Abstract
While neurostimulation—stimulation of the nervous system using electrical current—has been used to treat chronic pain, its use treating postsurgical pain has been limited. Here, we report on the clinical application of a novel investigational lead to provide analgesia following total knee arthroplasty. In 5 subjects, leads were inserted percutaneously using ultrasound guidance within 0.5 to 3.0 cm of the femoral and/or sciatic nerve(s). With the delivery of current, pain decreased an average of 63% at rest, with 4 of 5 subjects having relief of > 50%. During passive and active knee flexion, pain decreased an average of 14% and 50%, with 0/3 and 1/2 subjects attaining > 50% relief, respectively. Ultrasound‐guided percutaneous peripheral nerve stimulation may be a practical modality for the treatment of postsurgical pain.
Keywords: neuromodulation, percutaneous peripheral nerve stimulation, peripheral nerve stimulator, helical lead, small‐diameter open‐coiled helical lead, postoperative pain
Introduction
In the United States, approximately 700,000 total knee arthroplasty (TKA) procedures were performed in 2010, and this number is expected to grow to 3.5 million procedures per year by 2030.1 Approximately half (30% to 90%) of all TKA patients experience moderate to severe pain during the first month after TKA surgery,2, 3, 4, 5, 6 and over half remain on opiates during this time frame.6 Additionally, up to 35% of patients experience moderate to severe chronic pain of greater than 2 months following surgery.5, 7, 8, 9, 10, 11, 12, 13, 14 Therefore, there is a clear need to improve analgesia in both the short and longer terms following knee arthroplasty. Peripheral nerve stimulation (PNS) may offer just such a modality. While there are multiple theories describing the mechanism of action of PNS for the treatment of pain,15 Melzack and Wall's “gate control theory” is the most common.16 Per this theory, large‐diameter myelinated afferent peripheral nerve fibers are triggered with the use of electrical current and in turn impede communication of pain signals to the central nervous system from small‐diameter pain fibers at the level of the spinal cord (the “gate”).16, 17
While neurostimulation has been used to treat chronic pain of multiple etiologies for many decades with spinal cord and peripheral nerve stimulators,18 its use in treating early postsurgical pain has been limited for a variety of reasons. Introduction of conventional electric stimulation leads within the peripheral nervous system has historically required invasive surgery to expose the nerve for both insertion and removal.19 These procedures require a physician with neurosurgical training, are time consuming, and carry a risk of nerve damage stemming from encasement within a fibrous capsule that can adhere to the nerve.20 There have been attempts to mitigate these limitations using transcutaneous electrical nerve stimulation, but they have been largely unsuccessful,21 often because the stimulation intensities required by skin surface electrodes to activate pain‐relieving fibers located deep below the skin surface can activate cutaneous nerve endings, causing discomfort and/or pain.22, 23, 24
To bypass cutaneous nerve endings while avoiding surgical implantation, very small leads were developed that allowed “blind,” or surface landmark‐guided, percutaneous insertion directly into a muscle to stimulate terminal fibers of the peripheral nerve innervating the muscle.25 Subsequently, the use of ultrasound to percutaneously insert leads adjacent to peripheral nerves was described in cadavers.26, 27 Soon after, this technique was reported in patients for chronic pain states.28 However, these reports of ultrasound‐guided percutaneous PNS involved an off‐label use of a cylindrically shaped lead designed for insertion in the epidural space of the spinal cord and was limited exclusively to smaller sensory nerves.28 Nonetheless, these early studies were encouraging and demonstrated the need for a stimulation system and methods to enable easier insertion, shorter procedure duration, and a reliably high potential for efficacy with low risk of nerve damage, which may enhance the application of this stimulation modality for postsurgical pain.
A percutaneous PNS system and approach are under clinical investigation that is designed to address these needs and allow rapid deployment to large, proximal, mixed peripheral nerves (ie, possessing motor and sensory fibers) usually targeted for postsurgical regional anesthesia.29 While the ultrasound‐guided deployment of this system has been reported for the treatment of chronic pain conditions,30, 31, 32 the feasibility of this technique must still be determined for the treatment of postsurgical pain. The present report describes a proof‐of‐concept study of this novel system and approach to provide immediate neurostimulation‐induced analgesia within the femoral and sciatic nerve distributions following TKA.
Methods
This proof‐of‐concept study followed Good Clinical Practice and was conducted within the ethical guidelines outlined in the Declaration of Helsinki. Institutional review board (IRB) approval (MetroHealth Medical Center IRB, MetroHealth Medical Center, Cleveland, OH, U.S.A.; Western IRB, puyallup, WA, U.S.A., for the Center for Clinical Research, Winston‐Salem, NC, U.S.A.) and investigational device exemption were obtained, as needed. All subjects provided written, informed consent, and all devices used in the study were used in an investigational manner.
Subjects included adults (≥ 21 years of age) following primary, unilateral, TKA with surgically related knee pain inadequately controlled with oral analgesics (≥ 3 on an 11‐point numerical rating scale (NRS) of the Brief Pain Inventory Short Form, Question 3: “Pain at its worst in the last 24 hours”). Key exclusion criteria included an infection of the affected limb or other factors that increase the risk of infection, confounding pain conditions unrelated to the clinical indication for the knee arthroplasty (eg, fibromyalgia), nerve damage to the affected limb, the presence of implanted deep brain or cardiac stimulators, and an increased risk of bleeding (eg, bleeding disorder).
