Abstract
We have previously demonstrated that mice expressing human complement receptor type 2 (CR2/CD21) during the CD43+/CD25- late pro-B cell stage of B cell development have marked changes in their subsequent B cell ontogeny. Here, we show that the humoral immune response to the T cell dependent antigen, sheep red blood cells (SRBC) can be moderately enhanced with the addition of human CR1 (driven by the lambda promoter/enhancer transgene) to endogenous mCR1/CR2 expression on the B cell surface but that hCR1 expression alone (on the mouse CR1/2 deficient background) has no effect on the humoral immune response or general B cell development. Furthermore, expression of hCR1 had no recuperative effect on the markedly altered B cell phenotype noted with premature expression of hCR2 (either in the presence or absence of endogenous mCR1/2). We conclude that hCR1 alone cannot replace the role of CR2 in mice and that the effects of premature hCR2 expression during BCR development are not significantly altered by the addition of hCR1 at that developmental stage or beyond; thus hCR2 signaling in the mouse remains dominant over subsequent input from either hCR1 or endogenous receptors.
Keywords: Complement Receptor, B cell, Humoral Immune response, Anergy, Marginal zone
Introduction
Complement Receptor type 1 (CR1, CD35, C3b/C4b receptor) is a highly polymorphic multifunctional glycoprotein expressed primarily on erythrocytes and leukocytes. It contains both common blood group antigens and alleles associated with increased susceptibility to malaria or protection from development of SLE (Krych-Goldberg & Atkinson, 2001). CR1 binds C3b and C4b (also with lower affinity iC3b and C3dg) and can function as an immune adherence receptor as well as act as powerful inhibitor of both the classical and the alternative pathway C3- and C5-convertases. It achieves this through both decay-accelerating capacity and co-factor activity and is important in the breakdown of C3b to C3dg, which is a major ligand for complement receptor type 2 (CR2; CD21) (Holers et al, 1986). CR1 has been demonstrated to have both inhibitory effect and enhancing effects on B cell proliferation/differentiation (Hivroz et al, 1991; Jozsi et al, 2002; Tsokos et al, 1984). Further, simultaneous ligation of BCR and CR1 has been shown to be associated with reduced autoantibody levels (Iking-Konert et al, 2004; Voynova et al, 2008) and CR1 stimulation on phagocytes can lead to production of proinflammatory cytokines such as Il-1β (Bacle et al, 1990; Thieblemont et al, 1993). Thus, CR1 plays multiple roles in immune cell function and modulates the effects of complement activation at several levels.
In man (and other primates) CR1 and CR2 are two distinct genes but in mouse, CR1 and CR2 are products of alternative splicing of a single Cr2 gene (Kurtz et al, 1990; Molina et al, 1990). Despite this fundamental difference, there remain significant similarities in function across the species. For instance, expression of CR2 is tightly regulated (Takahashi et al, 1997) both in cell type (majority expressed on B cells and follicular dendritic cells) and particularly with respect to stage in B cell development (expression restricted to transitional and mature B cells). CR2 in both mouse and man associates primarily with CD19 to form an important B cell signaling complex. Simultaneous cross-linking of BCR and CR2/CD19 by iC3d and C3dg coated antigens dramatically lowers the threshold for B cell activation (Dempsey et al, 1996; Mongini et al, 1997). Association of human CR1 and CR2 in signaling complexes on B cells has also been shown and might be important in modulating signals (Tuveson et al, 1991). CR1 in both mouse and man is expressed by follicular-dendritic cells (and B cells), where its role in the retention of opsonised antigens in germinal centers is probably highly important in maintenance of immunological memory (Krych-Goldberg & Atkinson, 2001). The similarity in CR1 intrinsic function in both mouse and man has come to light during the development of a soluble human recombinant CR1 (sCR1) as a C inactivating therapeutic (Mulligan et al, 1992; Ramaglia et al, 2008; Weisman et al, 1990a; Weisman et al, 1990b). hCR1 can bind to mouse C3b and has co-factor function (against hC4b) with mouse factor I (Alexander et al, 2010; Kai et al, 1980) and crucially, sCR1 was shown to be a viable C regulatory protein in the murine system (Pemberton et al, 1993). However, there are also distinct differences between CR1 function in mouse and man. For instance, CR1 in man has a broad expression pattern, including most peripheral blood cells except platelets, natural killer cells and the majority of T lymphocytes (Fearon, 1980). Furthermore, CR1 on erythrocytes, in man, acts as an immune adherence receptor that binds C3b/C4b-opsonized immune complexes (IC) and ferries them to the liver and spleen for removal, a function carried out by factor H in the murine system (Alexander et al, 2001; Alexander & Quigg, 2007).
