Abstract
Segmentectomy has become a widely adopted surgical procedure, with recent reports describing the use of video-assisted thoracoscopic surgery (VATS) and robotic surgery. A major feature of segmentectomy is that it requires a three-dimensional (3D) understanding of the patient’s pulmonary structure and a thorough preoperative analysis of the patient’s individual anatomy. Here, we present our method for VATS segmentectomy based on 3D-computed tomography (3D-CT), with a review of the literature. We focus on techniques for 3D-CT reconstruction, analyses of the individual anatomy and anomalies, preoperative simulations of the procedure and surgical margin, and intraoperative navigation with 3D-CT images. We also reference the roles of members of our multi-disciplinary surgical team.
Keywords: Segmentectomy, video-assisted thoracoscopic surgery (VATS), three-dimensional-computed tomography (3D-CT)
Introduction
Lung segmentectomy has become a common surgical procedure in thoracic surgery. It is an alternative treatment for primary lung cancer, especially for small-sized tumors or for patients unfit to undergo a standard lobectomy and who thus require sublobar resection. Although segmentectomy can preserve lung function, its non-inferiority regarding the oncological outcome is still a matter of debate (1-4). Surgical approaches such as video-assisted thoracoscopic surgery (VATS) and robotic surgery have also been reported for segmentectomy (5-8). In addition to primary lung cancer, segmentectomy is performed for metastatic lung lesions arising from other malignancies such as colon cancer (9,10). Reports have also described the use of segmentectomy for the treatment of non-malignant diseases, including inflammatory pseudotumors, pulmonary arteriovenous fistulas, congenital bronchial atresia, intralobar pulmonary sequestration, and severe hemoptysis (11-15).
Compared with lobectomy, anatomical segmentectomy requires a thorough assessment of each patient’s thoracic anatomy, notably of the peripheral intersegmental veins. Thoracic surgeons thus must be familiar with the branching patterns of pulmonary vessels and bronchi. However, this remains a challenge because pulmonary anatomy has numerous variations and anomalies. Fortunately, advances in multidetector-row computed tomography (MDCT) and volume-rendering reconstruction software have allowed the reconstruction of three-dimensional (3D) images (16). 3D-CT imaging is a powerful tool for thoracic surgeons to determine pulmonary anatomy in a more intuitive manner when compared with conventional CT images. 3D-CT images help surgeons better understand the pulmonary anatomy of each patient before and during surgical procedures (17-21).
However, there are still technical and anatomical pitfalls that must be overcome to achieve a safe and precise segmentectomy. Here, we present our method for VATS segmentectomy based on 3D-CT angiography and bronchography (3D-CTAB), along with a review of the literature.
Patient selection and workup for 3D-CTAB reconstruction
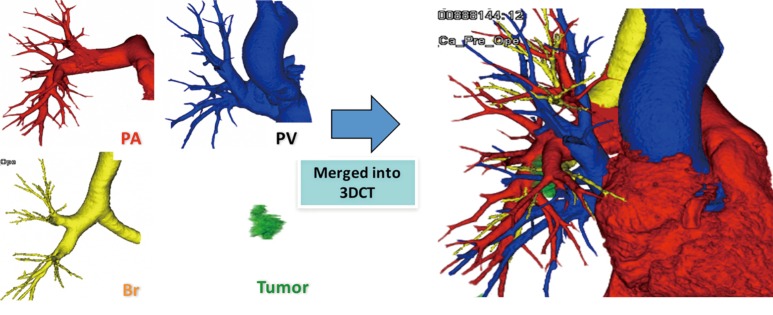
Our method for 3D-CTAB image reconstruction was previously reported (22). Briefly, we use 64-channel MDCT (SOMATOM Definition Flash; Siemens Healthcare, Berlin, Germany) for scanning. A total of 35 mL of contrast agent is mechanically injected at a rate of 5 mL/s, followed by the immediate injection of 20 mL of saline. Volume data from both the arterial and venous phases are transferred to a workstation running volume-rendering reconstruction software (Ziostation2; Ziosoft, Tokyo, Japan). The data are then converted into a 3D-CT angiographic format. The 3D reconstruction of the bronchi uses morphology-based two-dimensional segmentation of axial images, followed by the manual selection of segments. 3D images are first processed by radiology technicians and subsequently validated by thoracic surgeons (Figure 1).
Figure 1.
