Abstract
Background
To determine the association between extreme values of first trimester markers and adverse pregnancy outcomes.
Methods
A retrospective cohort study of 916 women who underwent first-trimester combined screening during 2015 was performed. Extreme values of NT, pregnancy-associated plasma protein-A (PAPP-A) and free β-hCG, and their association with adverse pregnancy outcomes were analyzed.
Results
Low PAPP-A (<10th percentile) was associated with an increased risk for preeclampsia (adjusted odds ratio (AOR) 4.13), fetal growth restriction (AOR 3.94) and abruptio placentae (AOR 52.63). Abnormally low or high free β-hCG, high PAPP-A or increased NT was not associated with an increased risk for adverse outcomes.
Discussion
PAPP-A <10th percentile could be associated with an increased risk for adverse outcomes. However, the majority of patients with these events do not have abnormal PAPP-A and few patients with PAPP-A <10th percentile will have an adverse outcome.
Keywords: Abruptio placentae, fetal growth, human chorionic-gonadotrophin beta-subunit, nuchal translucency, pregnancy-associated plasma protein-A, preeclampsia
Introduction
Maternal serum markers obtained in first trimester Down syndrome screening include pregnancy-associated plasma protein-A (PAPP-A) and free beta-human chorionic gonadotropin (free β-hCG). The combined test (sonographic measurement of fetal nuchal translucency (NT) and maternal serum markers) detects 85 percent of Down syndrome fetuses at a five percent false positive rate.1,2 Although the primary goal of this screening is to detect these disorders, recent studies describe the association between individual extreme levels of Down syndrome screening markers and adverse pregnancy outcomes, including preterm birth, fetal growth disorders, hypertensive disorders and spontaneous abortion.3–15 The aim of this study was to determine the association and predictive ability of extreme values of PAPP-A, free β-hCG and NT with adverse pregnancy outcomes.
Methods
A retrospective cohort study was performed including women with a singleton gestation who underwent first-trimester screening for aneuploidy between 11 weeks 0 days and 13 weeks 6 days of gestation (fetal crown-rump length between 45 and 84 mm) at Alto Minho Local Healthcare Unit, Viana do Castelo, Portugal, during 2015. Women who had a fetal demise before 22 weeks of gestation or whose fetuses were confirmed to have chromosome abnormalities or other malformation were excluded. Likewise, records with incomplete outcome data were not included. The three first-trimester markers PAPP-A level, free β-hCG and NT were evaluated. Values for the two serum markers were obtained by a certificated laboratory and NT measurements were obtained by a maternal fetal medicine specialist with Fetal Medicine Foundation certification. Information on maternal sociodemographics, medical and obstetrical history, gestational age at examination, pregnancy course and delivery outcome were collected. In a primary analysis, extreme values of NT (≥2.5 mm), PAPP-A (<1st, 5th and 10th percentiles, >90th, 95th and 99th percentiles) and free β-hCG (<1st, 5th and 10th percentiles, >90th, 95th and 99th percentiles) were evaluated, as well as their association with adverse pregnancy outcomes. Percentiles were defined using FASTER study6 (Table 1). In a second analysis, women with a PAPP-A <10th percentile multiples of median (MoM) were compared to those with a PAPP-A ≥10th percentile. In the final adjusted models, some variables were considered to be confounders for preeclampsia, namely, maternal age, parity and chronic hypertension. Gestational hypertension, chronic hypertension, pregestational diabetes and preeclampsia were considered for fetal growth restriction; and maternal age, parity, gestational hypertension and chronic hypertension, gestational age at delivery and infant sex were considered for abruptio placentae. Performance characteristics, which included the adjusted odds ratios (AOR) and 95% confidence interval (CI), sensitivity, specificity and positive predictive value (PPV) and negative predictive value (NPV) for PAPP-A were estimated for each outcome.
Table 1.
Relationship between analytic percentiles and multiples of the median (FASTER trial).
| Percentile | PAPP-A (MoM) | Free β-hCG (MoM) |
|---|---|---|
| 1st | 0.24 | |
| 5th | 0.35 | |
| 10th | 0.44 | |
| 90th | 2.94 | |
| 95th | 3.91 | |
| 99th | 6.56 |
MoM: multiples of median; β-hCG: free beta-human chorionic gonadotropin; PAPP-A: pregnancy-associated plasma protein-A.