A femoral and/or sciatic nerve lead was inserted, depending on where the majority of pain originated: Subjects with anterior knee pain received a femoral placement, and subjects with posterior knee pain received a sciatic lead. Subjects were positioned either supine or in the lateral decubitus position for femoral and sciatic insertions, respectively. Subjects had their ipsilateral limb prepared with chlorhexidine gluconate/isopropyl alcohol solution and sterile drapes at the level of the inguinal crease or over the posterior upper leg for femoral and sciatic insertions, respectively.
Lead Placement Technique
A portable ultrasound (M‐Turbo; SonoSite, Bothell, WA, U.S.A.; or Logiq e or Venue 40, GE, Fairfield, CT, U.S.A.), linear array transducer (HFL38x, SonoSite; or 12L‐RS, GE), or curved array transducer (C60x; Sonosite) within a sterile transducer sleeve were utilized for lead insertion. Both nerves were imaged in a transverse cross‐sectional (short axis) view, at the inguinal crease for femoral leads (n = 4), and between the ischial tuberosity and greater trochanter (n = 1) or on the posterior aspect of the thigh proximal to the popliteal fossa (n = 1) for sciatic leads. A local anesthetic skin wheal was raised lateral to the ultrasound transducer. A 7.5‐cm, 25‐gauge or 12.5‐cm, 24‐gauge monopolar needle electrode (SPR Therapeutics, Cleveland, OH, U.S.A.) was used to rapidly identify optimal lead locations and deliver test stimulation before lead insertion. The tip of the monopolar needle electrode was inserted through the skin wheal and within the plane of the ultrasound transducer (in‐plane technique) and positioned approximately 0.5 to 1.0 cm from the femoral nerve (Figure 1) or approximately 1.0 to 3.0 cm from the sciatic nerve. An electrical stimulator was attached to the monopolar needle electrode and delivered test stimulation (100 Hz, 15 to 200 μicroseconds, 0.2 to 20 mA) to verify that a comfortable sensation (eg, paresthesia) within the region(s) of pain could be induced without evoking muscle contractions or discomfort. If too superficial an electrode placement was suggested with uncomfortable local subcutaneous sensations, the needle electrode was advanced until the undesired sensations resolved. The needle electrode was withdrawn if muscle contractions and/or discomfort distal to the site of stimulation were induced, until the contractions and/or discomfort ceased.
Figure 1.
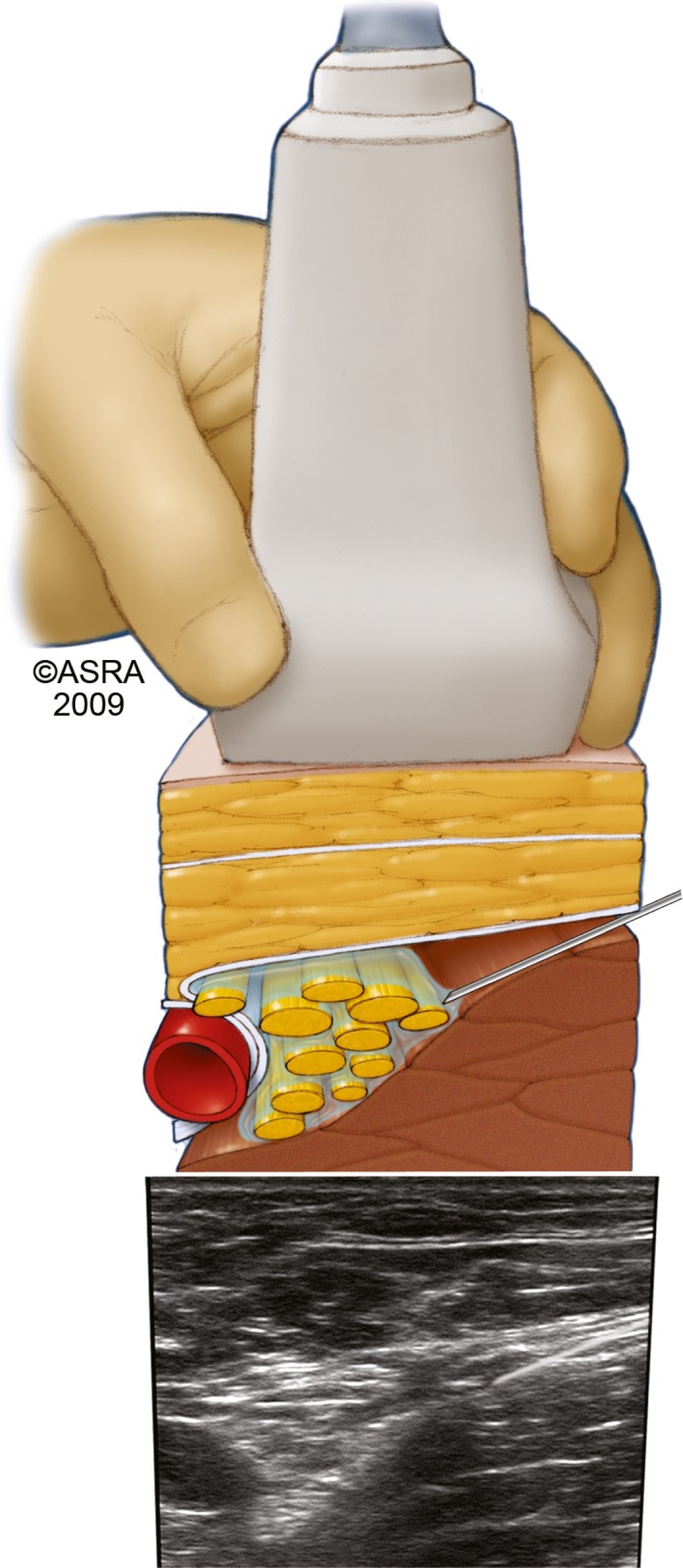
A needle inserted within the ultrasound plane anterolateral to the femoral nerve viewed in short axis.
