Abstract
Minimally invasive thoracic surgery, when compared with open thoracotomy, has been shown to have improved perioperative outcomes as well as comparable long-term survival. Robotic surgery represents a powerful advancement of minimally invasive surgery, with vastly improved visualization and instrument maneuverability, and is increasingly popular for thoracic surgery. However, there remains debate over the best robotic approaches for lung resection, with several different techniques evidenced and described in the literature. We delineate our method for total port approach with four robotic arms and discuss how its advantages outweigh its disadvantages. We conclude that it is preferred to other robotic approaches, such as the robotic assisted approach, due to its enhanced visualization, improved instrument range of motion, and reduced potential for injury.
Keywords: Lung cancer surgery, minimally invasive surgery, robotic surgery, total port approach
Introduction
Compared to thoracotomy, thoracic minimally invasive surgery—such as video-assisted thoracoscopic surgery (VATS) (1-3) and robotic surgery (4-8)—offer improved perioperative outcomes as well as similar long-term survival for patients with early-stage non-small cell lung cancers. Robotic surgery offers several advantages over VATS, such as replacing restricted, two-dimensional images with magnified, high-definition, three-dimensional visualization while greatly enhancing surgical instrument maneuverability and precision (1,4,9). Given these advancements, robotic thoracic surgery has swelled in popularity, with robotic lung resections tripling over the last 2 years (10).
Despite the growing popularity of robotic thoracic surgery worldwide, published comparisons of different technical methods applied to robotic surgery remain scarce. Such information is vital to identify, better understand, and thereby improve upon best practices. This allows for the establishment of technical standards that can help, among other things, improve outcomes and reduce operative duration and cost. In particular, there remains much discussion regarding what constitutes an optimal robotic approach and its associated port placement, with techniques cited ranging from incomplete port approaches with VATS access incisions to total port approaches with three versus four arms (11-13). Naturally, it is important to expound upon the technical details involved as well as the respective advantages and disadvantages that each technique may provide (8). The objective of this paper is to illustrate our preferred port placement for a total port approach with four robotic arms and discuss its relative advantages and disadvantages for robotic lung resections.
Port placement
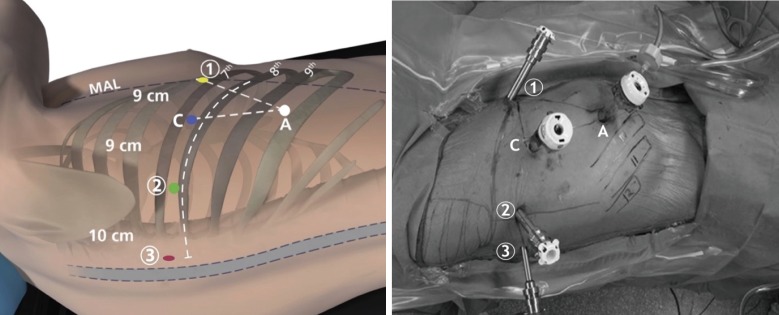
In our total port approach, we utilize all four arms of the da Vinci Si or Xi robot. Our port positioning and placement is systematic and optimized for robotic arm maneuverability (8,14,15) (Figure 1). For the da Vinci Si system, we use two 8 mm ports (left and right robotic arm ports), a 12 mm port (camera), and a 5 mm port (fourth robotic arm port—we use the smallest size port because it is all that is required for the fourth arm instrument, allowing us to minimize pain); for the Xi system, all the ports are 8 mm ports. We also utilize a 12 mm assistant port that can be used for stapling and exchange of items such as rolled-up sponges and vessel loops. The assistant port is also important in case sudden or catastrophic bleeding occurs. The following is a description of port placement for a right-sided resection.
Figure 1.
Total port approach with four-port placement for right-sided pulmonary lobectomy with da Vinci Si robotic arms 1, 2, 3, camera [C], and access port [A] [Reprinted with permission (15)].