Outcome measurements
The outcome measurements examined included preterm birth, gestational hypertension, preeclampsia, third trimester fetal growth restriction, low birth weight, preterm premature rupture of membranes (PPROM), abruptio placentae, gestational diabetes, macrosomia and oligohydramnios. Preterm birth was defined, according to World Health Organization, as a delivery occurring before 37 weeks of pregnancy. Gestational hypertension was defined as new onset of hypertension (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg) at ≥20 weeks of gestation, in the absence of proteinuria or new signs of end-organ dysfunction. The blood pressure readings were documented on at least two occasions at least four hours apart, according to the American College of Obstetricians and Gynecologists (ACOG). Preeclampsia was defined as new onset of hypertension and either proteinuria or end-organ dysfunction after 20 weeks of gestation. Criteria for diagnosis were: systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, and proteinuria ≥0.3 g in a 24 h urine specimen or protein:creatinine ratio ≥0.3, or signs of end-organ dysfunction (platelet count <100,000/µL, serum creatinine >1.1 mg/dL or doubling of the serum creatinine, elevated serum transaminases to twice normal concentration), according to ACOG. Third trimester fetal growth restriction was defined as a fetus whose estimated weight was below the 10th percentile for gestational age, calculated by ASTRAIA software, after 27 weeks 0 days of gestation. Low birth weight was defined as a birth weight of a liveborn of less than 2500 g, regardless of gestational age, according to ACOG. PPROM was defined as the rupture of the membranes before the onset of labor until 37 weeks of gestation. Abruptio placentae was defined as bleeding at the decidual-placental interface that causes partial or complete placental detachment prior to delivery of the fetus. The diagnosis was primarily clinical and confirmed by ultrasound or intra-operatory visualization of placental detachment. Gestational diabetes was diagnosed if fasting glucose level ≥92 and <126 mg/dL at first trimester or at least one value of plasma glucose concentration ≥92, 180 and 153 mg/dL (for fasting, 1- and 2-h post glucose load glucose values, respectively), after performing a 75 g oral glucose tolerance test at 24–28 weeks of gestation.16 Macrosomia was defined as a birth weight greater than 4500 g. Oligohydramnios was defined as amniotic fluid index ≤5 cm or the absence of a single deepest pocket <2 × 1 cm2.17
Statistical analysis
Continuous variables were described using median and interquartile ranges and categorical variables using frequencies. Continuous variables were compared between groups using Mann–Whitney U test and dichotomous variables using Fisher’s exact test or Chi-square test, as appropriate. In the final phase, logistic regression analysis was performed to evaluate possible confounding or adjustment factors. OR and 95% CI were calculated. Statistical analyses were performed with the SPSS package (SPSS Inc., Chicago IL, version 23). Statistical significance was defined as p value <0.05.
Results
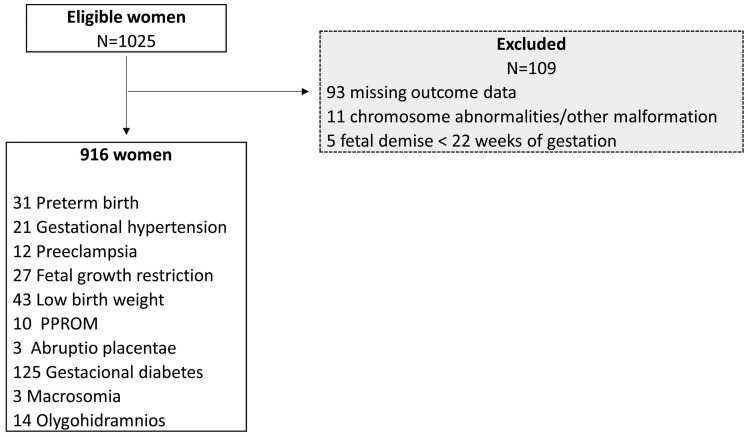
The study population included all women whom PAPP-A level, free β-hCG and NT were available (). One hundred and nine (10.63%) women were excluded (Figure 1). The patients included in this study did not differ significantly in sociodemographics from those who were excluded and population’s characteristics, with relevant patient history, demographic data and obstetric history are summarized in Table 2.
Figure 1.
Flow chart of study population selection.
PPROM: preterm premature rupture of membranes.
Table 2.