The monopolar needle electrode was subsequently withdrawn and replaced with a 12.5‐cm, 20‐gauge needle using the same skin entry point and in‐plane ultrasound approach (Figure 2). The final needle tip location was placed in the same location as the optimal position of the monopolar electrical needle tip. A preloaded, monopolar, helically coiled, insulated lead (MicroLead™; SPR Therapeutics) was deployed by withdrawing the needle over the lead. The lead was subsequently attached to an external stimulator (SPR Therapeutics), and a surface return electrode was placed on the ipsilateral limb or abdomen. Accurate lead placement was confirmed with subject reports of comfortable sensations over the region(s) of pain without eliciting muscle contractions.
Figure 2.
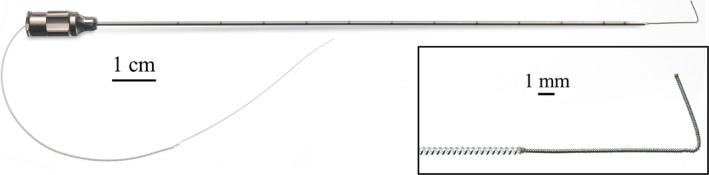
A small‐diameter (0.2‐mm), open‐coiled, helical electrical lead with an anchoring wire preloaded within the 12.5‐cm, 20‐gauge insertion needle (MicroLead™, SPR Therapeutics, Cleveland, OH, U.S.A.). Inset: A small‐diameter (0.2‐mm), open‐coiled, helical electrical lead with an anchoring wire (MicroLead™).
At the point of exit, the lead was formed into a loop and affixed to the skin with a sterile occlusive dressing. For the remainder of clinical evaluation, the stimulator was used to deliver stimulation. To limit the placebo effect, the screen of the stimulator was hidden from the subjects’ view and, as a result, they could not see the stimulation parameters or if stimulation was on or off. At the end of testing, stimulation was turned off, the occlusive dressing was removed, and the lead was removed using gentle traction. A small sterile bandage was applied at the lead exit site.
Endpoints
Baseline pain and range‐of‐motion (ROM) outcome measures were administered immediately prior to lead insertion (ie, stimulation off) to minimize the confounding effect of the procedure. Outcomes with stimulation on were assessed after the lead placement procedure. Pain outcomes at rest and during passive and active ROM were assessed with a 0 to 10 NRS, where 0 and 10 represent “no pain” and “the worst imaginable pain,” respectively. For the resting state assessment, participants were positioned supine with the knee positioned at the most comfortable angle. For the assessment of pain during passive ROM, participants were positioned supine and the knee was passively ranged from maximal tolerable extension to maximal tolerable flexion. The same approach was used for active ROM, except the participant actively ranged the knee. Maximal tolerable passive and active knee ROM was measured with a standard goniometer.
Results
Five subjects were enrolled (Table 1). At the time of enrollment, 3 subjects (Site I, subjects A to C) were receiving care at a skilled nursing facility or acute inpatient rehabilitation unit (6 to 9 days following total knee replacement), while 2 subjects (Site II, subjects D and E) had prolonged postsurgical pain (> 90 days following total knee replacement). Leads were inserted in all subjects, and electrical current produced comfortable sensations in the distributions of the targeted nerves without evoking motor responses in muscles innervated by the femoral or sciatic nerves.
Table 1.
Subject Characteristics
| Site | Subject | Days Since Surgery | Age (years) | Sex | BMI (kg/m2) | Leg |
|---|---|---|---|---|---|---|
| I | A | 8 | 60 | Male | 26 | Right |
| I | B | 9 | 62 | Female | 29 | Right |
| I | C | 6 | 56 | Female | 36 | Right |
| II | D | 92 | 48 | Male | 30 | Left |
| II | E | 97 | 39 | Female | 39 | Right |
BMI, body mass index.