We place the ports in the seventh (upper or middle lobectomy) or eighth (lower lobectomy) intercostal space. The fourth robotic arm is located 2–3 cm from the spine, the left robotic arm port is located 10 cm away from that port, the camera port is located 9 cm from the left robotic arm port, and the right robotic arm is located 9 cm away from the camera port (Figure 1). The port locations are marked beforehand, although slight changes to these locations are often necessary once the intrathoracic anatomy is visualized. The first port to be placed is the camera port [C]. To verify pleural space entry, a camera is introduced into the port before insufflating the thoracic cavity with warmed, humidified carbon dioxide to inferiorly displace the diaphragm and maximize the cavity size. Next, in an effort to reduce postoperative pain, we administer a subpleural paravertebral block of ribs three to eleven using 0.25% bupivacaine with epinephrine via a 21-gauge needle. Then, the fourth robotic arm port (labeled “3” in Figure 1) is placed. This port is inserted two ribs beneath the oblique fissure (often over the top of the eighth rib for upper lobectomy and over the top of the ninth rib for lower lobectomy) at a maximally posterior location about 2 cm anterior to the spinous processes of the vertebral bodies; it will control the second left hand instrument. The camera is then placed through the fourth robotic arm port before inserting the final two robotic ports for the left (labeled “2” in Figure 1) and right (labeled “1” in Figure 1) robotic arms under direct vision. The assistant port is a 12 mm port and is inserted just superior to the diaphragmatic fibers—and hence as anteroinferior in the chest as possible—while being triangulated between the camera and right robotic arm ports. This isosceles triangle positioning maintains excellent robotic arm maneuverability while securing adequate space for the bedside assistant.
There is a degree of flexibility in the assistant port’s position, if warranted anatomically, as it can also be triangulated between the left robotic arm [2] and camera port [C]. In either case, the purpose is to make this isosceles triangle maximally wide and deep, thereby allowing for both extensive robotic arm dexterity and space for the bedside assistant to work. Lastly, for the Si system, the camera port [C] incision is enlarged to a 12 mm double-cannulated port, enabling it to admit the robotic camera.
Once the ports have been secured, the Si robot is steered at a fifteen-degree angle over the patient’s shoulder and its arms are attached to the respective ports (one through four) as noted (8,14,15). For the Xi system, the robot can approach the operating room table perpendicular to the patient, after which the beam is rotated to the proper position.
The robotic instruments we use most commonly for lung segmentectomy are a bipolar curved tip dissector in right robotic arm, a Cadiere grasper in the left robotic arm, a lung grasper (Si system) or tip-up fenestrated grasper (Xi system) in the fourth robotic arm, and a zero-degree camera in the camera port.
Advantages and disadvantages
The total port approach for robotic surgery for lung resections comes with several distinct advantages over a robotic assisted approach. The total port approach is by definition a completely closed environment (8,14,15). This allows for the introduction of warm humidified carbon dioxide for thoracic insufflation, providing a myriad of benefits. Among them, it spares the lungs from exposure to the operating room’s cool, dry air (15). It also expands the thoracic cavity by decreasing the size of the lung parenchyma and pushing the diaphragm inferiorly (15). As a result, the space with which to visualize the thoracic anatomy—including mediastinal nodal views—is augmented (11). Moreover, the space in which robotic instruments can be manipulated is optimized, enabling more efficient and effective surgery. Pushing the diaphragm downwards with carbon dioxide insufflation also reduces potential for injury to it intraoperatively (15). We further believe that the use of carbon dioxide insufflation saves time, both by improving visualization (as aforementioned) as well as decreasing bleeding from the lung parenchyma via increased intrathoracic pressure.
Our port placement facilitates this enhanced visualization, and we take full advantage of the expanded room by using four robotic arms, equipping the surgeon to retract the lung with the fourth arm rather than relying on the assistant to do so. Given that retraction is critical for properly exposing hilar structures to be dissected, isolated, and divided, we believe that this also saves time and increases our level of efficiency compared to a three-arm technique. We additionally verify each port’s insertion point from the interior (after the first port) to reduce potential for injury, and try to place ports two, three, and four along the same rib in part to avoid damaging multiple intercostal neurovascular bundles (11,15). We use a zero-degree camera, which has less torque than a thirty-degree one, to further decrease the chances of intercostal nerve injury (8,14,15). Vitally, the total port approach eschews the morbidity of a utility thoracotomy incision and avoids the inefficiency of regularly switching from robotic to VATS resection during the operation (12,13).
