Abstract
Background
Internet-delivered, behavioral interventions represent a cost-effective, broadly disseminable approach for teaching persons with multiple sclerosis (MS) the theory-based skills, techniques, and strategies for changing physical activity.
Objectives
This pilot, randomized controlled trial examined the efficacy of a newly developed Internet website based on e-learning approaches that delivered a theory-based behavior intervention for increasing physical activity and improving symptoms, walking impairment, and neurological disability.
Methods
Participants with MS (N = 47) were randomly assigned into behavioral intervention (n = 23) or waitlist control (n = 24) conditions delivered over a six-month period. Outcomes were administered before and after the six-month period using blinded assessors, and data were analyzed using analysis of covariance in SPSS.
Results
There was a significant, positive intervention effect on self-reported physical activity (P = 0.05, = 0.10), and non-significant improvement in objectively measured physical activity (P = 0.24, = 0.04). There were significant, positive effects of the intervention on overall (P = 0.018, = 0.13) and physical impact of fatigue (P = 0.003, = 0.20), self-reported walking impairment (P = 0.047, = 0.10), and disability status (P = 0.033, = 0.11). There were non-significant improvements in fatigue severity (P = 0.10, = 0.06), depression (P = 0.10, = 0.07) and anxiety (P = 0.06, = 0.09) symptoms, and self-reported disability (P = 0.10, = 0.07).
Conclusions
We provide evidence for the efficacy of an Internet-based behavioral intervention with content delivered through interactive video courses grounded in e-learning principles for increasing physical activity and possibly improving secondary outcomes of fatigue, depression, anxiety, and walking impairment/disability in persons with MS.
Keywords: Multiple sclerosis, physical activity, behavior change, theory, e-learning
Introduction
Physical activity is reduced among persons with multiple sclerosis (MS) compared with the general population.1 This is alarming considering the low rate of physical activity in the general population2 and evidence that physical activity declines over time in MS.3 Of note, fewer than 20% of persons with MS meet public health guidelines for moderate-to-vigorous physical activity (MVPA).4
The standard approach for promoting physical activity in MS has involved structured, supervised exercise training.5 That approach has resulted in evidence of considerable benefit,6 but it has not altered the rate of physical activity over the past 25 years.1 Researchers have proposed a paradigm shift away from structured exercise training, toward lifestyle physical activity behavior change using behavioral interventions.7,8 Such behavioral interventions teach people the skills, techniques, and strategies based on a theory of behavior change for modifying and self-regulating physical activity.
Researchers have developed and tested a behavioral intervention delivered through an Internet website based on social cognitive theory (SCT)7 for changing physical activity in MS.8,9 The first randomized controlled trial (RCT) included a dedicated Internet website and indicated that the intervention group self-reported a large increase in physical activity over a three-month period.10 Such results were replicated in a follow-up RCT using objectively measured physical activity.11 The next RCT refined the dedicated Internet website and added one-on-one video chat sessions with behavioral coaches, and the modifications resulted in an increase in physical activity that was sustained for three months after the intervention ended.12 One recent RCT included the Internet website and one-on-one video coaching and demonstrated improvements in MVPA and symptoms of fatigue, depression, and anxiety over a six-month period.13
The major weakness of those behavioral interventions was the primary reliance upon content delivered through a text-based medium in which participants read text and passages for learning about principles of behavior change. Such a delivery modality offered a passive, non-interactive, and non-immersive approach for learning about skills, techniques, and strategies necessary for modifying and self-regulating physical activity. The text-based medium and Internet platform were suboptimal for delivery on smartphones and tablet devices. Those weaknesses can be addressed through advances in e-learning software that permit the development of interactive video courses that replace text-based delivery and work on all devices automatically. The interactive video courses provide an engaging user experience through pre-built and customizable interactions, slide layers and triggers, and a more immersive, engaging learner experience. This modern e-learning approach increases the interest, processing, and accessability of information by users with a likely greater impact of the behavioral intervention.
This pilot RCT examined the efficacy of a newly developed Internet website based on modern e-learning approaches that delivered a SCT-based behavior intervention for increasing physical activity in persons with MS. The primary endpoints were self-reported and objectively measured MVPA, and the secondary endpoints were symptoms, walking impairment, and neurological disability. The tertiary endpoints focused on compliance metrics and formative evaluation.