Outcomes were assessed within approximately 2 hours after optimal lead location, and stimulation parameters were determined. Percutaneous PNS decreased pain on an average of 63% (mean NRS decreasing from 5.8 to 2.5) at rest immediately after lead placement and stimulation initiation, with 4 of 5 subjects having a > 50% improvement (Table 2). Also, the proportion of subjects with pain at rest < 4 (ie, mild pain) increased with stimulation turned on (4 of 5; 80%) compared to with stimulation turned off (1 of 5; 20%).33 Pain during passive knee ROM with stimulation turned on was reduced on an average of 14% (n = 3) compared to with stimulation turned off (Table 3). In addition, pain during active knee ROM with stimulation turned on was reduced on an average of 50% (n = 2), including one subject with mild pain with stimulation turned off (NRS score = 3) who experienced 67% pain relief with stimulation turned on (NRS score = 1). Although neither maximum passive nor active knee range of motion was consistently affected (Table 4), stimulation enabled passive ROM to be performed with less pain and/or greater knee flexion and enabled active ROM to be assessed with approximately the same degree of knee flexion with less pain compared to with stimulation turned off. All leads were removed without difficulty.
Table 2.
Resting Pain Without and then With Percutaneous Peripheral Nerve Stimulation
| Subject | At Rest | ||
|---|---|---|---|
| Stimulation Off | Stimulation On | % Change | |
| A | 9 | 3 | 67 |
| B | 7 | 6 | 14 |
| C | 2 | 0.5 | 75 |
| D | 7 | 3 | 57 |
| E | 4 | 0 | 100 |
| Mean | 5.8 | 2.5 | 63 |
Pain evaluated using a numeric rating scale (scale of 0 to 10).
Table 3.
Dynamic PAIN Without and then With Percutaneous Peripheral Nerve Stimulation
| Subject | During Passive Range of Motion | During Active Range of Motion | ||||
|---|---|---|---|---|---|---|
| Stimulation Off | Stimulation On | % Change | Stimulation Off | Stimulation On | % Change | |
| A | 7 | 4 | 43 | NC | NC | NC |
| C | 4 | 5 | −25 | 3 | 1 | 67 |
| D | 8 | 6 | 25 | 6 | 4 | 33 |
| Mean | 6.3 | 5.0 | 14 | 4.5 | 2.5 | 50 |
Pain evaluated using a numeric rating scale (range 0 to 10). Subjects B and E did not complete range‐of‐motion testing.
NC, not collected.
Table 4.
Knee Range of Motion Without and then With Percutaneous Peripheral Nerve Stimulation
| Subject | Passive Range of Motion | Active Range of Motion | ||||
|---|---|---|---|---|---|---|
| Stimulation Off | Stimulation On | Change | Stimulation Off | Stimulation On | Change | |
| A | 75 | 90 | 15 | NC | NC | NC |
| C | 48 | 58 | 10 | 44 | 45 | 1 |
| D | 115 | 114 | −1 | 112 | 113 | 1 |
| Mean | 79 | 87 | 8 | 78 | 79 | 1 |
Data are presented in degrees. Subjects B and E did not complete range‐of‐motion testing.
NC, not collected.
Discussion
This proof‐of‐concept case series provides evidence that an open, helically coiled PNS lead permits ultrasound‐guided percutaneous insertion without a surgical incision for the treatment of postsurgical pain. The lead design allows insertion 0.5 to 3.0 cm remote from large, mixed sensory/motor nerves while still inducing postsurgical analgesia with the application of electrical current. Clinical access to a system and technique that allows placement of this investigational lead under ultrasound guidance could provide a new analgesic modality for treating postsurgical pain because it takes advantage of the prevalence of physicians trained in ultrasound‐guided regional anesthesia and the pervasiveness of ultrasound machine availability.
In many regards, neurostimulation has many advantages compared to current postsurgical analgesic modalities. The most common postsurgical analgesics—opioids—commonly induce nausea, vomiting, constipation, pruritus, sedation, and respiratory depression. Epidural local anesthetic infusions provide potent analgesia, but cannot be used for the upper extremity, require hospitalization, have a relatively short duration when used for acute pain, and are associated with their own set of undesirable side effects such as urinary retention, motor weakness, hypotension, and risk of epidural hematoma when used in conjunction with many anticoagulants. Although continuous peripheral nerve blocks provide potent site‐specific analgesia,29 when applied to the lower extremities they may induce motor, sensory, and proprioception deficits that could possibly increase the risk of falling up to 4 to 5 times over baseline.34 Furthermore, although they may be used outside of the hospital, their duration is usually limited to 3 to 4 days because of both the risk of infection and local anesthetic reservoir exhaustion.35 In addition, their dislodgement rate, fluid leakage, and the burden on patients carrying a portable infusion pump and half liter of local anesthetic—along with the previously listed limitations—have led some leaders in regional anesthesia to conclude that this technique is often “effective, but unrealistic”36, and calls within the surgical literature to abandon continuous peripheral nerve blocks have resulted.37, 38
In contrast, using the novel stimulation system and ultrasound‐guided techniques to provide percutaneous PNS has the potential to deliver postsurgical analgesia free of the major limitations of opioid analgesics, epidural infusions, and continuous peripheral nerve blocks. Combined with an external stimulator small and light enough to be worn on the body (Figure 3), percutaneous PNS is free from the bulk and weight of infusion pumps and anesthetic reservoirs, and may be utilized in the ambulatory setting (Figure 4).31 Recently, a percutaneous PNS system (including the lead and external stimulator used in the present study) received U.S. Food and Drug Administration 510(k) clearance for up to 30 days in the back and/or extremities for the symptomatic relief of chronic, intractable pain and acute pain, including postsurgical and post‐traumatic pain. If future research confirms delivery of adequate analgesia with an acceptably low adverse event profile, this modality could hypothetically transform postsurgical analgesia—and, specifically, ultrasound‐guided regional analgesia—as it has been practiced using opioids, local anesthetics, and medication adjuvants for over 100 years.39
Figure 3.