Patients’ characteristics by PAPP-A <10th percentile MoM versus PAPP-A ≥10th percentile MoM.
| All (N = 916) | PAPP-A <10th percentile (N = 103) | PAPP-A ≥10th percentile (N = 813) | p-Value | |
|---|---|---|---|---|
| Maternal age (years) | 31 (28–35) | 31 (29–34) | 31 (28–35) | |
| Parity | 1 (0–1) | 1 (0–1) | 1 (0–1) | |
| Previous preterm birth | 9 (1%) | 2 (1.9%) | 7 (0.9%) | |
| Pregestational diabetes | 8 (0.9%) | 3 (2.9%) | 5 (0.6%) | |
| Chronic hypertension | 20 (2.2%) | 3 (2.9%) | 7 (2.1%) | |
| Ultrasound marker NT ≥2.5 mm | 70 (7.6%) | 2 (1.9%) | 68 (8.4%) | |
| Serum marker Free β-hCG (MoM) | 0.889 (0.608–1.389) | 0.68 (0.49–0.966) | 0.919 (0.629–1.414) | |
| First trimester combined risk >1:300 | 26 (2.8%) | 9 (8.7%) | 17 (2.1%) | |
| Amniocentesis | 30 (3.3%) | 6 (5.8%) | 24 (3.0%) | |
| Delivery | ||||
| Duration of pregnancy (weeks) | 39 (38–40) | 39 (38–40) | 39 (39–40) | |
| Spontaneous labor | 582 (63.5%) | 64 (62.1%) | 518 (63.7%) | |
| No operative delivery | 516 (56.3%) | 39 (37.9%) | 456 (56.1%) | |
| Male sex | 445 (48.6%) | 64 (62.1%) | 407 (50.1%) | |
| Birth weight (g) | 3282.5 (2980–3560) | 3120 (2907.5–3527.5) | 3300 (2990–3571) | |
| 1-minute Apgar <7 | 26 (2.8%) | 2 (1.9%) | 24 (3.0%) | |
| 5-minute Apgar <7 | 2 (0.2%) | 0 (0.0%) | 2 (0.3%) |
Data are given as frequencies (percent) or median (quartiles).
Among the 916 women, 31 (3.4%) had a preterm birth, 21 (2.3%) developed gestational hypertension, 12 (1.3%) had preeclampsia, 27 (2.9%) had third trimester fetal growth restriction, 43 (4.7%) had a low birth weight newborn, 10 (1.1%) had a PPROM, 3 (0.3%) had abruptio placentae, 125 (13.6%) developed gestational diabetes, 3 (0.3%) had a macrosomic newborn and 14 (1.5%) had oligohydramnios. Only low PAPP-A level was associated with adverse pregnancy outcome. There was a statistically significant association between: PAPP-A <10th percentile and preeclampsia (adjusted odds ratio, AOR, 4.13, CI 1.15–4.80); low PAPP-A (<1st, 5th and 10th percentiles) and third trimester fetal growth restriction (AOR 8.62, 4.76, 3.94, respectively); and PAPP-A <10th percentile and abruptio placentae (AOR 52.63, 1.41–100.0) (Tables 2 and 3). For fetal growth restriction, there was an increased risk for an adverse outcome as the PAPP-A level became increasingly extreme, from 10th to 1st percentile. Because low PAPP-A (<10th percentile) level had the most significant associations with adverse pregnancy outcomes, the characteristics of two groups were compared (Table 2) and the sensitivity, specificity and positive and NPVs for PAPP-A <0.52 MoM was calculated (Table 3). Concerning PAPP-A <10th percentile, it was observed for preeclampsia 33.3% sensitivity with 3.88% PPV; for third trimester fetal growth restriction 33.3% sensitivity with 8.74% PPV; and for abruptio placentae 66.67% sensitivity, with 1.94% PPV (Table 4).
Table 3.
