd, Taisho Toyama Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Company Ltd.
M. T. and M. S. are employees of Sanofi K.K. R. G. is an employee of Kowa Company, Ltd.
K. K. is an advisor to, received honoraria for lectures from, and received scholarship grants from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Sumitomo Dainippon Pharma, Fujifilm Pharma, Kissei Pharmaceutical, Kowa, MSD, Novartis Pharma, Ono Pharmaceutical, Sanofi K.K., Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Taisho Toyama Pharmaceutical and Daiichi Sankyo.
Author contributions
Sanofi K.K. and Kowa Company, Ltd. were the sponsors of the study, and were responsible for the design and coordination of the study, monitoring clinical sites, collecting and managing data, and performing all statistical analyses. Y. T. and K. K. contributed to conception and design of the study, and participated in reviewing and editing the manuscript. M. T., M. S. and R. G. contributed to design of the study protocol, operated the study and reviewed the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Terauchi Y, Tamura M, Senda M, Gunji R and Kaku K. Efficacy and safety of tofogliflozin in Japanese patients with type 2 diabetes mellitus with inadequate glycaemic control on insulin therapy (J‐STEP/INS): results of a 16‐week randomized, double‐blind, placebo‐controlled multicentre trial. Diabetes Obes Metab. 2017;19:1397–1407. https://doi.org/10.1111/dom.12957
Funding Information Sanofi K.K. and Kowa Company, Ltd. were the sponsors of the study, and were responsible for the design and coordination of the study, monitoring clinical sites, collecting and managing data, and performing all statistical analyses.
REFERENCES
- 1. Halban PA, Polonsky KS, Bowden DW, et al. β‐cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37:1751‐1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sorli C, Heile MK. Identifying and meeting the challenges of insulin therapy in type 2 diabetes. J Multidiscip Healthc. 2014;7:267‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Massi‐Benedetti M, Orsini‐Federici M. Treatment of type 2 diabetes with combined therapy: what are the pros and cons? Diabetes Care. 2008;31(suppl 2):S131‐S135. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Approaches to glycemic treatment. Diabetes Care. 2016;39(suppl 1):S52‐S59. [DOI] [PubMed] [Google Scholar]
- 5. Eskesen S, Kelsberg G, Hitchcock K, Lo V. Clinical inquiries. What is the role of combination therapy (insulin plus oral medication) in type 2 diabetes? J Fam Pract. 2006;55:1001‐1003. [PubMed] [Google Scholar]
- 6. Aschner P, Sethi B, Gomez‐Peralta F, et al. Insulin glargine compared with premixed insulin for management of insulin‐naïve type 2 diabetes patients uncontrolled on oral antidiabetic drugs: the open‐label, randomized GALAPAGOS study. J Diabetes Complications. 2015;29:838‐845. [DOI] [PubMed] [Google Scholar]
- 7. Sato S, Saisho Y, Kou K, et al. Efficacy and safety of sitagliptin added to insulin in Japanese patients with type 2 diabetes: the EDIT randomized trial. PLoS One. 2015;10:e0121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jung CH, Jang JE, Park JY. A novel therapeutic agent for type 2 diabetes mellitus: SGLT2 inhibitor. Diabetes Metab J. 2014;38:261‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mudaliar S, Polidori D, Zambowicz B, et al. Sodium–glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care. 2015;38:2344‐2353. [DOI] [PubMed] [Google Scholar]
- 11. Kilov G, Leow S, Thomas M. SGLT2 inhibition with dapagliflozin: a novel approach for the management of type 2 diabetes. Aust Fam Physician. 2013;42:706‐710. [PubMed] [Google Scholar]
- 12. Poole RM, Prossler JE. Tofogliflozin: first global approval. Drugs. 2014;74:939‐944. [DOI] [PubMed] [Google Scholar]
- 13. Tanizawa Y, Kaku K, Araki E, et al. Long‐term safety and efficacy of tofogliflozin, a selective inhibitor of sodium‐glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open‐label, randomized controlled trials. Expert Opin Pharmacother. 2014;15:749‐766. [DOI] [PubMed] [Google Scholar]
- 14. Ikeda S, Takano Y, Cynshi O, et al. A novel and selective sodium‐glucose cotransporter‐2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:984‐993. [DOI] [PubMed] [Google Scholar]