3D-CTAB image. Reconstructed images of arteries (red), veins (blue), bronchi (yellow), and tumor (green) are merged into a single 3D-CTAB image that can be observed from all directions. PA, pulmonary artery; PV, pulmonary vein; B, bronchus; 3D-CTAB, 3D-CT angiography and bronchography.
More than 95% of pulmonary arteries are correctly detected by 3D-CTAB [reported values: 98.7% (22), 95.2% (23), 95% (24), and 97.8% (25)]. Thus, the technique may be considered feasible. According to these studies, undetected pulmonary arteries were 1–2 mm in diameter (22-25).
Cases excluded from 3D-CTAB reconstruction are patients who were unable to undergo intravenous injection of a contrast agent (e.g., due to low renal function or because of an allergy to the substance). Furthermore, bronchi or vessels could not be clearly detected in some cases, due to obstruction by enlarged lymph nodes or centrally located tumors.
Preoperative preparation of anatomical segmentectomy by 3D-CTAB
We reconstruct 3D-CTAB images for all lung cancer patients who undergo segmentectomy in order to classify the patient’s anatomical pattern, verify any anatomical anomalies, plan the surgical procedure, and evaluate surgical margins. The anatomical patterns are diverse, and the spatial configuration of the arteries, veins, and bronchi makes the entire picture even more difficult to understand. 3D-CTAB allows a 360-degree view of lung anatomy, and leads to rapid and intuitive recognition of individual anatomical patterns (22,26,27). By recognizing the branching pattern and spatial configuration of a patient’s anatomy, 3D-CTAB allows the surgeon to plan anatomical segmentectomy preoperatively by deciding which intersegmental veins should be used as landmarks, which artery/vein/bronchi should be dissected, and in what order. It is also valuable when evaluating surgical margins and planning surgery for impalpable or centrally located tumors.
Classification of anatomy and planning of the procedure
The bronchovascular anatomy of the lung can be classified into several patterns (28,29). For example, the pulmonary arteries of the right upper lobe (RUL) are classified into four patterns based on the combination of trunks superior, trunks inferior, and ascending artery. Based on an analysis of 3D-CTAB images, we previously reported that the most common type was the “trunks superior + ascending artery” type, seen in 71.9% of cases (22). Next, the pulmonary veins of the RUL are classified into four patterns, based on the combination of anterior and central veins. The most commonly reported type was the “anterior with central (Iab)” type, seen in 54% of cases. The remaining “anterior with central (Ib)” type, “anterior” type, and “central” type, were seen in 26%, 12%, and 7% of cases, respectively (22,26). Lastly, the bronchi are broadly classified into four patterns, based on whether they are bifurcated, trifurcated, quadrivial, or have a defect in the B1 bronchi. The most common type in our previous study was the trifurcated type, seen in 44.1% of cases (22).
When planning anatomical segmentectomy, such arteries, veins, and bronchi must be preoperatively assessed because the procedure for anatomical segmentectomy differs according to the branching pattern. In particular, veins should be analyzed up to more peripheral branches; that is, intersegmental veins (V1b, V2a, and V2c for the RUL). Intersegmental veins are landmarks during segmentectomy because they indicate the boundaries of the targeted lung segment. Previous reports have described the benefits of 3D angiography for recognizing these intersegmental veins (17,30). For example, when planning S2 or S3 segmentectomy, V2c is the segmental vein separating S2 and S3. For patients with an “anterior with central” or “central” type pattern, V2c drains into the central vein; thus, the central vein should be denuded from the hilum to identify V2c. On the other hand, for patients with an “anterior” type pattern, V2c drains into the anterior vein; thus, the anterior vein should be denuded to identify V2c. The surgeon should especially recognize the branching pattern of these intersegmental veins and identify which vein should be used as a landmark and at what level the veins should be dissected. We previously proposed a simplified vein model of the RUL to aid surgeons in planning RUL segmentectomy (26).
The approach itself is also different between upper lobe and lower lobe segmentectomy (17,31). Briefly, for upper lobe segments (and the S6 segment) we usually determine the veins first, dissect the segmental artery next, and then dissect the segmental bronchus after jet ventilation. On the other hand, for lower lobe segments, the branching pattern of veins is quite diverse so we dissect the segmental artery first, dissect the bronchus next, and then dissect the intersegmental plane and remaining veins.