Relationship between low PAPP-A levels (<1st, 5th and 10th percentiles) and adverse pregnancy outcomes.
| Outcome | 10th percentile N = 103 | ≥10th percentile N = 813 | p-Value | <5th percentile N = 47 | ≥5th percentile N = 869 | p-Value | <1st percentile N = 10 | ≥1st percentile N = 916 | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Preterm birth | 1 (1%) | 30 (3.7%) | 0 (0%) | 31 (3.6%) | 0 (0%) | 31 (3.4%) | |||
| GH | 4 (3.9%) | 17 (2.1%) | 4 (8.5%) | 17 (2.0%) | 1 (10%) | 20 (2.2%) | |||
| Preeclampsia | 4 (3.9%) | 8 (1%) | 1 (2.1%) | 11 (1.3%) | 0 (0%) | 12 (1.3%) | |||
| FGR | 9 (8.7%) | 18 (2.2%) | 5 (10.6%) | 22 (2.5%) | 2 (20%) | 25 (2.8%) | |||
| Low birth weight | 8 (7.8%) | 35 (4.3% ) | 5 (10.6%) | 38 (4.4%) | 2 (20%) | 41 (4.5%) | |||
| PPROM | 0 (0%) | 10 (1.2%) | 0 (0%) | 10 (1.2%) | 0 (0%) | 10 (1.1%) | |||
| Abruptio placentae | 2 (1.9%) | 1 (0.1%) | 1 (2.1%) | 2 (0.2%) | 0 (0%) | 3 (0.3%) | |||
| Gestational diabetes | 15 (14.6%) | 110 (13.5%) | 6 (12.8%) | 119 (13.7%) | 3 (30%) | 122 (13.5%) | |||
| Macrosomia | 0 (0%) | 3 (0.4%) | 0 (0%) | 3 (0.3%) | 0 (0%) | 3 (0.3%) | |||
| Oligohydramnios | 1 (1%) | 13 (1.6%) | 0 (0%) | 14 (1.6%) | 0 (0%) | 14 (1.5%) |
FGR: fetal growth restriction; GH: gestational hypertension; PPROM: preterm premature rupture of membranes. Figures in bold were statistically significant.
Table 4.
Adverse outcomes associated with PAPP-A <10th percentile ( MoM).
| Outcome | AOR | 95% CI | p-Value | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Preeclampsia | 1.15–14.80 | ||||||
| Fetal growth restriction | 1.68–9.26 | ||||||
| Abruptio placentae | 1.41–100.0 |
Discussion
The aim of this study was to analyze the association of extreme values of PAPP-A, free β-hCG and NT with adverse pregnancy outcomes. This study suggests that an unexplained low level of PAPP-A (<10th percentile) during first-trimester can be associated with adverse outcomes, namely preeclampsia, fetal growth restriction and abruptio placentae, as was suggested by previous studies. However, there was not always an increased risk as the PAPP-A level became increasingly extreme and probably the use of <10th percentile threshold can be more clinically useful. In this study, low levels of free β-hCG (<1st, 5th and 10th percentiles) were not associated with adverse outcomes. Other studies demonstrated an association between low levels of free β-hCG (<0.4 or 0.5 MoM) and birthweight below the 5th or 10th percentile (OR 1.6–1.7).4,6 If PAPP-A and free β-hCG reflect placentation quality, low levels should be associated with deficient placentation that occurs in preeclampsia and fetal growth restriction. On the other hand, PAPP-A is known to be an insulin-like growth factor-binding protein protease and to increase the bioavailability of insulin-like growth factor (IGF), that plays an important role in fetal growth18 by mediating trophoblast invasion, and to regulate steroidogenesis and glucose and amino acid transport in the chorionic villi.19 The mechanism involved with most adverse outcomes such as preeclampsia and fetal growth restriction may be related to the protease activity of PAPP-A affecting free IGF concentrations in early pregnancy, and probably this marker is the best predictor for these outcomes. There was no relationship between high PAPP-A or free β-hCG levels and adverse outcome. These results are in agreement with previous publications.4,6,7,10,20 If free β-hCG is increased when oxygen tension is low, as in preeclampsia and fetal growth restriction, this could be a plausible theory to explain adverse outcomes in these women. However, for the time being, there is no known consistent association. Lastly, if impaired establishment of utero-placental circulation during the first trimester may influence the physiological decrease of NT thickness in late first trimester, increased fetal NT measurement should be associated with adverse outcomes. Tsai et al.21 conclude that the sensitivity of fetal NT measurement in first trimester is not sufficient as a single marker for predicting the risk of preeclampsia. In this study this association has not been demonstrated probably because NT is a late marker in first trimester and this work included women who underwent first-trimester screening earlier.