However, the total port approach does carry some disadvantages when compared to a robotic assisted approach. Perhaps most significantly, the total port approach’s completely closed environment does not allow for inserting a finger into the chest (15). This is traditionally used for direct palpation of a nodule or the lung, helping to locate an area of interest. That said, several alternate methods—such as electromagnetic navigational bronchoscopy with tattooing using a marker such as methylene blue or indigo carmine—allow for precise nodule targeting without necessitating direct palpation, and can be readily accommodated with our approach (16). In addition, near-infrared imaging of intravenously-administered indocyanine green can be used to detect lung nodules; this capability is integrated into the da Vinci Xi platform (17). Finally, if it is indeed felt to be imperative in select cases, our technique does not contraindicate an additional small access incision for this purpose. While there is voiced concern for a supposed inappropriateness of a completely portal operation—with reasons ranging from cumbersome dissection to increased risk for catastrophic bleeding—we have demonstrated this to simply not be the case, with a 10% postoperative complication rate, 2% major morbidity rate, and 0% 90-day mortality rate in our published consecutive series of one-hundred planned robotic segmentectomies (7,15). In terms of three-arm versus four-arm approaches, there is the hypothetical disadvantage of added pain from the additional incision made in the chest for the four-arm approach. That said, there have been no studies comparing pain levels or narcotic usage between the two techniques. In addition, there can be greater potential for collision of instruments outside the body when using the additional arm; this can, however, be minimized with proper attention to the spacing and placement of ports, as we have detailed. The advantages and disadvantages of a totally portal four-arm robotic approach to lobectomy are shown in Table 1.
Table 1. Advantages and disadvantages of totally portal four-arm approach to robotic lobectomy.
| Advantages |
| Carbon dioxide insufflation |
| Warmed, humidified air |
| Improved visualization |
| Decreased bleeding |
| Decreased risk for diaphragmatic injury |
| Fourth arm available for retraction |
| Smaller incision than if utility port used |
| No need to switch between robotic and VATS techniques |
| Disadvantages |
| No way to palpate the lung |
| Added pain from fourth incision |
| Increased risk of collisions with extra arm |
VATS, video-assisted thoracoscopic surgery.
Discussion
Minimally invasive surgery offers improved perioperative outcomes as well as similar long-term survival when compared with open thoracotomy in the treatment of early-stage non-small cell lung cancers (1-3,8). Specifically, robotic lobectomy as compared to open thoracotomy has been shown to have decreased rates of morbidity—including air leak, blood loss, blood transfusions, and chest tube duration—as well as reduced length of hospital stay (10,18). Robotic lung surgery offers improved visualization with its magnified, high-definition, three-dimensional images, allowing for a level of anatomic appreciation that cannot be replicated by VATS or even open thoracotomy. In addition, robotic arms and their instruments can be manipulated with more degrees of articulation than their VATS counterparts. With the advent of robotic stapling, the argument that VATS has a greater variety or range of instruments has become weaker. The ergonomics of robotic surgery, where the surgeon is sitting at the console rather than standing, and the motion scaling that decreases the tremor of the surgeon’s hands, are other benefits of robotic technology. Disadvantages of robotic surgery include cost, potentially increased duration of operation, and complexity in terms of logistical needs, training, and equipment.
Just as with VATS lobectomy, multiple techniques for robotic lobectomy exist. Dylewski and Ninan have described a completely portal three-arm approach in 74 patients (11). Veronesi and Melfi have described an incompletely portal robotic assisted approach that utilizes four robotic arms as well as a VATS access incision in 54 patients (12). Gharagozloo, meanwhile, has reported on a hybrid approach in 100 patients (13). Our extensive experience in robotic surgery for lung resections, including 520 robotic lobectomies between February 2010 and December 2015, has allowed us to develop and fine-tune a regimented process for port placement via the total port approach with four robotic arms. In essence, our strategy is optimized to achieve several goals: (I) to operate with an emphasis on safety and minimizing postoperative complications; (II) to maximize maneuverability for robotic instruments and improve operative precision; (III) to improve efficiency and reduce operating room time and cost; and (IV) to improve patient outcomes.
We favor a completely portal four-arm approach for the benefits outlined in this paper. By maintaining a completely closed environment, the thorax can be insufflated with warm, humidified carbon dioxide, safely enlarging the operative environment while helping protect the lungs. This enables enhanced visualization and facilitates efficient and effective surgery. However, it also carries some disadvantages, including the presence of an additional incision, greater potential for instrument collisions for the inexperienced practitioner (four-port versus three-port technique), and the inability to directly palpate the lung or a nodule secondary to the completely closed environment (completely portal versus a utility incision technique). Notably, though, alternatives for nodule identification exist and are becoming more widely studied and adopted.