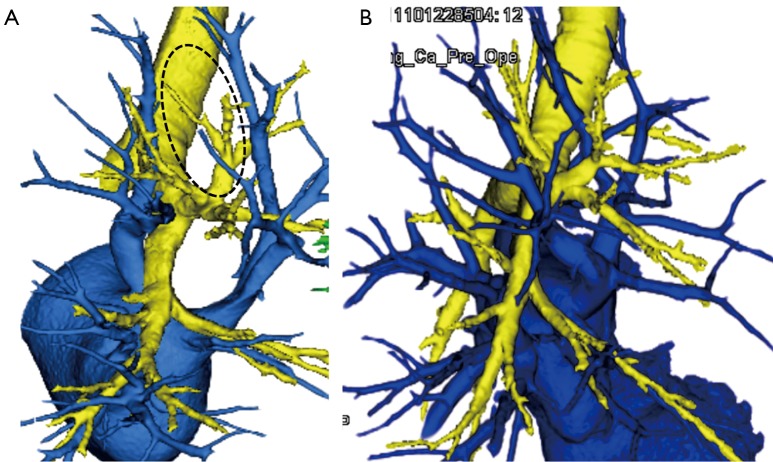
Preoperative 3D-CTAB is also useful for detecting anatomical patterns that are unfit for anatomical segmentectomy. For example, anatomical segmentectomy of RUL segments using intersegmental veins as landmarks is not recommended when the intersegmental V2a vein is defective (Figure 2A) or when the intersegmental veins run across segments and do not clearly delineate borders between the upper lobe segments (Figure 2B).
Figure 2.
Identification of cases unfit for anatomical segmentectomy. (A) A case with no intersegmental vein between S1 and S3 (the dotted area shows a defect in V2a); (B) a case with complex intersegmental veins that cross over segments and do not mark the boundaries of segments.
Evaluation of the surgical margin
3D-CTAB is also used to assess the surgical margin between the tumor and planned resection line. We measure the distance between the edge of the tumor and intersegmental veins using conventional and 3D-CT images (17). A sufficient surgical margin is essential to prevent loco-regional recurrence, especially for limited resections such as segmentectomy. Previous reports have described the utility of the preoperative measurement of a “virtual safety margin” with 3D-CT (32,33).
When treating primary lung cancer, segmentectomy can usually be selected when enough surgical margin can be secured. If the surgical margin is insufficient we change the surgical plan from single segmentectomy to another procedure (e.g., segmentectomy + subsegmentectomy, subsegmentectomy + subsegmentectomy, or lobectomy). For metastatic lung tumors, we usually set up a surgical margin of at least 1 cm. There may be exceptions for centrally located metastatic tumors, especially in small segments such as S7, because we cannot secure 2 cm of lung parenchyma between the tumor and hilum. In such a case, we denude and “skeletonize” the vessels and bronchi that constitute the borders of the resection plane. This method may also be applied to pure ground-glass nodules, which supposedly do not have an invasive component.
Technically, the surgical margin measured by conventional CT or 3D-CTAB may differ from the actual surgical margin of the resected lung because the former is measured in an inflated state while the latter is measured in a deflated state. Indeed, when we compare the actual surgical margin on the lung after resection with that planned by conventional CT and 3D-CT images, we experience up to 5 mm of difference. Therefore, to secure a postoperative surgical margin of 2 cm, we prefer to set up the planned surgical margin to more than 2.5 cm.
Another benefit of planning segmentectomy with 3D-CTAB is its utility for small impalpable and centrally located tumors. If we can preoperatively confirm by 3D-CTAB that the tumor is confined to a single segment with adequate surgical margins, we can theoretically and confidently resect the lesion by performing anatomical segmentectomy, using intersegmental veins as landmarks.
Procedure: anatomical segmentectomy guided by 3D-CTAB
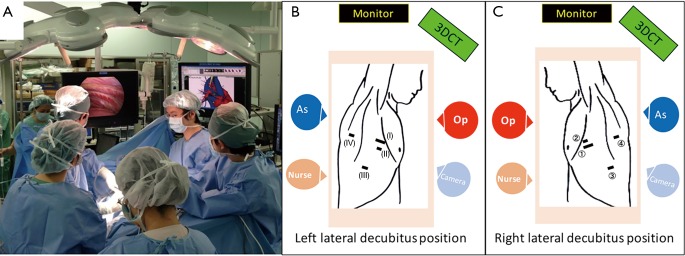
The intraoperative identification of veins, arteries, and bronchi is imperative for safe anatomical segmentectomy. 3D-CTAB allows the spatial presentation of vessels and bronchi, and it guides us through the surgical procedure. Spatial recognition is especially useful for atypical or “difficult” segmentectomies (20). We always place the 3D-CTAB image next to the thoracoscopic monitor during surgery and constantly confirm that the actual anatomy correlates with the 3D-CTAB images (Figure 3). Intraoperative discussion between surgeons can easily take place whenever there is a possible misconception of the anatomy.