Preeclampsia
Previous studies suggested low PAPP-A as a marker for subsequent development of preeclampsia.4,6,13,22–24 In this work, the resulting AOR for preeclampsia was significantly higher than the results from other studies, with a fourfold increased risk. Smith et al.4 examined the relationship between PAPP-A 5th percentile and preeclampsia and found a statistically significant increased risk (OR 2.3). Dugoff et al.6 found an increased risk of preeclampsia (OR 1.34) when PAPPA was 10th percentile. In the present study, the 10th percentile threshold was the only one statistically significant, probably given the small number of cases in PAPPA level <5th percentile group. PAPP-A <10th percentile demonstrated low sensitivity and PPV, limiting its clinical utility.
Fetal growth restriction
Low serum PAPP-A level may represent early onset placental dysfunction. In this study, the resulting AOR for fetal growth restriction was higher than the results from other studies, with a threefold increase risk. There was an increased risk of fetal growth restriction as the PAPP-A level became increasingly extreme. Dugoff et al.6 found an increased risk of fetal growth restriction (OR 2.15) when PAPP-A was <10th percentile and maternal serum free β-hCG was mildly reduced in pregnancies that subsequently developed fetal growth restriction. Goetzinger et al.25 concluded that black race (OR 2.9), β-hCG MoM >90th percentile (OR 1.6), and PAPP-A MoM <5th percentile (OR 2.8) were significant predictors for fetal growth restriction. The association between low PAPP-A levels and fetal growth restriction has been consensual in other studies, however the use of free β-hCG is unsatisfactory until today.20,26 PAPP-A <10th percentile demonstrated low sensitivity and PPV. Smith et al.4 concluded that the sensitivity of detecting fetal growth restriction for a first trimester PAPP-A level below the 5th percentile is low and PAPP-A as single marker is probably an insufficient screening tool. The same group concluded later that women with low levels of PAPP-A between 10 and 14 weeks and high levels of AFP between 15 and 21 weeks, were more likely to deliver a low birth weight infant, probably reflecting the combined effect of two independent pathophysiologic processes in early placental development.27 In this study, for PAPP-A <1st percentile (<0.28 MoM) and PAPP-A <5th percentile (<0.42 MoM) PPVs were 20% and 10,6%, respectively, similar to the results of Krantz et al.7 However, the majority of patients with these adverse outcomes do not have a PAPP-A <5th and these results should be carefully interpreted.
Abruptio placentae
Some authors have questioned if low PAPP-A may be a marker for development of abruption placenta. In this study, the 10th percentile threshold was the only one statistically significant and the resulting OR for abruptio placentae was very high, with a fiftyfold increase risk, but with only with a low PPV (1.94). Until this study, no significant increase in relative risk has been noted for abruptio placentae.24,28 Low levels of PAPP-A in the first-trimester have been associated with abnormal placental morphometry. There is also an evidence linking abnormal trophoblastic invasion of the decidua, thrombotic changes in the spiral arteries and abruption-induced preterm births.29 Although the majority of studies were not focused on abruptio placentae, low PAPP-A can be associated with this outcome. Blumenfeld et al.30 suggested that first-trimester PAPP-A <5th percentile was associated with placental abruption (AOR 1.9). In this study, due to the small sample size, there were no possible conclusions, however this association can be analysed in future studies.
No significant increase in relative risk was noted for other adverse outcomes evaluated including: preterm birth; low birth weight; PPROM; gestational diabetes; macrosomia; and oligohydramnios.
Limitations
This study has several limitations. Outcome data were not available for 93 women, but the patients evaluated did not differ significantly from those who were excluded. The median maternal age of this study population was high. However, maternal age was not significantly different between women with low versus high PAPP-A levels. Sample size was insufficient to analyze some rarer outcomes.
Conclusion
This study suggests that women with abnormal PAPP-A level, less than 10th percentile, could have a higher risk for preeclampsia, fetal growth restriction and possibly for abruptio placentae. For fetal growth restriction, the lower the MoM value of PAPP-A, the higher the chances of an adverse outcome. However, the majority of patients with these adverse outcomes do not have a low PAPP-A and, therefore, the sensitivity is low. In addition, few patients with a low PAPP-A actually have an adverse outcome (low PPV). There is no consensus yet as to how these pregnancies should be managed. In the absence of such evidence, no changes in clinical practice are indicated.12 The Society of Obstetricians and Gynecologists of Canada Genetics Committee recommends that obstetricians should establish a care plan that takes into account the specific risks of each patient, including patient education, ultrasonography to assess fetal growth and amniotic fluid volume, cervical length, second trimester uterine artery Doppler examination and fetal surveillance.12 Further investigation is needed to determine whether if any type of monitoring and intervention protocol would improve pregnancy outcome when abnormal markers are detected.