Methods
Participants
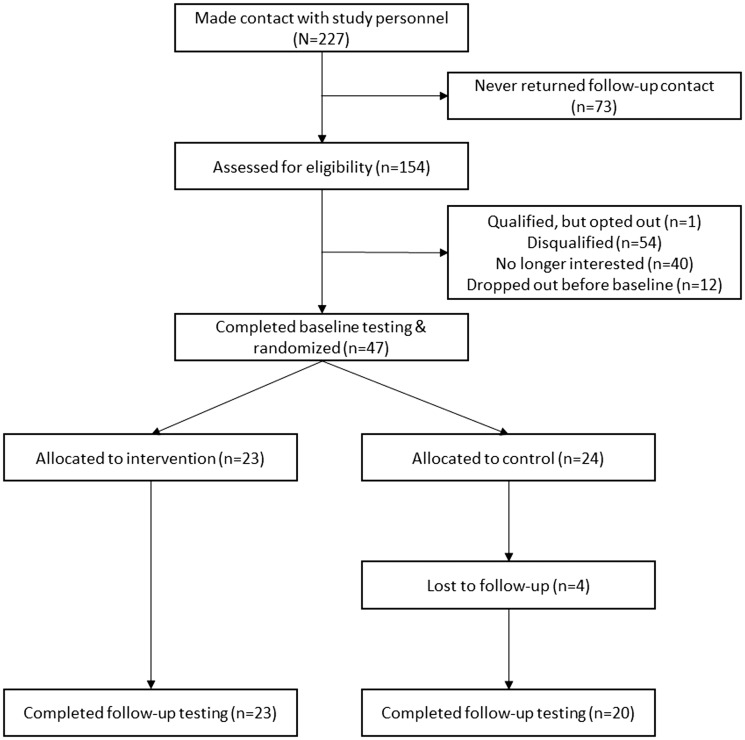
The CONSORT diagram is provided in Figure 1. We recruited participants by distribution of a flyer through the Greater Illinois Chapter of the National Multiple Sclerosis Society. The flyer described the study as an opportunity to participate in programs for improving health among persons with MS. This resulted in 227 persons contacting the study personnel about the study. We conducted a screening for eligibility based on inclusion criteria among 154 persons (67.8% of those who contacted the study personnel). The inclusion criteria were: (a) diagnosis of MS;14 (b) relapse free in the past 30 days; (c) Internet access; (d) willingness to complete the questionnaires, wear the accelerometer, and undergo randomization; (e) non-active during the previous six months; (f) ability to ambulate with or without assistance; and (g) age between 18 and 64 years. We further only included participants who had a minimal risk for engaging in a physical activity program based on the physical activity readiness questionnaire.15 Of the 154 who were assessed for eligibility, 47 participants completed baseline testing (31.5%) and were randomly assigned into one of two conditions, behavioral intervention (n = 23) or waitlist control (n = 24).
Figure 1.
CONSORT flow diagram.
Measures
Physical activity
We included the Godin leisure-time exercise questionnaire (GLTEQ)16 and ActiGraph model GT3X+ accelerometers (Actigraph Corporation, FL, USA) as measures of MVPA. The GLTEQ includes three items that measure the frequency of engagement in mild, moderate, or strenuous physical activity for at least 15 minutes during one’s leisure time in the previous week. We computed the health contribution score (HCS) for the GLTEQ by multiplying the frequency of strenuous and moderate activity by 9 and 5 metabolic equivalents of task, respectively, and then summing the weighted scores.17 The total HCS score ranges between 0 and 98; higher scores reflect greater self-reported MVPA.18 The ActiGraph model GT3X+ accelerometers provided an objective measure of MVPA during the previous week. The accelerometer was placed in a pouch on an elastic belt worn around the waist during the waking hours of a seven-day period. The data from the accelerometer were downloaded, processed into one-minute epochs using ActiLife (Actigraph Corporation), and then scored for wear time and minutes/day of MVPA based on activity count cutpoints for MS.19 Only data from valid days (wear time ≥600 minutes) were included in the analyses; we averaged the data over two or more valid days for the outcome of minutes/day of MVPA.20
Fatigue impact and severity
The impact and severity of fatigue were measured using the modified fatigue impact scale (MFIS)21 and fatigue severity scale (FSS),22 respectively. The MFIS includes 21 items that correspond with three subscales representing the physical, cognitive, and psychosocial impact of fatigue on daily activities over the previous month. The item scores are summed for the physical (possible range of scores 0–36), cognitive (possible range of scores 0–40), and psychosocial (possible range of scores 0–8) subscales. The total MFIS score is calculated by summing scores for all items (possible range of scores 0–84); higher scores indicate a greater impact of fatigue on daily activities. The FSS includes nine items that measure perceived fatigue severity over the past week. The scores for the nine items are averaged (possible range of scores 1–7); higher scores indicate more severe fatigue.
Depression and anxiety
The hospital anxiety and depression scale (HADS)23 includes 14 items that measure the frequency of anxiety (seven items) and depressive (seven items) symptoms over the past four weeks. The item scores are summed into a total score for anxiety and depressive symptom subscales (possible range of scores 0–21); higher scores indicate more frequent symptoms of anxiety and depression.
Pain
The short form of the McGill pain questionnaire (SF-MPQ)24 includes 15 items that measure pain during the past four weeks. The individual item scores are summed into an overall score (possible range of scores 0–45); higher scores reflect worse pain.
Walking impairment
The 12-item, multiple sclerosis walking scale (MSWS-12)25 provided a patient-reported outcome for measuring walking impairment during the past two weeks. The item scores are summed and then transformed into an overall scale (possible range of scaled scores 0–100); higher scores reflect greater perceived walking impairment.