Figure 3.
Arrangement of the surgical team and equipment. Intraoperative photo (A) and scheme (B) of right-sided surgery. The patient is placed in a left-lateral decubitus position. The main surgeon stands at the ventral side, the assistant with the thoracoscope at the caudal side, and the main assistant at the dorsal side. 3D-CTAB images are positioned next to the main monitor. Four ports are used: (I) a 2- to 3-cm access port at the 4th or 5th ICS of the anterior axillary line for the operator’s right hand, (II) a 5-mm port at the 5th or 6th ICS of the anterior axillary line for the operator’s left hand, (III) a 10-mm camera port at the 7th ICS of the middle axillary line, and (IV) a 1- to 1.5-cm access port at the 6th ICS of the posterior axillary line for the assistant. (C) For left-sided surgery, the patient is placed in a right-lateral decubitus position and the operator’s port-positions are reversed (the access port at the 5th or 6th ICS and the 5-mm port at the 4th or 5th ICS). As, assistant; Op, operator; ICS, intercostal space; 3D-CTAB, 3D-CT angiography and bronchography.
The key steps of anatomical segmentectomy for the upper lobe are as follows: we first identify the central part of the intersegmental plane by denuding the appropriate intersegmental veins towards the periphery, we next identify the peripheral part of the intersegmental plane on the surface of the lung by creating a demarcation line and dissect the intersegmental plane towards the hilum, and we dissect the remaining lung parenchyma and complete dissection of the intersegmental plane (VATS right S3 anatomical segmentectomy, Figure 4).
Figure 4.

VATS right S3 anatomical segmentectomy (34). Presentation of S3 segmentectomy. The branching pattern was “anterior with central (Ib)” type. First, we identify the branches of the superior pulmonary vein. We denude the central vein and identify the intersegmental V2c vein. We then denude the anterior vein and identify the intersegmental V1b vein. We next dissect the segmental A3 artery, selectively inflate S3 via B3, and create the demarcation line. We dissect the central part and then the peripheral part of the intersegmental plane. We dissect the remaining lung parenchyma by using bipolar sealing system and finally extract the S3 segment. VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/1571
Identification of the central part of the intersegmental plane
Identification and dissection of the central part of the intersegmental plane is achieved by dissecting the lung parenchyma along intersegmental veins. Intraoperative 3D-CTAB navigation is used to identify these intersegmental veins that form the boundaries of segments. Because the branching pattern of pulmonary veins is diverse (22,26), precise planning and intraoperative navigation of the pulmonary vessels that require denuding is essential.
Interestingly, the introduction of VATS surgery and advances in the quality of thoracoscopes have led to high-definition and magnified images of anatomical structures. We have noticed during VATS segmentectomy that intersegmental planes can be visualized when denuding intersegmental veins at the hilum (Figure 5).
Figure 5.
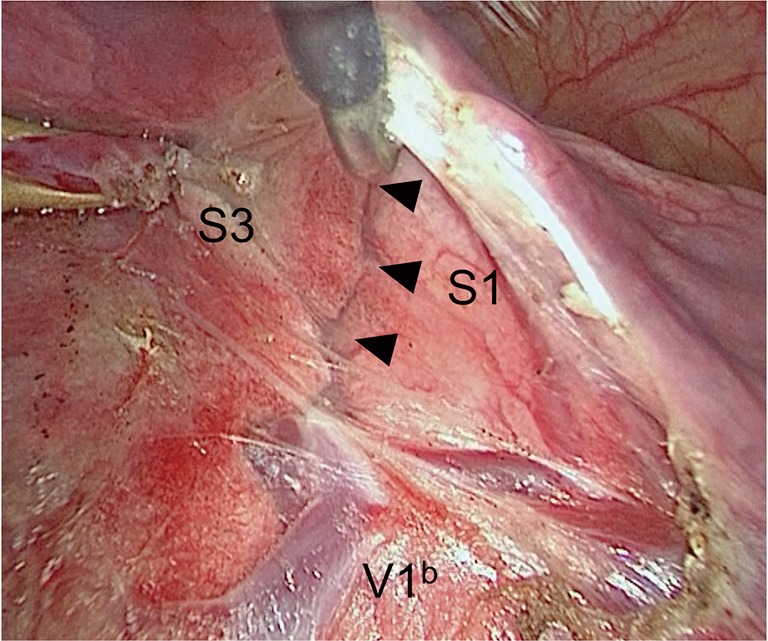
Identification of the central part of the intersegmental plane. V1b is the intersegmental vein running along the intersegmental plane between S1 and S3. The intersegmental plane can be visualized (arrowheads along the intersegmental plane between S1 and S3) when denuding the intersegmental veins at the hilum in a peripheral direction.