Targeted deletion of the Cr2 gene in mice has demonstrated that mCR1/2 protein expression is important for B cell development and function. The mCR1/2 deficient mice have an expanded marginal zone B cell population (Haas et al, 2002) and a poor germinal center/memory cell/adaptive immune response to T-dependent antigens (Ahearn et al, 1996; Haas et al, 2002; Molina et al, 1996). Furthermore, mCR1/2 deficient mice, when crossed to autoimmune susceptible stains develop signs of disease much more rapidly (Wu et al, 2002). We have previously created mice expressing hCR2 using a P1 genomic DNA construct (P1.hCR2) (Marchbank et al, 2000) or hCR2 cDNA under the control of a B cell specific lambda promoter/enhancer mini-gene (lambda CR2) (Marchbank et al, 2002) and took advantage of CR1/2 deficient mice (Molina et al, 1996) to begin to investigate the individual roles of CR2 and CR1 in B cell fate. The P1 hCR2 study yielded mice with low level expression of hCR2 at the correct developmental stage, giving essentially a wild type phenotype with expression of hCR2 giving a small recovery of immune response when compared with CR1/2 deficient mice (Marchbank et al, 2000). On the other hand, the lambda CR2 transgene mice displayed marked changes compared to wild type and CR1/2 deficient animals, particularly those that expressed the highest levels of human CR2 protein (henceforth hCR2hi)(Marchbank et al, 2002). The hCR2hi mice displayed marked reduction in peripheral blood B cell numbers, a reduction in IgG isotype antibodies, loss of follicular and reciprocal expansion of ‘innate’ B cell populations in the spleen and as well as a marked reduction in the immune response to both T dependent and T independent antigens beyond that seen in CR1/2 deficient mice. Indeed, the hCR2hi mice were subsequently shown to be highly resistant to collagen induced arthritis and have a degree of protection from developing systemic autoimmune disease as a result of these changes (Kulik et al, 2007; Pappworth et al, 2009; Twohig et al, 2007; Twohig et al, 2009). All these alterations in B cell function are almost certainly a result of the premature expression of CR2 on the B cell surface during B cell development in the bone marrow (Kulik et al, 2007), which irreversibly alter the signaling potential of these B cells whether in the presence or absence of endogenous mCR1/2 expression or signaling (Birrell et al, 2005; Kulik et al, 2007; Marchbank et al, 2002).
Hence, we have developed mice expressing human complement receptor 1 (CR1*1 allotype) with the aim of establishing whether the presence of hCR1 could alter mouse B cell development and function; whether the lambda transgene has an negative impact on B cell function and whether the combination of hCR1 and hCR2 in the murine system can rectify the negative effects on humoral immune response seen in the CR1/2 knockout and the hCR2hi mice.
Materials and Methods
Cells
Peripheral blood lymphocytes (PBL) were isolated from blood collected into 20 μl of heparin following a tail vein nick and washed once in cold PBS. Bone marrow B cells were collected by flushing mouse femurs with cold PBS. Splenocytes were isolated from whole spleens by disrupting them between two frosted glass slides in PBS buffer and transfer to 15 ml conical tubes on ice. Once large debris had settled, the supernatant was transferred to a new tube. Cells were pelleted by centrifugation and washed once with staining buffer (PBS, 1% FCS, 0.02% sodium azide). Samples were incubated with 0.5-1 ml of red blood cell (RBC) lysis buffer (0.83% NH4Cl, 0.1% KCO3, 0.1mM EDTA) and incubated at RT for 1-2 min. The cells were then washed with 1 ml staining buffer. Cells were then counted and 1-3 x 106 cells/ml were used per analysis. Cells were stained as described below.