Acknowledgements
The authors would like to acknowledge the maternal fetal medicine specialist with Fetal Medicine Foundation certification Domingos Ribeiro, Elisabete Gonçalves and Sandra Ferreira in the first trimester echographic evaluation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Ethical approval was obtained from de Clinical Research Ethics Committee of the Alto Minho Local Healthcare Unit Hospital.
Guarantor
MSG
Contributorship
All five authors were involved in writing and reviewing the submitted manuscript.
References
- 1.Malone FD, Canick JA, Ball RH, et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N Engl J Med 2005; 353: 2001–2011. [DOI] [PubMed] [Google Scholar]
- 2.Palomaki GE, Lambert-Messerlian GM, Canick JA. A summary analysis of Down syndrome markers in the late first trimester. Adv Clin Chem 2007; 43: 177–210. [PubMed] [Google Scholar]
- 3.Ong CY, Liao AW, Spencer K, et al. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG 2000; 107: 1265–1270. [DOI] [PubMed] [Google Scholar]
- 4.Smith GCS, Stenhouse EJ, Crossley JA, et al. Early pregnancy levels of pregnancy-associated plasma protein A and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab 2002; 87: 1762–1767. [DOI] [PubMed] [Google Scholar]
- 5.Tul N, Pusenjak S, Osredkar J, et al. Predicting complications of pregnancy with first-trimester maternal serum free-βhCG, PAPP-A and inhibin-A. Prenat Diagn 2003; 23: 990–996. [DOI] [PubMed] [Google Scholar]
- 6.Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (The FASTER Trial). Am J Obs Gynecol 2004; 191: 1446–1451. [DOI] [PubMed] [Google Scholar]
- 7.Krantz D, Goetzl L, Simpson JL, et al. Association of extreme first-trimester free human chorionic gonadotropin-β, pregnancy-associated plasma protein A, and nuchal translucency with intrauterine growth restriction and other adverse pregnancy outcomes. Am J Obs Gynecol 2004; 191: 1452–1458. [DOI] [PubMed] [Google Scholar]
- 8.She BQ, Chen SC, Lee FK, et al. Low maternal serum levels of pregnancy-associated plasma protein-A During the first trimester are associated with subsequent preterm delivery with preterm premature rupture of membranes. Taiwan J Obs Gynecol 2007; 46: 242–247. [DOI] [PubMed] [Google Scholar]
- 9.Barrett SL, Bower C, Hadlow NC. Use of the combined first-trimester screen result and low PAPP-A to predict risk of adverse fetal outcomes. Prenat Diagn 2008; 28: 28–35. [DOI] [PubMed] [Google Scholar]
- 10.Pihl K, Sørensen TL, Nørgaard-Pedersen B, et al. First-trimester combined screening for Down syndrome: prediction of low birth weight, small for gestational age and pre-term delivery in a cohort of non-selected women. Prenat Diagn 2008; 28: 247–253. [DOI] [PubMed] [Google Scholar]
- 11.Spencer K, Cowans NJ, Molina F, et al. First-trimester ultrasound and biochemical markers of aneuploidy and the prediction of preterm or early preterm delivery. Ultrasound Obs Gynecol 2008; 31: 147–152. [DOI] [PubMed] [Google Scholar]
- 12.Gagnon A, Wilson RD, Audibert F, et al. Obstetrical complications associated with abnormal maternal serum markers analytes. J Obs Gynaecol Can 2008; 30: 918–49. [DOI] [PubMed] [Google Scholar]
- 13.Poon LCY, Maiz N, Valencia C, et al. First-trimester maternal serum pregnancy-associated plasma protein-A and pre-eclampsia. Ultrasound Obs Gynecol 2009; 33: 23–33. [DOI] [PubMed] [Google Scholar]
- 14.Goetzinger KR, Cahill AG, Macones GA, et al. Association offirst-trimester low PAPP-A levels with preterm birth. Prenat Diagn 2010; 30: 309–313. [DOI] [PubMed] [Google Scholar]
- 15.Huang T, Hoffman B, Meschino W, et al. Prediction of adverse pregnancy outcomes by combinations of first and second trimester biochemistry markers used in the routine prenatal screening of Down syndrome. Prenat Diagn 2010; 30: 471–477. [DOI] [PubMed] [Google Scholar]
- 16.Direção Geral da Saúde. Norma da DGS: Diagnóstico e conduta na Diabetes Gestacional. Direção Geral da Saúde, 2011. Available at: http://www.saudereprodutiva.dgs.pt/diabetes-gestacional/norma-da-diabetes-gestacional.aspx.