Disability
The patient determined disease steps (PDDS)26 scale and the Expended Disability Status Scale (EDSS)27 characterized the overall level of disability of the sample and captured possible change in disability. The PDDS is a single, nine-point item for reporting neurological impairment on a scale of 0 (normal) to 8 (bedridden) with a midpoint of score of 4 (early cane). The EDSS was based on a neurological examination performed by a Neurostatus certified examiner. The EDSS score provides an overall clinical rating of neurological impairment with anchors of 0 (normal neurological exam) to 10 (death due to MS) with benchmark scores of 4 (fully ambulatory) and 6 (intermittent or unilateral constant assistance).
Formative evaluation
Participants in the behavioral intervention provided program evaluation using a study-specific instrument. The first six questions evaluated satisfaction with website components (e.g. interactive video courses, tracker, voices, forums), one-on-one chats, and the entire program using a scale of 1 (completely unsatisfied) to 5 (completely satisfied) with a mid-point of 3 (neutral). The last question evaluated agreement with the statement, ‘I would recommend the program to others with MS’, and was rated using a scale of 1 (strongly disagree) to 5 (strongly agree) with a mid-point of 3 (neutral).
Procedures
The study procedures were reviewed and approved by a university institutional review board. Interested participants contacted the project coordinator who described the study and what it entailed, and then conducted a screening for inclusion criteria over the phone. We then distributed the informed consent document among participants who satisfied inclusion criteria through email, postal mail, or fax. Participants completed enrollment by returning a signed copy of this document. Once enrolled, the project coordinator contacted participants and scheduled a baseline testing session.
During the baseline testing session, participants completed a battery of measures that included a demographic/clinical characteristic scale and self-report assessments of symptoms and walking impairment. We measured height and weight using a scale stadiometer (Detecto, Webb City, MO, USA), and conducted a neurological examination for the generation of an EDSS score and administered the PDDS. All data were collected by treatment-blinded assessors. We provided the participant with verbal instructions and a demonstration for wearing the accelerometer. Participants were asked to maintain current, usual activities while wearing the accelerometer around the waist on the non-dominant hip for seven days, during all waking hours, except while engaging in water activities. Participants completed the GLTEQ after wearing the accelerometer, and returned those study materials in a pre-stamped and pre-addressed envelope. Participants were randomly assigned into either the behavioral intervention or control condition using a random numbers generator and concealed allocation (i.e. sealed envelopes). Participants received notification of group assignment and instructions for participation through email and postal mail. The intervention group further received study materials (Yamax SW-200 Digiwalker pedometer, a log book for recording daily pedometer steps, and study website log-in information). Participants completed the same assessments following the 6-month period, and those in the behavioral intervention condition further provided formative evaluation. Follow-up data were again collected by treatment-blinded assessors. Participants in the control condition received the behavioral intervention after the follow-up assessment.
Behavioral intervention
The behavioral intervention consisted of two primary components, namely a dedicated Internet website and one-on-one video chats with a behavioral coach. The Internet website was programed in Drupal 8.0 for accessibility, and underwent modification from previous versions based on the research team’s experiences with MS and focus group feedback, as well as formative evaluation from pilot testing for efficacy in multiple published RCTs.10–13 The most extensive modification involved transitioning from the primarily text-based presentation of material in previous versions of the behavioral intervention10–13 into interactive video courses designed based on e-learning principles. We further included the physical activity ‘tracker’ feature directly into the website itself rather than as an external tool. This allowed participants to directly enter weekly step counts and graphically monitor activity levels within the Internet website. The Internet website was password protected and hosted on a research-based server.
Internet website
The Internet website represented a medium for disseminating information on the skills, techniques, resources, and strategies for becoming and staying physically active with MS. The primary content of the Internet website was based on SCT and represented the transformation of an effective and empirically validated, face-to-face intervention approach that increased adherence with supervised exercise training28 in persons with MS.29 The content was delivered through interactive video courses based on e-learning principles. The interactive video courses were developed using Storyline in Articulate 360 (Articulate, New York, NY, USA) based on operationalizing principal elements of SCT including self-efficacy, outcome expectations, impediments, and goal-setting. The interactive video courses followed a similar template of introduction and overview, primary content, and take home message and were developed for an 8th grade reading level and with steady presentation of animations. The interactive video courses received formative evaluation prior to the intervention from representative persons with MS and were organized into four ‘modules’ for the behavioral intervention, namely: getting started; planning for success; beating the odds; and sticking with it. The getting started module included interactive video courses on the benefits of physical activity for persons with MS, instructions for becoming physically active, completing daily activity logs, and self-monitoring of behavior via a Yamax SW-200 pedometer and an embedded website feature called tracker for reporting weekly physical activity. The planning for success module included interactive video courses on goal setting and feedback again via a Yamax SW-200 pedometer and tracker, developing realistic outcome expectations, and increasing self-efficacy. The beating the odds module included interactive video courses on identifying barriers to physical activity and strategies of overcoming barriers as well as developing social support. The sticking with it module included interactive video courses on maintaining an active lifestyle and prevention of relapse into a sedentary lifestyle. The interactive video courses included embedded, supplementary options of ‘learn more’ and ‘resources’. The ‘learn more’ option included passive videos on content that aligned with the course, and the ‘resources’ option included research articles for documenting the statements within the courses; manuals on stretching, aerobic, and resistance modes of physical activity; and worksheets and questionnaires for further developing the personal relevance of the stories. The interactive video courses along with embedded options became ‘accessible’ in a titrated fashion of seven times during the first two months, four times during the second two months, and twice during the final two months of the intervention.