Identification of the peripheral part of the intersegmental plane
Recognizing the intersegmental plane is an important step in anatomical segmentectomy, and several techniques have been established. The most common method is to create a demarcation line between the inflated and deflated lung, a technique that we have adopted (Figure 6). Initially, a demarcation line was created by inflating the preserved lung after resecting the bronchus of the targeted segment, which remains deflated. Tsubota reported a different method, where the targeted segment was kept inflated by tying the segmental bronchus after inflation and trapping the air within (35). Okada et al. further improved the method by applying bronchofiberscopic jet ventilation to the selected segment (36). Other techniques have been proposed to inflate or keep the targeted segment inflated, including direct inflation by inserting a butterfly needle into the segmental bronchus (37), or ligation of the segmental bronchus with a slip-knot after inflation (38). For all methods, the branching pattern of the segmental bronchus has to be correctly recognized (22).
Figure 6.
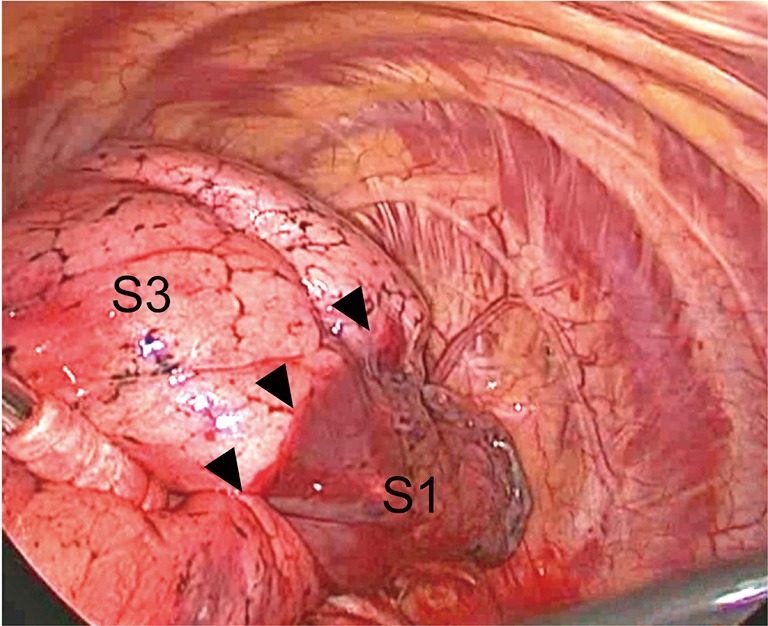
Identification of the peripheral part of the intersegmental plane. The targeted segment (S3) is inflated with bronchofiberscopic jet ventilation, which creates a demarcation line between segments (arrowheads along the intersegmental plane between S1 and S3). The bronchus (B3) is immediately resected and sealed after inflation.
Additional methods using thermography or a combination of indocyanine green (ICG) and infrared thoracoscopy have been developed (39-44). Ligation of the segmental artery followed by an intravenous injection of ICG creates a demarcation line at the surface of the pleura, leaving only the targeted segment unstained (40-42). Inversely, an injection of ICG to the segmental bronchus followed by ligation of the segmental vein creates a demarcation line by keeping the targeted segment stained. Transbronchial injection of ICG allows identification of the intersegmental plane not only at the surface but also within the lung parenchyma (43,44). For either method, the segmental artery or segmental artery/bronchus/vein must be correctly recognized intraoperatively.
Dissection of the intersegmental plane
The optimal method for dissecting intersegmental planes remains a matter of debate. Current options include electrocautery, staplers, or electrothermal bipolar sealing system. The effect of each technique on bleeding and air leakage from the intersegmental plane, or the impact on postoperative complications and respiratory functions, has previously been discussed (45-48). We usually use a combination of both electrocautery and staplers, with the use of conventional electrocautery when dissecting the peripheral intersegmental plane, a hook-type electrocautery device for central denudation of intersegmental vessels, and a bipolar sealing system or staplers for dissecting the remaining lung parenchyma.