A stimulator small enough to be simply adhered to the skin during use (SPR Therapeutics, Cleveland, OH, U.S.A.).
Figure 4.
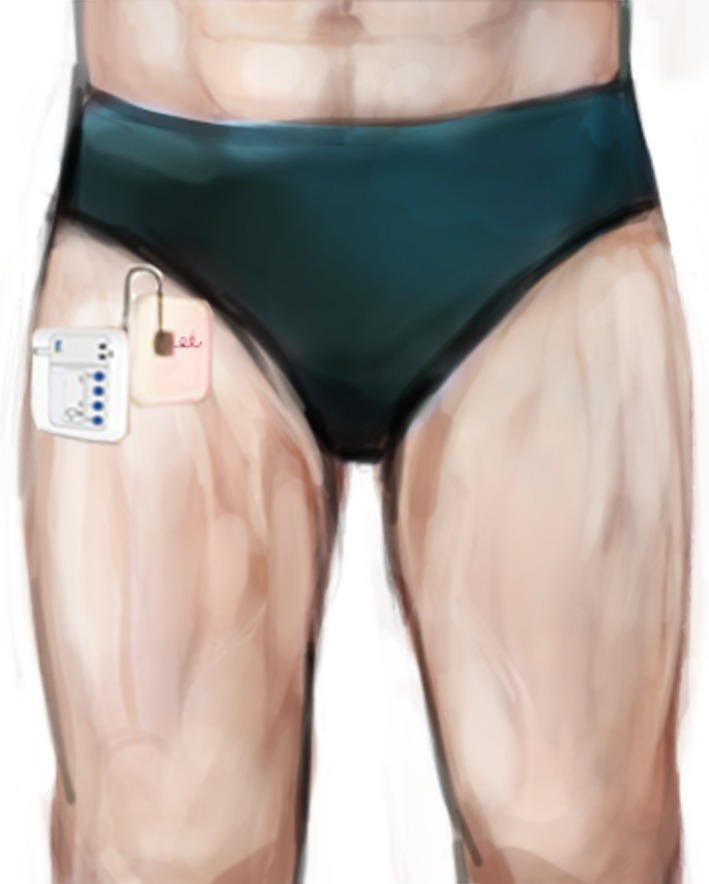
Setup for percutaneous peripheral nerve stimulation of the femoral nerve (SPR Therapeutics, Cleveland, OH, U.S.A.; used with permission from Brian M. Ilfeld, MD, MS).
The envisioned therapy is intended to provide continuous stimulation to relieve perioperative and acute postsurgical pain. The ability to provide continuous (24 hours/day) and comfortable percutaneous PNS has been demonstrated previously for the treatment of chronic pain,30, 31 and additional research is underway to determine the feasibility of continuous stimulation in patients following surgery. Also, in the present proof‐of‐concept study, stimulation produced immediate pain relief in subjects with early (6 to 9 days following TKA) or prolonged (> 90 days following TKA) postsurgical pain. This suggests that percutaneous PNS has the potential to provide pain relief following hospital discharge and to be used as needed to foster nonopiate analgesia while patients recover at home. Effective nonopiate postoperative pain management may play a role in reducing length of stay as a function of reduced opiate‐related side effects and may even increase the proportion of patients discharged directly to home rather than to an in‐patient rehabilitation center or a skilled nursing facility. Further, percutaneous PNS may be used prior to surgery to reduce preoperative pain, which is associated with greater postoperative pain.40, 41, 42 Additional studies are underway to evaluate further the effectiveness of percutaneous PNS in the management of acute, postacute, and chronic pain.30, 31
Compared to existing methods of PNS, the present technique for ultrasound‐guided percutaneous PNS is designed to provide advantages that may make it suitable for the treatment of postsurgical pain. Conventional PNS typically requires surgically exposing the nerve to place leads directly on or adjacent (< 2 mm) to the nerve, as well as to adjust, replace, or remove the leads. Such an invasive surgical procedure to treat short‐term pain would likely be considered by most to be inappropriate. Previous techniques for ultrasound‐guided percutaneous PNS also required a surgical incision to deploy the lead, and smaller sensory nerve branches had to be targeted to generate suitable pain relief (often requiring multiple leads) to avoid the generation of muscle contractions that limited the therapeutic window over which PNS could be delivered.28 In contrast, the present technique requires no surgical incision and may target large, mixed (ie, motor and sensory) nerves as well as small sensory nerves. Stimulating the femoral and sciatic nerve trunks rather than their distal branches allows fewer leads to cover the areas of pain following TKA and enables the leads to be located farther away from the surgical field (and thus, less likely to interfere with the surgical procedure and theoretically reducing the risk of infection).