Antibodies
Purified, biotin and allophycocyanin (APC) conjugated mAb 171 (anti-hCR2, IgG1 isotype) (Guthridge et al, 2001) and mAb 543 (anti-hCR1, ECACC); biotin mAb 7e9 (anti murine CR2, IgG) (Kinoshita et al, 1988) and IgG1 isotype control were produced in the laboratory following standard methods. 2.4G2 (anti-mCD16/mCD32, Fc Block), Phycoerythrin (PE) conjugated B-Ly-4 (anti-hCR2), anti-CD35 (anti-hCR1); anti- CD43, anti-IgG1 and anti-CD1d; APC, fluorescein isothiocyanate (FITC) or PerCP conjugated RA3-6B2 (anti-mCD45R, B220), FITC conjugated anti-IgG2a/c, IgG2b, IgG3, Gl-7 and anti-CD23, biotin anti-CD138 (Syndecan – 1) and anti-CD24; Streptavidin (SA)- APC and Quantibrite beads were all obtained from Pharmingen (BD, Oxford, UK). Cy5.5- conjugated anti-IgM or IgG, SA-FITC and SA-PE were obtained from Jackson Immunoresearch Laboratories (Stratech Scientific, UK). Purified goat anti-mouse IgG/IgM, alkaline phosphatase (AP) conjugated goat anti–mouse Ig isotype secondaries were obtained from Caltag-Medsystems Ltd (Buckingham, UK).
Transgenic mice
All mice in this study are on the C57BL/6 (B6) genetic background with or without the transgenes and/or genetic deletions described below.
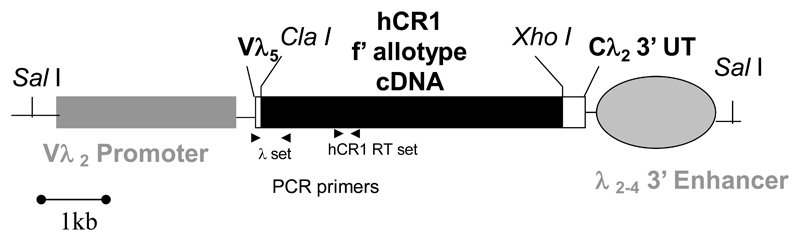
In order to generate the hCR1 cDNA transgene, we introduced a short multiple cloning site including Xho I and Cla I restriction sites into the unique Pme I (cDNA cloning) site of the vλ2-4 vector (a kind gift from Prof J. Hagman, Denver, USA) using standard techniques. This vector was then double digested with Xho-I and Cla-I prior to ligation with a 6.5kb Cla I to Xho I fragment isolated by sequential digest of a CDM8 vector containing the full cDNA sequence for human CR1 (F allotype; a kind gift from Prof. J. Atkinson, St Louis, USA). Purified vλ hCR1 DNA was then cut with Sal I to release the transgene and purified from low melt point agar gel prior to injection into FVB/n mouse ova. Pups were screened using hCR1 specific PCR, Briefly, 3 μl of DNA solution underwent standard PCR using the following primer set (vλ 5’-GTGAATTAAGGCCTGGACTTCACTT -3’ and hCR1-r 5’-TTATCCCAGGTTCCATCCAC -3’; at cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min. PCR reactions were carried out for 35 cycles. Genotyping was also confirmed by flow cytometric analysis of PBL.
The lambda human CR2 transgenic mice (hCR2hi) used in this study were generated and screened by PCR and/or flow cytometry as previously described (Marchbank et al, 2002; Marchbank et al, 2000). Additional mice used are the Cr2-/- mice (confirmed by PCR or flow cytometry using b7E9). All mice used were age and sex matched littermates from in house colonies maintained at Cardiff University and subsequently at Newcastle University as appropriate. All experiments reported herein were carried out on F4-8 backcross and age matched C57BL/6 mice purchased from Harlan (UK) were used as wild type (B6; mCR2+/+ hCR1- hCR2-) control mice where appropriate. All experiments were carried out under strict compliance with Home office (UK) animal licenses as appropriate.