The tracker feature of the website was designed for self-monitoring and goal-setting within the website itself, and the data were directly accessible by the behavioral coaches for discussions within the one-on-one video chats. Tracker included four elements of ready (directly entering baseline week of step counts), set (setting a step count goal for the program), go (directly entering step counts weekly during the program), and see (graphical display of step counts for monitoring progress over the program compared with the goal). This was further supplemented with direct, on-screen feedback regarding progress with the behavioral goal on a weekly basis. We again received formative evaluation from representative persons with MS for refinement of the new tracker feature prior to implementation.
The website further included ‘voices’ or audio files of individual persons with MS discussing physical activity that overlaid loops of pictures of the narrator. The ‘voices’ changed weekly and were included for the purpose of engaging people with MS in social modeling as a source of self-efficacy information based on SCT. The Internet website further included an ongoing and seeded participant ‘forum’ for discussions of physical activity behavior change among participants (another form of vicarious experience). The website was supported by automated e-mail announcements about updates and tips of the week and included information on news (late-breaking research results) and events that further supplemented the website content.
One-on-one video chats
The one-on-one video chats supported adherence with the intervention, discussion and elaboration of website material, supportive accountability, and reporting adverse events/injuries; these further provided social persuasion for promoting behavior change. The video chats were modeled based on TeleCoach for maximizing adherence with Internet interventions, provided an ongoing interaction between the behavioral coach and the participant, and were conducted face to face through Skype. The video chats were semi-scripted and based on principles of supportive accountability. The video chats consisted of an ongoing review of goal setting and progress toward goal attainment through tracker as well as discussion of strategies and facilitators of behavioral change based on SCT and current website content. The chats occurred seven times during the first two months, four times during the second two months, and twice during the final two months of the intervention; this aligned with the accessibility of new interactive video courses. The video chats further allowed for inquiry and reporting on adverse and serious adverse events. The behavioral coaches were doctoral-level graduate students who had undertaken coursework in behavior change theory and training on the application of SCT for MS.
Compliance
Participant compliance with the behavioral intervention was recorded in two ways: (a) weekly step count upload (weekly compliance with the Internet website); and (b) video chat session attendance (compliance with the one-on-one video chats). Both measures were expressed as percentage compliance based on the number of weekly uploads completed or the number of sessions attended out of the total number of opportunities for uploads (24 possible uploads) or sessions offered (13 possible sessions).
Control condition
This study included a waitlist control condition for capturing the effects of repeated test administration and passage of time when examining the outcomes of our newly developed behavioral intervention. Such an approach was considered acceptable as we were evaluating a novel behavioral intervention approach based on modern e-learning, and this condition is considered reasonable for monitoring health/wellbeing of participants when compared with controls.30 Participants in the waitlist control condition were offered the behavioral intervention upon completition of the study.
Data analysis
All analyses were conducted using SPSS Statistics 24.0 (SPSS Inc., Chicago, IL, USA). Data were presented as mean score and standard deviation, unless otherwise noted. We conducted independent samples t-tests and chi-square difference tests to determine initial differences in pre-trial characteristics/outcomes between the conditions. The effect of the behavioral intervention on physical activity, symptomatic, and walking/disability outcomes was examined using analysis of covariance (ANCOVA) on post-trial scores with condition (intervention or control) as the between-subjects factor and pre-trial outcome scores as covariate. We expressed effect sizes for the F-statistic as partial eta squared (). The effect sizes were interpreted as small, moderate, and large based on guidelines of 0.01, 0.06, and 0.14, respectively.31
Results
Participants
Pre-trial characteristics of participants in the behavioral intervention and control conditions are presented in Table 1. Overall, the sample was middle-aged (51.9 years), primarily female (76%), and had a relapsing–remitting multiple sclerosis (RRMS) disease course (85%) with a mean disease duration of 13.2 years. The level of disability of the sample was moderate (median, interquartile range (IQR) PDDS 2.0, 3.0; median, IQR EDSS 3.5, 2.0) and most participants (85%) were on a disease-modifying therapy. There were no significant differences between groups in demographic/clinical characteristics, physical activity, symptomatic outcomes, and walking/disability outcomes pre-trial. Of the 47 participants randomly assigned into behavioral intervention and control conditions, 23 completed the behavioral intervention (100%) and 20 completed the control (83%).