Our total port approach with four arms, and the meticulousness with which we have adjusted and detailed our port placement, has developed over several years of efforts to improve our robotic lung resections. This documented experience is a strength of this paper. A necessary limitation of this paper is that it relies largely on the experience at a single institution, making its generalizability unproven. In addition, the fact remains that the objective benefits of one robotic lobectomy technique over another have not been systematically studied. That being said, we have striven to expound upon not only the intricacies of our preferred port placement but also the reasoning behind our decisions, so as to allow surgeons to adapt our model to individual patients as they deem appropriate. Likewise, we have offered some potential modifications for select situations. Future study from multiple thoracic robotic surgeons and multiple centers is needed to further explore the advantages and disadvantages of the total port approach with four robotic arms as compared to its alternatives. We have attempted to position this paper as a basis from which thoracic robotic surgeons can expand upon, improve, and then document their refinements to our techniques, and we look forward to their input.
Conclusions
In conclusion, robotic thoracic surgery is a growing field in which there remains a great need for demonstrably effective and efficient technical methods. We have elucidated our strategy for the total port approach with four robotic arms and explained why it is our favored approach for robotic lung resections.
Acknowledgements
None.
Footnotes
Conflicts of Interest: OI Ramadan: none; B Wei: Medtronic—speaker; RJ Cerfolio: Intuitive Surgical—proctor, speaker, lecturer; Ethicon—speaker, teacher; Community Health Services—consultant; KCL—consultant; Bovie—consultant; C-SATS—consultant.
References
- 1.Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. 10.1200/JCO.2008.18.2733 [DOI] [PubMed] [Google Scholar]
- 3.Rocco G, Internullo E, Cassivi SD, et al. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin 2008;18:235-47. 10.1016/j.thorsurg.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Velez-Cubian FO, Ng EP, Fontaine JP, et al. Robotic-Assisted Videothoracoscopic Surgery of the Lung. Cancer Control 2015;22:314-25. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura H. Systematic review of published studies on safety and efficacy of thoracoscopic and robot-assisted lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2014;20:93-8. 10.5761/atcs.ra.13-00314 [DOI] [PubMed] [Google Scholar]
- 6.Rinieri P, Peillon C, Salaün M, et al. Perioperative outcomes of video- and robot-assisted segmentectomies. Asian Cardiovasc Thorac Ann 2016;24:145-51. 10.1177/0218492315627556 [DOI] [PubMed] [Google Scholar]
- 7.Bryant AS, Rudemiller K, Cerfolio RJ. The 30- versus 90-day operative mortality after pulmonary resection. Ann Thorac Surg 2010;89:1717-22; discussion 1722-3. [DOI] [PubMed]
- 8.Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. 10.1016/j.jtcvs.2011.07.022 [DOI] [PubMed] [Google Scholar]
- 9.Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. 10.1016/j.jtcvs.2005.07.031 [DOI] [PubMed] [Google Scholar]
- 10.Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. 10.1016/j.athoracsur.2013.07.117 [DOI] [PubMed] [Google Scholar]
- 11.Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. 10.1016/j.ejcts.2010.01.047 [DOI] [PubMed] [Google Scholar]
- 12.Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. 10.1016/j.jtcvs.2009.10.025 [DOI] [PubMed] [Google Scholar]
- 13.Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. 10.1016/j.athoracsur.2009.04.039 [DOI] [PubMed] [Google Scholar]
- 14.Cerfolio RJ. Total port approach for robotic lobectomy. Thorac Surg Clin 2014;24:151-6, v. 10.1016/j.thorsurg.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 15.Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 1095-6. 10.1016/j.athoracsur.2015.08.092 [DOI] [PubMed] [Google Scholar]
- 16.Bolton WD, Howe H, 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. 10.1016/j.athoracsur.2014.04.085 [DOI] [PubMed] [Google Scholar]
- 17.Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014;98:1223-30. 10.1016/j.athoracsur.2014.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams RD, Bolton WD, Stephenson JE, et al. Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann Thorac Surg 2014;97:1893-8; discussion 1899-900. [DOI] [PubMed]