- 17.Kehl S, Schelkle A, Thomas A, et al. Single deepest vertical pocket or amniotic fluid index as evaluation test for predicting adverse pregnancy outcome (SAFE trial): a multicenter, open-label, randomized controlled trial. Ultrasound Obs Gynecol 2016; 47: 674–679. [DOI] [PubMed] [Google Scholar]
- 18.Bale LK, Conover CA. Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J Endocrinol 2005; 186: 325–331. [DOI] [PubMed] [Google Scholar]
- 19.Sun IYC, Overgaard MT, Oxvig C, et al. Pregnancy-associated plasma protein A proteolytic activity is associated with the human placental trophoblast cell membrane. J Clin Endocrinol Metab 2002; 87: 5235–5240. [DOI] [PubMed] [Google Scholar]
- 20.Spencer K, Yu CKH, Cowans NJ, et al. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free β-hCG and with second-trimester uterine artery Doppler. Prenat Diagn 2005; 25: 949–953. [DOI] [PubMed] [Google Scholar]
- 21.Tsai MS, Lee FK, Cheng CC, et al. Association between fetal nuchal translucency thickness in first trimester and subsequent gestational hypertension and preeclampsia. Prenat Diagn 2002; 22: 747–751. [DOI] [PubMed] [Google Scholar]
- 22.Kang JH, Farina A, Park JH, et al. Down syndrome biochemical markers and screening for preeclampsia at first and second trimester: correlation with the week of onset and the severity. Prenat Diagn 2008; 28: 704–709. [DOI] [PubMed] [Google Scholar]
- 23.Yaron Y, Heifetz S, Ochshorn Y, et al. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenat Diagn 2002; 22: 778–782. [DOI] [PubMed] [Google Scholar]
- 24.Ranta JK, Raatikainen K, Romppanen J, et al. Decreased PAPP-A is associated with preeclampsia, premature delivery and small for gestational age infants but not with placental abruption. Eur J Obstet Gynecol Reprod Biol 2011; 157: 48–52. [DOI] [PubMed] [Google Scholar]
- 25.Goetzinger KR, Singla A, Gerkowicz S, et al. The efficiency of first-trimester serum analytes and maternal characteristics in predicting fetal growth disorders. Am J Obs Gynecol 2009; 201: 412.e1–412.e6. [DOI] [PubMed] [Google Scholar]
- 26.Zhong Y, Tuuli M, Odibo AO. First-trimester assessment of placenta function and the prediction of preeclampsia and intrauterine growth restriction. Prenat Diagn 2010; 30: 293–308. [DOI] [PubMed] [Google Scholar]
- 27.Smith GCS, Shah I, Crossley JA, et al. Pregnancy-associated plasma protein A and alpha-fetoprotein and prediction of adverse perinatal outcome. Obstet Gynecol 2006; 107: 161–166. [DOI] [PubMed] [Google Scholar]
- 28.Karim JN, Sau A. Low pregnancy associated plasma protein-A in the 1st trimester: Is it a predictor of poor perinatal outcome? J Obs Gynaecol 2013; 33: 351–354. [DOI] [PubMed] [Google Scholar]
- 29.Lockwood CJ, Kayisli UA, Stocco C, et al. Abruption-induced preterm delivery is associated with thrombin-mediated functional progesterone withdrawal in decidual cells. Am J Pathol 2012; 181: 2138–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blumenfeld YJ, Baer RJ, Druzin ML, et al. Association between maternal characteristics, abnormal serum aneuploidy analytes, and placental abruption. Am J Obs Gynecol 2014; 211: 144.e1–144.e9. [DOI] [PubMed] [Google Scholar]