Table 1.
Participant characteristics and tests for pre-trial differences between randomized groups.
| Variable | Intervention (n = 23) | Control (n = 24) |
|---|---|---|
| Demographic | ||
| Age, years | 52.3 (10.3) | 51.4 (7.4) |
| Sex, female/male | 21/2 | 19/5 |
| Height, cm | 164.9 (6.7) | 169.6 (7.5) |
| Weight, kg | 78.9 (19.3) | 83.4 (21.3) |
| Body mass index, kg/m2 | 29.1 (7.5) | 29.0 (7.4) |
| Clinical | ||
| PDDS, median (IQR) | 2.0 (4.0) | 2.0 (2.0) |
| EDSS, median (IQR) | 3.5 (1.5) | 3.5 (2.0) |
| Disease duration, years | 14.4 (10.4) | 12.1 (8.7) |
| Disease course, RRMS/SPMS/PPMS | 20/2/1 | 20/3/1 |
| DMT use, yes/no | 19/4 | 21/3 |
| Physical activity | ||
| GLTEQ health contribution score | 10.8 (14.0) | 12.7 (14.8) |
| MVPA, minutes | 13.9 (9.2) | 17.5 (18.3) |
| Symptoms | ||
| MFIS | 44.5 (14.2) | 42.7 (17.5) |
| FSS | 5.1 (1.0) | 4.9 (1.2) |
| HADS depression | 5.7 (3.3) | 5.7 (3.2) |
| HADS anxiety | 5.2 (3.7) | 5.5 (3.2) |
| SF-MPQ | 10.0 (9.8) | 10.9 (10.4) |
| Walking | ||
| MSWS-12 | 34.1 (29.7) | 33.6 (26.3) |
Values are means (SD) unless otherwise noted.
DMT: disease-modifying therapy; EDSS: Expanded Disability Status Scale; FSS: fatigue severity scale; GLTEQ: Godin leisure-time exercise questionnaire; HADS: hospital anxiety and depression scale; IQR: interquartile range; MFIS: modified fatigue impact scale; MSWS-12: multiple sclerosis walking scale-12; MVPA: moderate-to-vigorous physical activity; PDDS: patient-determined disease steps; PPMS: primary progressive multiple sclerosis; RRMS: relapsing–remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis; SF-MPQ: short-form McGill pain questionnaire.
Effects of the intervention
Physical activity
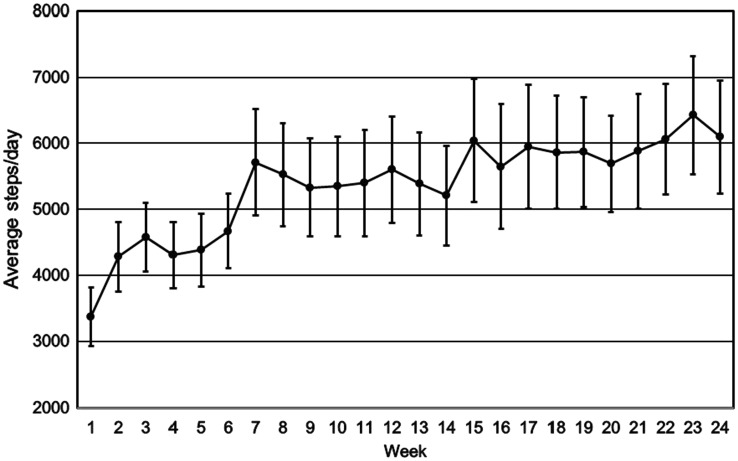
The means for weekly pedometer step counts over the six-month period for the behavioral intervention are provided in Figure 2. On average, participants took 3365 (2125) steps/day at the beginning of the intervention (week 1) and 6095 (4125) steps/day by the end of the six-month program (week 24); this represented an increase of 2730 steps/day over the six-month program (81% increase). The majority of the increase occurred early in the intervention (66% increase over the first 12 weeks), and physical activity levels were generally maintained, with a slight increase over the later part of the intervention (15% increase over the second 12 weeks).
Figure 2.
Average steps/day with standard error bars for the participants (N = 23) during the 24 weeks of the behavioral intervention.
Adjusted post-trial mean scores for physical activity controlling for pre-trial mean scores are provided in Table 2. Participants in the intervention group reported significantly higher GLTEQ HCS scores than the control group following the six-month period (F[1,39] = 4.10, P = 0.05, = 0.10). Regarding accelerometer-measured MVPA, participants in the intervention condition engaged in approximately eight more minutes of MVPA per day than controls following the six-month period, but the change did not reach statistical significance (F[1,39] = 1.40, P = 0.24, = 0.04).
Table 2.