- 15. Rosenstock J, Jelaska A, Zeller C, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78‐week randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2015;17:936‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fioretto P, Giaccari A, Sesti G. Efficacy and safety of dapagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in diabetes mellitus. Cardiovasc Diabetol. 2015;14:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pieber TR, Famulla S, Eilbracht J, et al. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4‐week, randomized, placebo‐controlled trial (EASE‐1). Diabetes Obes Metab. 2015;17:928‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S, Dapagliflozin 006 Study Group . Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124‐136. [DOI] [PubMed] [Google Scholar]
- 19. Devineni D, Morrow L, Hompesch M, et al. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14:539‐545. [DOI] [PubMed] [Google Scholar]
- 20. Cefalu WT, Riddle MC. SGLT2 inhibitors: the latest “new kids on the block”!. Diabetes Care. 2015;38:352‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishihara H, Yamaguchi S, Nakao I, et al. Efficacy and safety of ipragliflozin as add‐on therapy to insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a multi‐centre, randomized, placebo‐controlled, double‐blind study. Diabetes Obes Metab. 2016;18:1207‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Araki E, Onishi Y, Asano M, et al. Efficacy and safety of dapagliflozin in addition to insulin therapy in Japanese patients with type 2 diabetes: results of the interim analysis of 16‐week double‐blind treatment period. J Diabetes Invest. 2016;7:555‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inagaki N, Harashima S, Maruyama N, et al. Efficacy and safety of canagliflozin in combination with insulin: a double blind, randomized, placebo controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheen A. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet. 2015;54:691‐708. [DOI] [PubMed] [Google Scholar]
- 25. Wilding JP, Norwood P, T'joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin‐independent treatment. Diabetes Care. 2009;32:1656‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52‐week randomized trial. Diabetes Care. 2013;36:2508‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo‐controlled study. Diabetes Care. 2013;36:2154‐2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. International Diabetes Federation . Guideline for management of postmeal glucose. 2007. https://www.idf.org/webdata/docs/Guideline_PMG_final.pdf. Accessed December 20, 2016.
- 29. Yki‐Järvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care. 2001;24:758‐767. [DOI] [PubMed] [Google Scholar]
- 30. Al‐Goblan AS, Al‐Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neal B, Perkovic V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium‐glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403‐411. [DOI] [PubMed] [Google Scholar]
- 32. John M, Gopinath D, Jagesh R. Sodium‐glucose cotransporter 2 inhibitors with insulin in type 2 diabetes: clinical perspectives. Indian J Endocrinol Metab. 2016;20:22‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Savarese G, D'Amore C, Federici M, et al. Effects of dipeptidyl peptidase 4 inhibitors and sodium‐glucose linked cotransporter‐2 inhibitors on cardiovascular events in patients with type 2 diabetes mellitus: a meta‐analysis. Int J Cardiol. 2016;220:595‐601. [DOI] [PubMed] [Google Scholar]
- 34. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 35. Takae K, Nagata M, Hata J, et al. Serum uric acid as a risk factor for chronic kidney disease in a Japanese community – the Hisayama Study. Circulation J. 2016;80:1857‐1862. [DOI] [PubMed] [Google Scholar]
- 36. Chen JH, Chuang SY, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all‐cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum. 2009;61:225‐232. [DOI] [PubMed] [Google Scholar]
- 37. Wilding JP, Woo V, Soler NG, et al. Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Inter Med. 2012;156:405‐415. [DOI] [PubMed] [Google Scholar]
- 38. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815‐1823. [DOI] [PubMed] [Google Scholar]
- 39. Cefalu WT, Leiter LA, de Bruin TW, Gause‐Nilsson I, Sugg J, Parikh SJ. Dapagliflozin's effects on glycemia and cardiovascular risk factors in high‐risk patients with type 2 diabetes: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study with a 28‐week extension. Diabetes Care. 2015;38:1218‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suzuki K, Mitsuma Y, Sato T, Anraku T, Hatta M. Comparison of combined tofogliflozin and glargine, tofogliflozin added to insulin, and insulin dose‐increase therapy in uncontrolled type 2 diabetes. J Clin Med Res. 2016;8:805‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seufert J. SGLT2 inhibitors – an insulin‐independent therapeutic approach for treatment of type 2 diabetes: focus on canagliflozin. Diabetes Metab Syndr Obes. 2015;8:543‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1.