Precise dissection of the peripheral part of the intersegmental plane can be achieved by exerting adequate traction to the intersegmental plane. We create a 3D plane with the demarcation line at its center, which we call the “three-point support” dissection method (Figure 7). To avoid postoperative air leakage, additional coagulation of the intersegmental plane is performed with ball electrodes at soft coagulation mode (80 watt, VIO300D, ERBE Elektromedizin GmbH, Tübingen, Germany), and the dissected plane is routinely covered with polyglycolic acid (sheet-type Neoveil®, Gunze, Kyoto, Japan) and a fibrin sealant (Beriplast® P, CSL Behring Pharma, Tokyo, Japan). Patients with heavy emphysematous or interstitial changes, or with unclear demarcation lines, may be inadequate for the “three-point support” dissection method because correct dissection of the intersegmental plane is difficult and may be prone to postoperative air leakage. In such cases, we may consider the use of staplers to dissect the intersegmental planes.
Figure 7.

Intersegmental plane dissection (49). Dissection of the S1–S3 intersegmental plane by using “three-point support” dissection method. The assistant uses two thoracoscopic forceps (or one forceps and one lung forceps) and the surgeon, with his left hand, uses one thoracoscopic forceps to create a 3D plane. Dissection of the visceral pleura by electrocautery will lead to a natural dissociation of lung parenchyma at the intersegmental plane, and only slight electrocauterization will suffice to dissect and stop hemorrhage from the intersegmental plane. Available online: http://www.asvide.com/articles/1572
Role of members of our multi-disciplinary team
In anatomical segmentectomy guided by 3D-CTAB, the key members of the multi-disciplinary team includes the radiology technician, assistant surgeon, assistant holding the thoracoscope (usually a junior surgeon in training), and anesthesiologist. The role of the radiology technician is essential for adequate CT scanning and the processing of initial 3D-CTAB images. The two assistants participating in the anatomical segmentectomy will check the 3D-CTAB images along with the main surgeon and discuss the surgical procedure before surgery. 3D-CT has the additional benefits of educating junior surgeons, enabling them to experience the virtual operative view seen through a thoracoscope, and allowing the simulation of virtual surgical procedures before surgery (50). The role of the anesthesiologist is to inflate the targeted segment selectively using bronchofiberscopic jet ventilation, thus creating a demarcation line. Surgeons also verify from the surgical field that the bronchofiber is inserted into the appropriate bronchus before it is inflated by the anesthesiologist.
Conclusions
Recognition of anatomy based on 3D-CTAB is essential for safe and precise anatomical segmentectomy. It allows the surgeon to be guided in all steps of the procedure: (I) classification of individual anatomical patterns; (II) identification of anomalies; (III) identification of unsuitable cases for segmentectomy; (IV) preoperative simulation of surgical steps; and (V) intraoperative navigation for veins, arteries, and bronchi.
Although access to 3D-CTAB images in all patients undergoing VATS segmentectomy would be ideal, we believe that even if surgeons only have access to conventional CT images, knowledge of anatomical patterns and a repertoire of 3D-CTAB images can help surgeons classify the patient in front of them into a specific category, and thus enable the procedure to be simulated.
Acknowledgements
We thank the radiology physicians Yasuhiro Fukushima, Junya Fukuda, and Hiroyuki Takei for their reconstruction of the three-dimensional images. We also thank Dr. Toshiki Yajima, Dr. Takayuki Kosaka, Dr. Yoichi Ohtaki, Dr. Kai Obayashi, Dr. Yoko Azuma and Dr. Misaki Iijima for critically reading the manuscript.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60; discussion 61. 10.1016/S0003-4975(00)02223-2 [DOI] [PubMed] [Google Scholar]
- 2.Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. 10.1016/j.jtcvs.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 3.Ohtaki Y, Shimizu K. Anatomical thoracoscopic segmentectomy for lung cancer. Gen Thorac Cardiovasc Surg 2014;62:586-93. 10.1007/s11748-014-0409-7 [DOI] [PubMed] [Google Scholar]