Post-trial data from intervention and control conditions controlling for pre-trial outcome scores along with partial eta-squared value for condition effect.
| Outcome | Intervention (n = 23) | Control (n = 20) | |
|---|---|---|---|
| Physical activity | |||
| GLTEQ health contribution score | 27.2 (4.5) | 14.1 (4.7) | 0.10 |
| MVPA, minutes | 28.8 (5.0) | 20.2 (5.2) | 0.04 |
| Symptoms | |||
| MFIS total | 36.8 (2.2) | 44.7 (2.3) | 0.13 |
| MFIS physical | 16.1 (1.1) | 21.1 (1.2) | 0.20 |
| MFIS cognitive | 17.3 (1.1) | 19.5 (1.2) | 0.04 |
| MFIS psychosocial | 3.6 (0.2) | 4.0 (0.3) | 0.03 |
| FSS | 4.4 (0.2) | 4.9 (0.2) | 0.06 |
| HADS depression | 4.4 (0.5) | 5.7 (0.5) | 0.07 |
| HADS anxiety | 3.8 (0.6) | 5.4 (0.6) | 0.09 |
| SF-MPQ | 9.6 (1.0) | 10.1 (1.1) | 0.00 |
| Walking/mobility disability | |||
| MSWS-12 | 31.8 (2.5) | 39.3 (2.7) | 0.10 |
| EDSS | 3.7 (0.1) | 4.1 (0.1) | 0.11 |
| PDDS | 1.9 (0.2) | 2.4 (0.2) | 0.07 |
Values are adjusted means (standard error).
EDSS: Expanded Disability Status Scale; FSS: fatigue severity scale; GLTEQ: Godin leisure-time exercise questionnaire; HADS: hospital anxiety and depression scale; MFIS: modified fatigue impact scale; MSWS-12: multiple sclerosis walking scale-12; MVPA: moderate-to-vigorous physical activity; PDDS: patient-determined disease steps; SF-MPQ: short-form McGill pain questionnaire.
Symptoms and walking impairment/disability
Adjusted post-trial mean scores controlling for pre-trial mean score are presented in Table 2. Participants in the intervention group reported significantly lower overall MFIS scores than those in the control group following the six-month period (F[1,40] = 6.11, P = 0.018, = 0.13). Regarding MFIS subscales, the effect of the intervention was significant for the physical subscale (F[1,40] = 9.89, P = 0.003, = 0.20), but not cognitive (F[1,40] = 1.72, P = 0.20, = 0.04) or psychosocial (F[1,40] = 1.25, P = 0.27, = 0.03) subscales. Participants in the intervention group reported lower FSS scores than those in the control group following the six-month period, but the difference was not statistically significant (F[1,40] = 2.69, P = 0.10, = 0.06). Participants in the intervention condition reported lower HADS depression (F[1,40] = 2.90, P = 0.10, = 0.07) and anxiety (F[1,40] = 3.92, P = 0.06, = 0.09) scores than the control group after the six-month period, but neither change was statistically significant. There was no effect of the intervention on SF-MPQ scores (F[1,40] = 0.15, P = 0.70, = 0.00).
Participants in the intervention condition reported significantly lower MSWS-12 scores than those in the control condition after the six-month period (F[1,40] = 4.22, P = 0.047, = 0.10). There was a further statistically significant difference between groups in EDDS scores following the six-month period (F[1,39] = 4.91, P = 0.033, = 0.11) that favored reduced disability status in the intervention group. There was a non-significant difference between groups in PDDS scores following the study period (F[1,40] = 2.83, P = 0.10, = 0.07) that favored reduced self-reported disability status in the intervention group.
Compliance and adverse events
Overall compliance was excellent (97.4%). Compliance with uploading weekly step counts and attending video chat sessions was 99.1% and 95.7%, respectively. There were no adverse or serious adverse events.
Formative feedback
Table 3 contains data on the formative evaluation of the behavioral intervention. There was generally strong satisfaction for the overall program and its components, with the exception of the forums. Of the 19 persons providing feedback, 18 strongly agreed with recommending the program among other people with MS.
Table 3.
Formative evaluation of the behavioral intervention.
| Variable | Mean (SD) | No. of people reporting completely satisfied/strongly agree | % of people reporting completely satisfied/strongly agree |
|---|---|---|---|
| Satisfaction with the overall program | 4.7 (0.6) | 15 | 79 |
| Satisfaction with interactive video courses | 4.5 (0.6) | 11 | 58 |
| Satisfaction with tracker | 4.4 (0.8) | 12 | 63 |
| Satisfaction with voices | 4.4 (0.8) | 12 | 63 |
| Satisfaction with forums | 3.8 (1.0) | 6 | 32 |
| Satisfaction with chats | 4.5 (0.8) | 14 | 74 |
| Recommend program to others with MS | 4.9 (0.5) | 18 | 95 |
Nineteen of 23 participants who completed the behavioral intervention condition provided formative evaluation of the program and its components.
SD: standard deviation; MS: multiple sclerosis.