Another advantage over existing methods of PNS is the use of the coiled electrical lead, enabling stimulation to be delivered at a greater distance from the nerve. The electrical leads used in this study are designed to deliver monopolar stimulation (ie, return electrode located distant from the lead) rather than bipolar stimulation (ie, return electrode located nearby, often on the same lead), allowing the leads to activate the target nerve fibers even when inserted 0.5 to 3.0 cm away (commonly ≤ 2 mm for conventional leads).28 Allowing for a relatively remote and variable distance from the nerve theoretically permits faster insertion with a higher success rate under ultrasound guidance. In addition, the greater distance promotes selective stimulation of the required larger‐diameter myelinated sensory neurons43 without activating motor or smaller‐diameter sensory neurons that induce muscle contraction and discomfort, respectively (Figure 5).
Figure 5.
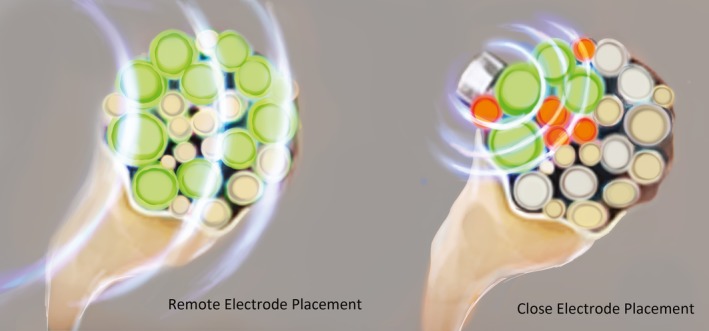
The therapeutic window and the ability to preferentially activate the targeted large nerve fibers across its diameter—without activating nontargeted pain or motor neurons—increase as the distance between the electrode and the nerve increases (used with permission from Brian M. Ilfeld, MD, MS).
In addition, the design of the leads used in this study conveys several theoretical advantages, all of which are intended to increase the applicability of neurostimulation in treating postsurgical pain. The leads were composed of a small‐diameter (0.2‐mm) wire formed into an open helical coil wound from a fluoropolymer‐insulated 7‐strand, type 316L stainless steel wire with a single anchor at the tip (see Figure 5). Its small size allows percutaneous insertion of the lead using a 20‐gauge needle and removal with simple traction. The helical design allows the lead to flex and stretch rather than shift when subjected to force and encourages tissue ingrowth between the coils to secure the lead in place. These features theoretically reduce the incidence of fracture and migration (which can lead to decreased analgesia or activation of cutaneous pain fibers, causing pain), as well as decreasing the risk of infection to 0.03 per 1,000 indwelling days. When used to treat pain and left indwelling for up to 60 days, to date there have been no infections reported in over 330 lead placements.44
Ultrasound‐guided percutaneous PNS has several limitations. The analgesic potency conveyed within the first few days following surgery remains unknown as this study involved subjects who underwent neurostimulation more than 5 days postsurgically. While the present case series utilized a small sample size (n = 5), the study was designed to demonstrate the proof of concept of percutaneous PNS for the treatment of postsurgical pain. Also, while subjects in the present study experienced clinically significant pain relief (≥ 50%) with stimulation turned on compared to stimulation turned off, subject C already had mild pain (< 4/10) with stimulation turned off at rest (see Table 2) as well as during active ROM (see Table 3).45, 46, 47 Although neurostimulation has been reported previously involving most peripheral nerves, studies investigating percutaneously inserted monopolar leads of various anatomic locations are underway to evaluate safety (eg, ability to reduce risks of falls relative to existing therapies), efficacy, and the potential placebo effect.
Additional research will further elucidate the relative benefits and risks of ultrasound‐guided percutaneous PNS in treating postsurgical pain. Nonetheless, this proof‐of‐concept case series suggests that the possibility exists in providing targeted, nonopioid postsurgical analgesia with minimal side effects. This would be a dramatic leap forward in the treatment of acute pain for the tens of millions of surgical procedures performed annually amenable to this technique.
Financial Support
Research reported in this publication was supported, in part, by the National Institute on Aging of the National Institutes of Health under Award Number R44AG052196. In addition, SPR Therapeutics (Cleveland, Ohio, U.S.A.) provided funding and the peripheral nerve electrical leads and stimulators used in this investigation. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding entities.
Conflict of Interests
SPR Therapeutics (Cleveland, Ohio, U.S.A.) provided funding and the peripheral nerve electrical leads and stimulators used in this investigation. Brian Ilfeld: Dr. Ilfeld's institution has received funding for his research from SPR Therapeutics (for studies other than the current investigation), Baxter Healthcare, Smiths Medical, Summit Medical, Teleflex Medical, Myoscience, and Pacira Pharmaceuticals. In addition, Dr. Ilfeld has also acted as a consultant to Pacira Pharmaceuticals. Stuart Grant: Dr. Grant's institution has received funding for his research from SPR Therapeutics, Cara Therapeutics, and Durrect. Dr. Grant also acts as a consultant to B.Braun Medical. Christopher Gilmore: Dr. Gilmore's institution has received funding for his research from SPR Therapeutics, and Dr. Gilmore has acted as a consultant for SPR Therapeutics. John Chae: Dr. Chae is a consultant and Chief Medical Advisor to SPR Therapeutics. He has received research grants from and owns equity in SPR Therapeutics. Richard Wilson: Dr. Wilson's institution has received funding for his research from SPR Therapeutics, and Dr. Wilson has acted as a consultant for SPR Therapeutics. Amorn Wongsarnpigoon: Dr. Wongsarnpigoon is an employee of SPR Therapeutics. Joseph W. Boggs: Dr. Boggs is an employee of SPR Therapeutics and owns equity in the company.