- 4.Okada M, Mimae T, Tsutani Y, et al. Segmentectomy versus lobectomy for clinical stage IA lung adenocarcinoma. Ann Cardiothorac Surg 2014;3:153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C-FJ, D'Amico TA. Open, thoracoscopic and robotic segmentectomy for lung cancer. Ann Cardiothorac Surg 2014;3:142-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linden D, Linden K, Oparka J. In patients with resectable non-small-cell lung cancer, is video-assisted thoracoscopic segmentectomy a suitable alternative to thoracotomy and segmentectomy in terms of morbidity and equivalence of resection? Interact Cardiovasc Thorac Surg 2014;19:107-10. 10.1093/icvts/ivu080 [DOI] [PubMed] [Google Scholar]
- 7.Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72; discussion 72. 10.1016/j.athoracsur.2015.06.112 [DOI] [PubMed] [Google Scholar]
- 8.Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiono S, Okumura T, Boku N, et al. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg. 2016 doi: 10.1093/ejcts/ezw322. [DOI] [PubMed] [Google Scholar]
- 10.Berry MF. Role of segmentectomy for pulmonary metastases. Ann Cardiothorac Surg 2014;3:176-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melloni G, Carretta A, Ciriaco P, et al. Inflammatory pseudotumor of the lung in adults. Ann Thorac Surg 2005;79:426-32. 10.1016/j.athoracsur.2004.07.077 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura H, Miwa K, Haruki T, et al. Pulmonary arteriovenous fistula with cerebral infarction successfully treated by video-assisted thoracic surgery. Ann Thorac Cardiovasc Surg 2008;14:35-7. [PubMed] [Google Scholar]
- 13.Igai H, Kamiyoshihara M, Nagashima T, et al. Anatomical segmentectomy for pneumothorax associated with congenital bronchial atresia. Eur J Cardiothorac Surg 2013;43:198. 10.1093/ejcts/ezs377 [DOI] [PubMed] [Google Scholar]
- 14.Inoue T, Oizumi H, Nakamura M, et al. Port-access thoracoscopic anatomical segmentectomy for pediatric intralobar pulmonary sequestration. Thorac Cardiovasc Surg Rep 2014;3:42-4. 10.1055/s-0034-1377065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiral H, Evman S, Tezel C, et al. Pulmonary resection in the treatment of life-threatening hemoptysis. Ann Thorac Cardiovasc Surg 2015;21:125-31. 10.5761/atcs.oa.14-00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin GD. 3-D imaging with MDCT. Eur J Radiol 2003;45 Suppl 1:S37-41. 10.1016/S0720-048X(03)00035-4 [DOI] [PubMed] [Google Scholar]
- 17.Shimizu K, Nakano T, Kamiyoshihara M, et al. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact Cardiovasc Thorac Surg 2012;15:194-6. 10.1093/icvts/ivs202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda N, Yoshimura A, Hagiwara M, et al. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg 2013;19:1-5. 10.5761/atcs.ra.12.02174 [DOI] [PubMed] [Google Scholar]
- 19.Akiba T. Utility of three-dimensional computed tomography in general thoracic surgery. Gen Thorac Cardiovasc Surg 2013;61:676-84. 10.1007/s11748-013-0336-z [DOI] [PubMed] [Google Scholar]
- 20.Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. 10.1016/j.jtcvs.2010.08.027 [DOI] [PubMed] [Google Scholar]
- 21.Chen-Yoshikawa TF, Date H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J Thorac Dis 2016;8:S295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagashima T, Shimizu K, Ohtaki Y, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2015;63:354-60. 10.1007/s11748-015-0531-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuhara K, Akashi A, Nakane S, et al. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg 2008;34:875-7. 10.1016/j.ejcts.2008.07.014 [DOI] [PubMed] [Google Scholar]
- 24.Akiba T, Marushima H, Harada J, et al. Importance of preoperative imaging with 64-row three-dimensional multidetector computed tomography for safer video-assisted thoracic surgery in lung cancer. Surg Today 2009;39:844-7. 10.1007/s00595-009-3965-1 [DOI] [PubMed] [Google Scholar]
- 25.Hagiwara M, Shimada Y, Kato Y, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery†. Eur J Cardiothorac Surg 2014;46:e120-6. 10.1093/ejcts/ezu375 [DOI] [PubMed] [Google Scholar]
- 26.Shimizu K, Nagashima T, Ohtaki Y, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg 2016;64:604-11. 10.1007/s11748-016-0686-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2017. [Epub ahead of print]. 10.1007/s11748-017-0754-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyden EA, Scannell JG. An analysis of variations in the bronchovascular pattern of the right upper lobe of 50 lungs. Am J Anat 1948;82:27-73. 10.1002/aja.1000820103 [DOI] [PubMed] [Google Scholar]