Discussion
This pilot RCT examined the efficacy of a newly developed Internet website based on modern e-learning approaches that delivered a SCT-based behavior intervention for increasing physical activity in persons with MS. There was evidence for a meaningful change in physical activity levels in the behavioral intervention condition. We observed a change in steps/day of 81% or 2730 steps/day by the end of the six-month program in the behavioral intervention. Those in the behavioral intervention further reported an increase in physical activity, based on the GLTEQ HCS, that was nearly 93% or 13.1 units greater than the control condition. The behavior intervention demonstrated an increase in objective physical activity, based on minutes per day of MVPA from accelerometry, that was nearly 43% or 8.6 minutes per day greater than the control condition. The mean post-trial values for the GLTEQ HCS (27.2) and minutes per day of MVPA (28.8 minutes per day) exceeded and nearly meet public health guidelines for physical activity of 24 units17 and 30 minutes per day of MVPA,4 respectively. The change of 2730 steps/day further exceeded guidelines for change in recommended levels of physical activity of 2500 steps/day.32
We examined changes in symptoms and walking impairment/disability with the behavioral intervention. The behavioral intervention yielded a significant reduction in fatigue impact, with a particularly large reduction in the physical impact of fatigue. There were further reductions in fatigue severity and the frequency of depression and anxiety symptoms after the behavioral intervention, although the changes only approached statistical significance. There was no evidence of change in pain symptoms, but there was a significant improvement in walking impairment and disability status; we did not examine intervention effects on functional system scores, as these were only collected for generating an overall EDSS score and not retained for data analysis, and therefore we cannot explain why the intervention yielded improvements in overall EDSS scores.
We were interested in the compliance data and formative feedback for this new e-learning Internet site and behavioral intervention. The data for overall compliance (97.4%) exceeded compliance (88.6%) in our previous RCT13 that had a less engaging, passive Internet intervention. Compliance data for uploading weekly step counts and attending video chat sessions were excellent, there were no adverse or serious adverse events, and all 23 participants assigned into the behavioral intervention completed the study. Regarding formative feedback, there was generally strong satisfaction for the overall program and its components, with the exception of the forums; this suggests that the forums might be redesigned or removed in subsequent RCTs of the behavioral intervention. Of the persons providing feedback, 95% strongly agreed with recommending the program among others with MS.
There are several important avenues for future research testing the efficacy of the behavioral intervention in a future, phase II RCT involving persons with MS. One avenue is monitoring mid-trial change in physical activity with self-report measures and accelerometry, as most of the change in steps/day occurred in the first 12 weeks. We further propose monitoring the long-term change in physical activity six or 12 months after completion of the program. We propose further monitoring secondary outcomes mid-trial for determining the time course of changes in symptoms and walking, and additionally consider long-term follow-up of the behavioral intervention for capturing sustainable effects. We see value in monitoring other outcomes such as comorbid conditions, as the change in physical activity may influence the risk for comorbid conditions.
There are several limitations of the current study. The sample consisted primarily of middle-aged women with RRMS who had mild-to-moderate disability. The sample was selected based, in part, on physical activity levels, but was not prescreened based on the presence of symptoms or dysfunction. The sample size was not determined based on a formal power analysis, but rather selected based on previous experience and general guidelines for sample sizes in pilot RCTs.33 This may have produced low statistical power that can reduce the likelihood that a statistically significant result reflects a true effect.34 The consequences might include an overestimation of effect sizes and/or low reproducibility of results.34 There was a large percentage of persons who underwent screening for eligibility, but did not participate in the RCT. This is not uncommon for pilot RCTs testing the efficacy of a new interventional approach for changing outcomes, as such designs often include tightly controlled inclusion/exclusion criteria. This does, however, limit the generalizability of our current approach and results for the general MS population, and the issue of generalizability can be addressed in a subsequent larger RCT examining intervention effectiveness wherein the inclusion/exclusion criteria are less stringent and permit an opportunity for understanding the broader impact of an interventional approach.
Overall, we provide evidence for the efficacy of an Internet-based behavioral intervention with content based on SCT and delivered through interactive video courses grounded in e-learning principles for increasing physical activity and possibly improving fatigue, depression, anxiety, and walking impairment/disability in persons with MS. This approach yielded a high level of compliance, complete adherence, no adverse events, and strong formative feedback and endorsement.
Conflicts of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was funded by a grant from the National Multiple Sclerosis Society (RG 5144A6/1).