RT-PCR Analysis
To analyze hCR1 gene mRNA expression in individual murine tissues, RNA was first isolated using the Trizol reagent (Gibco BRL, Rockville, MD) according to the manufactures instructions. hCR1 mRNA expression was analyzed using RT-PCR. 3 μg of total RNA from each tissue was first treated with 10 U DNase 1 (Amp Grade, Gibco) for 15 min at RT, followed by incubation with 1 μl of 25 mM EDTA for 10 min at 65°C. 1 μg was then utilized in each RT-PCR reaction. Reverse transcriptase reactions were primed with random primers according to manufacturer’s specifications (Gibco). Parallel samples with and without MuMLV reverse transcriptase were used to assure that templates were mRNA- and not DNA-derived (not shown). PCR was performed using the GeneAmp PCR kit (Perkin Elmer, Norwalk, CT) according to manufacturer’s directions using 2 mM MgCl2 and amplified 25 or 30 cycles, as appropriate, using the hCR1 RT oligonucleotides ; 5’-GCAGCTCTGCTAGTTATTGTG-3’ and 5’-ATTGGCGATGGTTGGGGG-3’ at 94°C x 1 min, 56°C x 45 sec, 72°C x 1 min. This oligonucleotide pair results in the creation of a 700 bp in the middle of the hCR1 gene (33, Diagram 1).
Diagram 1. The hCR1 lambda light chain transgene.
Shown is the lambda light chain mini gene and the position of the human CR1 (F allotype) cDNA. Also shown are the restriction digest sites for removal of the mini-gene from the pUC19 plasmid backbone and the position of the PCR primers used to detect the transgene.
We used a mGAPDH primer pair as a positive control for quality and relative quantity of the RNA collected from tissues analysed. These were 5’-GCCCATCACCATCTTCCAGGAGCG-3’ and 5’-GTCATATTTCTCGTGGTTCACACC-3’. PCR conditions were identical to above. This oligonucleotide pair results in the creation of a 200 bp spliced PCR product. Samples were electrophoresed on 1.5% agarose. Markers used were Easyladder Itm (Bioline, UK).
Flow cytometry
After RBC lysis, cells were washed and then re-suspended in 10 μg/ml of 2.4G2 antibody in order to block Fc receptors. After 15 min incubation on ice, cells were washed and re-suspended in 100 μl staining buffer containing primary Ab (0.1-3 μg/ml) and 1 μl anti-B220-FITC (or PerCP, or APC), where appropriate. Cells were incubated for 30 min on ice in the dark. After incubation, cells were washed in staining buffer 3 times and then incubated with the appropriate streptavidin conjugated fluorochrome to detect biotin labeled primary Abs. Following incubation, cells were washed as above and then resuspended in staining buffer containing 1% formaldehyde. Flow cytometry was carried using a FACSCalibur (BD, Oxford, UK).
Sheep red blood cell (SRBC) immunizations and serum immunoglobulin assays
SRBCs (TCS Bioscience, Buckinghamshire, UK) were washed 3 times and re-suspended to the required concentration in PBS. Mice were injected intraperitoneally with 500μl of SRBC in PBS at day 0 or subcutaneously with 100μl SRBC mixed 1:1 with complete Freund’s adjuvant as required. Mice were boosted with 100μl SRBC in incomplete Freund’s adjuvant at D28 as required. Serum was collected at day 0, 7,14,21,28 and 35 during time courses. For germinal center analysis, mice were immunized on day 0 and serum collected at day 10. Detection of Ab to SRBC was carried out using flow cytometry. SRBC were washed 3 times in PBS and re-suspended at a 0.05% v/v solution in flow buffer. 100μl of SRBC solution was dispensed on ELISA plates and heat inactivated (56°C for 30 min) mouse anti-sera added. Standard curves were generated with doubly diluted control anti-sera to confirm that test samples fell within the linear range of the assay for each isotype used and to allow relative units (RU) to be assigned. Test samples were titrated over 4 dilutions and incubated in duplicate at room temperature for 30 min and then washed 3 times in flow buffer. Plates were then incubated with 1/250 dilution of anti-mouse IgM or IgG-Cy5.5 for 30 min at room temperature. Alternatively, for Ig Isotype analysis 1/100 dilution of anti IgG2a/c, IgG2b or IgG3 –FITC or 1/500 dilution of anti-IgG1-PE (BD Pharmingen) was used. SRBC were washed 3 x and then immediately analysed. No fewer than 5 mice were used in each group. Non-linear regression curve fit analysis was used to generate RU using Prism Graphpad software (where 10,000 RU was used as highest dilution of standard sera). Response to PBS, as negative control, remained below detectable RU thresholds ≤ 20 RU and therefore is not shown.