- 29.Yamashita H. Variations in the pulmonary segments and the bronchovascular trees. In: Yamashita H. editor. Roentgenologic anatomy of the lung. Tokyo: Igakushoin, 1978:70-107. [Google Scholar]
- 30.Oizumi H, Endoh M, Takeda SI, et al. Anatomical lung segmentectomy simulated by computed tomographic angiography. Ann Thorac Surg 2010;90:1382-3. 10.1016/j.athoracsur.2009.11.062 [DOI] [PubMed] [Google Scholar]
- 31.Nomori H, Okada M. Illustrated anatomical segmentectomy for lung cancer. Springer, 2012. [Google Scholar]
- 32.Ueda K, Tanaka T, Hayashi M, et al. What proportion of lung cancers can be operated by segmentectomy? A computed-tomography-based simulation. Eur J Cardiothorac Surg 2012;41:341-5. 10.1016/j.ejcts.2011.05.034 [DOI] [PubMed] [Google Scholar]
- 33.Iwano S, Yokoi K, Taniguchi T, et al. Planning of segmentectomy using three-dimensional computed tomography angiography with a virtual safety margin: technique and initial experience. Lung Cancer 2013;81:410-5. 10.1016/j.lungcan.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 34.Shimizu K, Nakazawa S, Nagashima T, et al. VATS right S3 anatomical segmentectomy. Asvide 2017;4:262. Available online: http://www.asvide.com/articles/1571
- 35.Tsubota N. An improved method for distinguishing the intersegmental plane of the lung. Surg Today 2000;30:963-4. 10.1007/s005950070056 [DOI] [PubMed] [Google Scholar]
- 36.Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. 10.1016/j.jtcvs.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 37.Kamiyoshihara M, Kakegawa S, Morishita Y. Convenient and improved method to distinguish the intersegmental plane in pulmonary segmentectomy using a butterfly needle. Ann Thorac Surg 2007;83:1913-4. 10.1016/j.athoracsur.2006.06.052 [DOI] [PubMed] [Google Scholar]
- 38.Oizumi H, Kato H, Endoh M, et al. Techniques to define segmental anatomy during segmentectomy. Ann Cardiothorac Surg 2014;3:170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakamoto K, Kanzaki M, Mitsuboshi S, et al. A novel and simple method for identifying the lung intersegmental plane using thermography. Interact Cardiovasc Thorac Surg 2016;23:171-3. 10.1093/icvts/ivw076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. 10.1016/j.jtcvs.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 41.Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. 10.1016/j.jtcvs.2010.07.020 [DOI] [PubMed] [Google Scholar]
- 42.Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. 10.1093/ejcts/ezt565 [DOI] [PubMed] [Google Scholar]
- 43.Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. 10.1016/j.jtcvs.2012.01.079 [DOI] [PubMed] [Google Scholar]
- 44.Oh S, Suzuki K, Miyasaka Y, et al. New technique for lung segmentectomy using indocyanine green injection. Ann Thorac Surg 2013;95:2188-90. 10.1016/j.athoracsur.2012.12.068 [DOI] [PubMed] [Google Scholar]
- 45.Miyasaka Y, Oh S, Takahashi N, et al. Postoperative complications and respiratory function following segmentectomy of the lung - comparison of the methods of making an inter-segmental plane. Interact Cardiovasc Thorac Surg 2011;12:426-9. 10.1510/icvts.2010.253989 [DOI] [PubMed] [Google Scholar]
- 46.Ohtsuka T, Goto T, Anraku M, et al. Dissection of lung parenchyma using electrocautery is a safe and acceptable method for anatomical sublobar resection. J Cardiothorac Surg 2012;7:42. 10.1186/1749-8090-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao H, Tanaka T, Hayashi T, et al. Influence of stapling the intersegmental planes on lung volume and function after segmentectomy. Interact Cardiovasc Thorac Surg 2016;23:548-52. 10.1093/icvts/ivw188 [DOI] [PubMed] [Google Scholar]
- 48.Kuroda H, Dejima H, Mizumo T, et al. A new LigaSure technique for the formation of segmental plane by intravenous indocyanine green fluorescence during thoracoscopic anatomical segmentectomy. J Thorac Dis 2016;8:1210-6. 10.21037/jtd.2016.04.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimizu K, Nakazawa S, Nagashima T, et al. Intersegmental plane dissection. Asvide 2017;4:263. Available online: http://www.asvide.com/articles/1572
- 50.Solomon B, Bizekis C, Dellis SL, et al. Simulating video-assisted thoracoscopic lobectomy: a virtual reality cognitive task simulation. J Thorac Cardiovasc Surg 2011;141:249-55. 10.1016/j.jtcvs.2010.09.014 [DOI] [PubMed] [Google Scholar]