Acknowledgements
The authors appreciate the invaluable assistance of Jihad Jaffer, MD, Michael Bassett, MD, Margaret Maloney, RN (MetroHealth Medical Center, Cleveland, OH, U.S.A.), and Jamie Southern, LPN (Center for Clinical Research, Winston‐Salem, NC, U.S.A.), without which this study would not have been possible. In addition, the authors thank Haley Chung for her rendering of Figures 4 and 5.
Previous Presentations: This work was presented, in part, as a scientific abstract for the Annual Meeting of the North American Neuromodulation Society in Las Vegas, Nevada, December 11–12, 2015. This work was also presented, in part, as a scientific abstract for the Annual Meeting of the International Anesthesia Research Society in San Francisco, California, May 21–24, 2016.
References
- 1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. [DOI] [PubMed] [Google Scholar]
- 2. Strassels SA, McNicol E, Wagner AK, Rogers WH, Gouveia WA, Carr DB. Persistent postoperative pain, health‐related quality of life, and functioning 1 month after hospital discharge. Acute Pain. 2004;6:95–104. [Google Scholar]
- 3. Dahlen L, Zimmerman L, Barron C. Pain perception and its relation to functional status post total knee arthroplasty: a pilot study. Orthop Nurs. 2006;25:264–270. [DOI] [PubMed] [Google Scholar]
- 4. Choy WS, Lee SK, Kim KJ, Kam BS, Yang DS, Bae KW. Two continuous femoral nerve block strategies after TKA. Knee Surg Sports Traumatol Arthrosc. 2011;19:1901–1908. [DOI] [PubMed] [Google Scholar]
- 5. Brander VA, Stulberg SD, Adams AD, et al. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res. 2003;416:27–36. [DOI] [PubMed] [Google Scholar]
- 6. Andersen LO, Gaarn‐Larsen L, Kristensen BB, Husted H, Otte KS, Kehlet H. Subacute pain and function after fast‐track hip and knee arthroplasty. Anaesthesia. 2009;64:508–513. [DOI] [PubMed] [Google Scholar]
- 7. Visser EJ. Chronic post‐surgical pain: epidemiology and clinical implications for acute pain management. Acute Pain. 2006;8:73–81. [Google Scholar]
- 8. Callahan CM, Drake BG, Heck DA, Dittus RS. Patient outcomes following tricompartmental total knee replacement. A meta‐analysis. JAMA. 1994;271:1349–1357. [PubMed] [Google Scholar]
- 9. Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152:566–572. [DOI] [PubMed] [Google Scholar]
- 10. Ritter MA. Postoperative pain after total knee arthroplasty. J Arthroplasty. 1997;12:337–339. [DOI] [PubMed] [Google Scholar]
- 11. Peng L, Ren L, Qin P, et al. Continuous femoral nerve block versus intravenous patient controlled analgesia for knee mobility and long‐term pain in patients receiving total knee replacement: a randomized controlled trial. Evid Based Complement Alternat Med. 2014;2014:569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80:63–69. [DOI] [PubMed] [Google Scholar]
- 13. Baker PN, van der Meulen JH, Lewsey J, Gregg PJ. The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J Bone Joint Surg Br. 2007;89:893–900. [DOI] [PubMed] [Google Scholar]
- 14. Pinto PR, McIntyre T, Ferrero R, Araujo‐Soares V, Almeida A. Persistent pain after total knee or hip arthroplasty: differential study of prevalence, nature, and impact. J Pain Res. 2013;6:691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guan Y. Spinal cord stimulation: neurophysiological and neurochemical mechanisms of action. Curr Pain Headache Rep. 2012;16:217–225. [DOI] [PubMed] [Google Scholar]
- 16. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. [DOI] [PubMed] [Google Scholar]
- 17. Campbell JN, Taub A. Local analgesia from percutaneous electrical stimulation. A peripheral mechanism. Arch Neurol. 1973;28:347–350. [DOI] [PubMed] [Google Scholar]
- 18. Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17:515–550. [DOI] [PubMed] [Google Scholar]
- 19. Hassenbusch SJ, Stanton‐Hicks M, Schoppa D, Walsh JG, Covington EC. Long‐term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996;84:415–423. [DOI] [PubMed] [Google Scholar]
- 20. Picaza JA, Hunter SE, Cannon BW. Pain suppression by peripheral nerve stimulation. Chronic effects of implanted devices. Appl Neurophysiol. 1977;40:223–234. [DOI] [PubMed] [Google Scholar]
- 21. Rakel BA, Zimmerman MB, Geasland K, et al. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: a randomized, blinded, placebo‐controlled trial. Pain. 2014;155:2599–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hymes AC, Raab DE, Yonehiro EG, Nelson GD, Printy AL. Electrical surface stimulation for control of acute postoperative pain and prevention of ileus. Surg Forum. 1973;24:447–449. [PubMed] [Google Scholar]