Results
Creation of transgenic mice expressing hCR1
The vλ5 hCR1 cDNA Sal I digest fragment (diagram 1) was injected into fertilized mouse ova using standard techniques. PCR analysis of tail DNA from the resulting 34 mice (3 experiments) revealed the presence of 2 genotype positive mice. Analysis of peripheral blood leukocytes was carried out by flow cytometry. One of the two mice exhibited detectable staining for hCR1 on its B cells (figure 1a). The B220 negative lymphocyte population (primarily T cells) showed marginally above background staining for hCR1, indicating that these cells also express low levels of hCR1 (figure 1b).
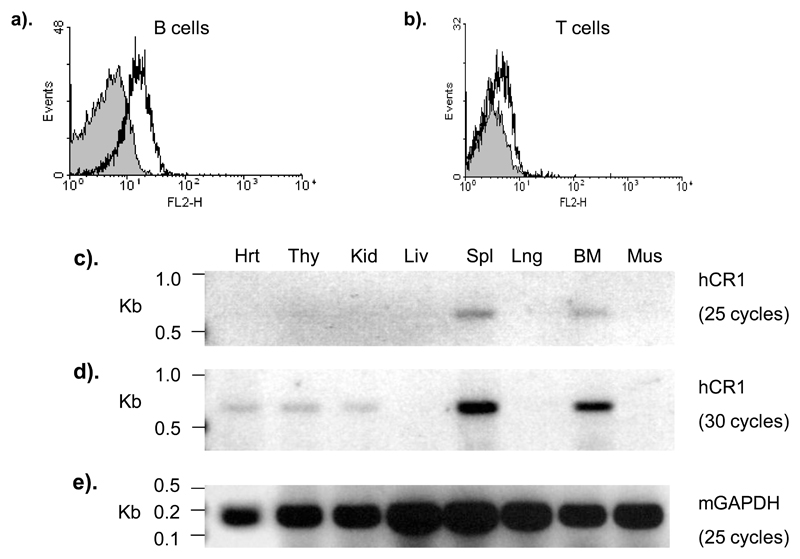
Figure 1. Expression of hCR1.
PBL were isolated from F1 mice by tail bleed and red cells were lysed. (a) B220+ cells (peripheral blood B cells) or (b) CD3e+ cells (peripheral blood T cells) were also stained with anti-hCR1–PE (black line) or anti-mIgG1-PE isotype control (grey filled). At least 5000 B or T cell events were collected and results are representative of triplicate analysis. cDNA was prepared from murine tissue as described in materials and methods. The hCR1 primer set (diagram 1) was used to establish the presence of hCR1 cDNA and samples were assayed after 25 (c) and 30 (d) cycles as described in materials and methods. mGAPDH specific primers were used to control for RNA quality and levels. These results are representative of 4 such experiments on littermates.
Using RT-PCR, expression of the hCR1 transgene was detected primarily in the lymphoid compartment, spleen, BM and to a lesser extent the thymus, as expected (figure 1d and 1e). However, there was also low level signal detected in the kidney and heart samples, which maybe from blood derived cells (figure 1e).
Transgenic mice expressing hCR1 have reduced B cell numbers
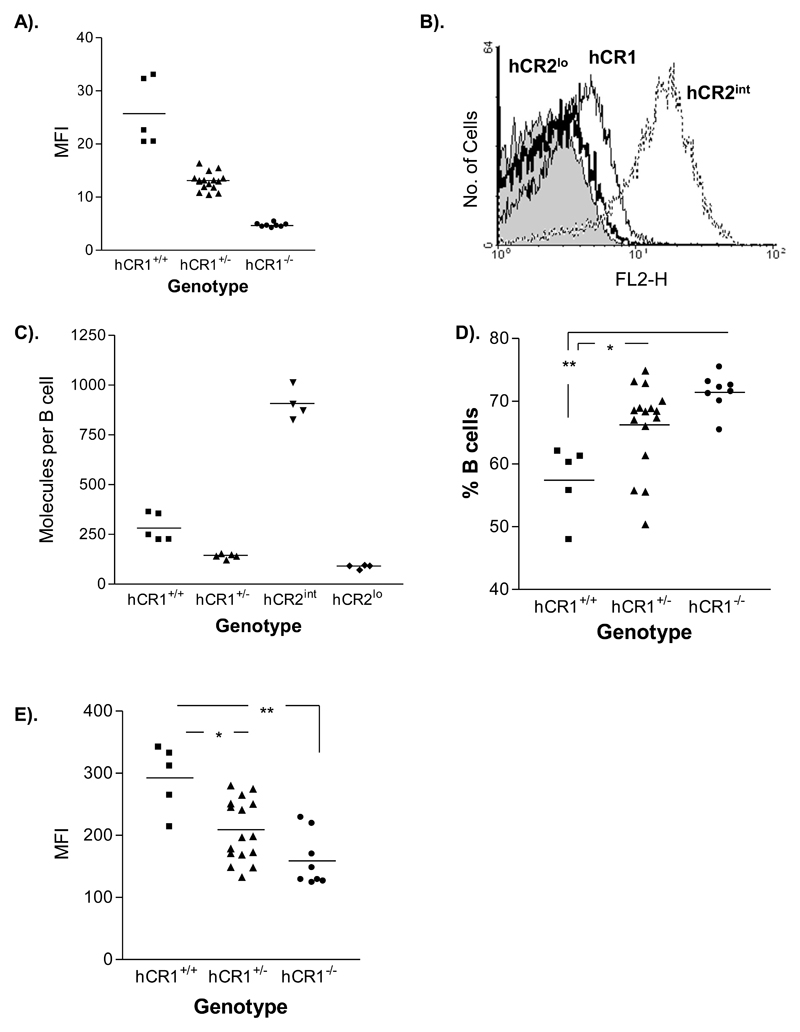
One of the defining features of the hCR2 tg mice is a loss of peripheral B cells as copy number of the lambda hCR2 transgene increases (Marchbank et al, 2002).Using PCR, we determined that there was only one or two copies of the transgene present in the genome of mice expressing hCR1 (data not shown). Homozygous expression of the transgene after cross breeding resulted in a doubling of detectable hCR1 protein on B cells (figure 2a, 2b and 2c). We determined the expression levels of hCR1 on the B cell population to be relatively low when compared to the previous lambda hCR2 transgenic mice (figure 2b and 2c). Peripheral B cell numbers in heterozygous hCR1 