References
- 1.Kinnett-Hopkins D, Adamson B, Rougeau K, et al. People with MS are less physically active than healthy controls but as active as those with other chronic diseases: an updated meta-analysis. Mult Scler Relat Disord 2017; 13: 38–43. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevalence of no leisure-time physical activity – 35 states and District of Columbia, 1988-2002. MMWR 2004; 53: 82–86. [PubMed] [Google Scholar]
- 3.Motl RW, McAuley E, Sandroff BM. Longitudinal change in physical activity and its correlates in relapsing-remitting multiple sclerosis. Phys Ther 2013; 93: 1037–1048. [DOI] [PubMed] [Google Scholar]
- 4.Klaren RE, Motl RW, Dlugonski D, et al. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil 2013; 94: 2342–2348. [DOI] [PubMed] [Google Scholar]
- 5.Lai B, Young HJ, Bickel CS, et al. Current trends in exercise intervention research, technology, and behavioral change strategies for people with disability: a scoping review. Am J Phys Med Rehabil 2017; in press. [DOI] [PubMed]
- 6.Motl RW, Sandroff BM. Benefits of exercise training in multiple sclerosis. Curr Neurol Neurosci Rep 2015; 15: 62. [DOI] [PubMed] [Google Scholar]
- 7.Bandura A. Health promotion by social cognitive means. Health Educ Behav 2004; 31: 143–164. [DOI] [PubMed] [Google Scholar]
- 8.Ellis T, Motl RW. Physical activity behavior change in persons with neurological disorders: overview and examples from Parkinson disease and multiple sclerosis. J Neurol Phys Ther 2013; 37: 85–90. [DOI] [PubMed] [Google Scholar]
- 9.Motl RW. Lifestyle physical activity in persons with multiple sclerosis: the new kid on the MS block. Mult Scler 2014; 20: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 10.Motl RW, Dlugonski D, Wójcicki TR, et al. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult Scler 2011; 17: 116–128. [DOI] [PubMed] [Google Scholar]
- 11.Dlugonski D, Motl RW, McAuley E. Increasing physical activity in multiple sclerosis: replicating Internet intervention effects using objective and self-report outcomes. J Rehabil Res Dev 2011; 48: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 12.Dlugonski D, Motl RW, Mohr DC, et al. Internet-delivered behavioral intervention to increase physical activity in persons with multiple sclerosis: sustainability and secondary outcomes. Psychol Health Med 2012; 17: 636–651. [DOI] [PubMed] [Google Scholar]
- 13.Pilutti LA, Dlugonski D, Sandroff BM, et al. Randomized controlled trial of a behavioral intervention targeting symptoms and physical activity in mutliple sclerosis. Mult Scler 2014; 20: 594–601. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire. Can J Sport Sci 1992; 17: 338–345. [PubMed] [Google Scholar]
- 16.Godin G, Shephard RJ. A simple method to assess exercise behaviour in the community. Can J Appl Sport Sci 1985; 10: 141–146. [PubMed] [Google Scholar]
- 17.Godin G. The Godin–Shephard leisure-time physical activity questionnaire. Health Fit J Can 2011; 4: 18–22. [Google Scholar]
- 18.Motl RW, Sandroff BM and Klaren RE. Validation of the health contribution score from the Godin Leisure-Time Exercise Questionnaire and its classification coding system using accelerometry in multiple sclerosis. Rehabil Psychol 2017; in press. [DOI] [PubMed]
- 19.Sandroff BM, Suh Y, Motl RW. Accelerometer output and its association with energy expenditure in persons with multiple sclerosis. J Rehabil Res Dev 2012; 49: 467–475. [DOI] [PubMed] [Google Scholar]
- 20.Motl RW, McAuley E, Klaren R. Reliability of physical activity measures over six months in adults with multiple sclerosis: Implications for designing behavioral interventions. Behav Med 2014; 40: 29–33. [DOI] [PubMed] [Google Scholar]
- 21.Ritvo PG, Fischer JS, Miller DM, et al. MSQLI: Multiple Sclerosis Quality of Life Inventory: a User’s Manual, New York, NY: National Multiple Sclerosis Society, 1997. [Google Scholar]
- 22.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 24.Melzack R. The short-form McGill Pain Questionnaire. Pain 1987; 30: 191–197. [DOI] [PubMed] [Google Scholar]
- 25.Hobart JC, Riazi A, Lamping DL, et al. Measuring the impact of MS on walking ability: the 12-item MS Walking Scale (MSWS-12). Neurology 2003; 60: 31–6. [DOI] [PubMed] [Google Scholar]
- 26.Hadjimichael O, Kerns RB, Rizzo MA, et al. Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain 2007; 127: 35–41. [DOI] [PubMed] [Google Scholar]
- 27.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 28.McAuley E, Courneya KS, Rudolph DL, et al. Enhancing exercise adherence in middle-aged males and females. Prev Med 1994; 23: 498–506. [DOI] [PubMed] [Google Scholar]
- 29.McAuley E, Motl RW, Morris KS, et al. Enhancing physical activity adherence and well-being in multiple sclerosis: a randomised controlled trial. Mult Scler 2007; 13: 652–659. [DOI] [PubMed] [Google Scholar]
- 30.Mohr DC, Spring B, Freedland KE, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother Psychosom 2009; 78: 275–284. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd edn. Hillsdale, NJ: Lawrence Earlbaum Associates, 1988.
- 32.Tudor-Locke C, Craig CL, Brown WJ, et al. How many steps/day are enough? for adults. Int J Behav Nutr Phys Act 2011; 8: 79.21798015 [Google Scholar]
- 33.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Statist 2005; 4: 287–291. [Google Scholar]
- 34.Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14: 365–376. [DOI] [PubMed] [Google Scholar]