- 23. VanderArk GD, McGrath KA. Transcutaneous electrical stimulation in treatment of postoperative pain. Am J Surg. 1975;130:338–340. [DOI] [PubMed] [Google Scholar]
- 24. Yu DT, Chae J, Walker ME, Hart RL, Petroski GF. Comparing stimulation‐induced pain during percutaneous (intramuscular) and transcutaneous neuromuscular electric stimulation for treating shoulder subluxation in hemiplegia. Arch Phys Med Rehabil. 2001;82:756–760. [DOI] [PubMed] [Google Scholar]
- 25. Yu DT, Chae J, Walker ME, Fang ZP. Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: a pilot study. Arch Phys Med Rehabil. 2001;82:20–25. [DOI] [PubMed] [Google Scholar]
- 26. Huntoon MA, Huntoon EA, Obray JB, Lamer TJ. Feasibility of ultrasound‐guided percutaneous placement of peripheral nerve stimulation electrodes in a cadaver model: part one, lower extremity. Reg Anesth Pain Med. 2008;33:551–557. [DOI] [PubMed] [Google Scholar]
- 27. Huntoon MA, Hoelzer BC, Burgher AH, Hurdle MF, Huntoon EA. Feasibility of ultrasound‐guided percutaneous placement of peripheral nerve stimulation electrodes and anchoring during simulated movement: part two, upper extremity. Reg Anesth Pain Med. 2008;33:558–565. [DOI] [PubMed] [Google Scholar]
- 28. Huntoon MA, Burgher AH. Ultrasound‐guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes. Pain Med. 2009;10:1369–1377. [DOI] [PubMed] [Google Scholar]
- 29. Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;113:904–925. [DOI] [PubMed] [Google Scholar]
- 30. Rauck RL, Kapural L, Cohen SP, et al. Peripheral nerve stimulation for the treatment of postamputation pain—a case report. Pain Pract. 2012;12:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rauck RL, Cohen SP, Gilmore CA, et al. Treatment of post‐amputation pain with peripheral nerve stimulation. Neuromodulation. 2014;17:188–197. [DOI] [PubMed] [Google Scholar]
- 32. Weiner RL. Occipital neurostimulation for treatment of intractable headache syndromes. Acta Neurochir Suppl. 2007;97:129–133. [DOI] [PubMed] [Google Scholar]
- 33. Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate‐to‐severe postoperative pain on the numeric rating scale: a cut‐off point analysis applying four different methods. Br J Anaesth. 2011;107:619–626. [DOI] [PubMed] [Google Scholar]
- 34. Ilfeld BM. Single‐injection and continuous femoral nerve blocks are associated with different risks of falling. Anesthesiology. 2014;121:668–669. [DOI] [PubMed] [Google Scholar]
- 35. Capdevila X, Bringuier S, Borgeat A. Infectious risk of continuous peripheral nerve blocks. Anesthesiology. 2009;110:182–188. [DOI] [PubMed] [Google Scholar]
- 36. Rawal N. American Society of Regional Anesthesia and Pain Medicine 2010 Gaston Labat Lecture: perineural catheter analgesia as a routine method after ambulatory surgery–effective but unrealistic. Reg Anesth Pain Med. 2012;37:72–78. [DOI] [PubMed] [Google Scholar]
- 37. Kandasami M, Kinninmonth AW, Sarungi M, Baines J, Scott NB. Femoral nerve block for total knee replacement—a word of caution. Knee. 2009;16:98–100. [DOI] [PubMed] [Google Scholar]
- 38. Feibel RJ, Dervin GF, Kim PR, Beaule PE. Major complications associated with femoral nerve catheters for knee arthroplasty: a word of caution. J Arthroplasty. 2009;24:132–137. [DOI] [PubMed] [Google Scholar]
- 39. van Zundert A, Helmstadter A, Goerig M, Mortier E. Centennial of intravenous regional anesthesia. Bier's block (1908–2008). Reg Anesth Pain Med. 2008;33:483–489. [DOI] [PubMed] [Google Scholar]
- 40. Lamacraft G. The link between acute postoperative pain and chronic pain syndromes. South Afr J Anaesth Analg. 2012;18:45–50. [Google Scholar]
- 41. Rakel BA, Blodgett NP, Bridget Zimmerman M, et al. Predictors of postoperative movement and resting pain following total knee replacement. Pain. 2012;153:2192–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. [DOI] [PubMed] [Google Scholar]
- 43. Grill WM, Mortimer JT. Stimulus waveforms for selective neural stimulation. IEEE Trans Biomed Eng. 1995;14:375–385. [Google Scholar]
- 44. Ilfeld BM, Gabriel RA, Saulino MF, et al. Infection rate of electrical leads used for percutaneous neuromuscular stimulation of the peripheral nervous system. Pain Practice[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. J Pain Symptom Manage. 2003;25:406–411. [DOI] [PubMed] [Google Scholar]
- 46. Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88:287–294. [DOI] [PubMed] [Google Scholar]
- 47. Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–414. [DOI] [PubMed] [Google Scholar]