mice were unaffected. However, with increasing hCR1 expression there was a small but significant reduction in the percentage of B cells in the blood (figure 2d). Interestingly, unlike hCR2 tg mice (Marchbank et al, 2002; Twohig et al, 2007), hCR1+/+ mice had a significant increase in the surface expression level of endogenous mCR1 (figure 2e) but showed no alteration in hCR1 expression levels after removal of C3 (Supplemental figure 1). In hCR2hi mice the reduction in B cell numbers is also associated with marked alterations in B cell subpopulations in the bone marrow and spleen with consequent changes in baseline immunoglobulin levels (Marchbank et al, 2002). However, full analysis of hCR1 mice revealed they were essentially the same as wild type littermates and carried no defects in these B cell compartments (Supplemental figures 2a, 2b and 3). This was despite the hCR1 protein being expressed at the same point in B cell development as hCR2 (data not shown).
Figure 2. hCR1 expression level and the effects of homozygousity.
(a) PBL were isolated from F4 mice by tail bleed and red cells were lysed. (a) B220+ cells (peripheral blood B cells) were then stained with anti-hCR1–PE (black line) or an isotype control, anti-mIgG1-PE (grey filled) to determine hCR1 expression on either heterozygote or homozygous littermates. Results are expressed as mean fluorescent intensity (MFI) under the area of the curve and mouse genotype is indicated. (b) Shows the fluorescent intensity of hCR1 or hCR2 on B cells after staining with appropriate PE conjugated antibodies. (c) Shows the absolute expression level of hCR1 and hCR2 as determined by use of directly conjugated primary antibodies and a Quantibrite bead (BD) standard curve. Fluorescent intensity of cells was converted into number of molecules per B cell using non-linear regression curve fit analysis according to manufacturer instructions. (d) Lymphoid cells (based on forward and side scatter profile and expression of B220 or CD3e) where gated and the percentage of B220+ cells (B cells) established within that gate. B cells are expressed as a percentage of total lymphocytes captured in that gate for each mouse genotype as identified. (e) B cells (B220+) were stained with biotin 8C12 and SA-APC to establish the expression level of murine CR1. 10,000 events were collected and results are representative of several experiments and triplicate analysis. *p< 0.05, **p<0.01.
Mice expressing hCR1 mount an improved humoral immune response to SRBC (T-dependent Ag)
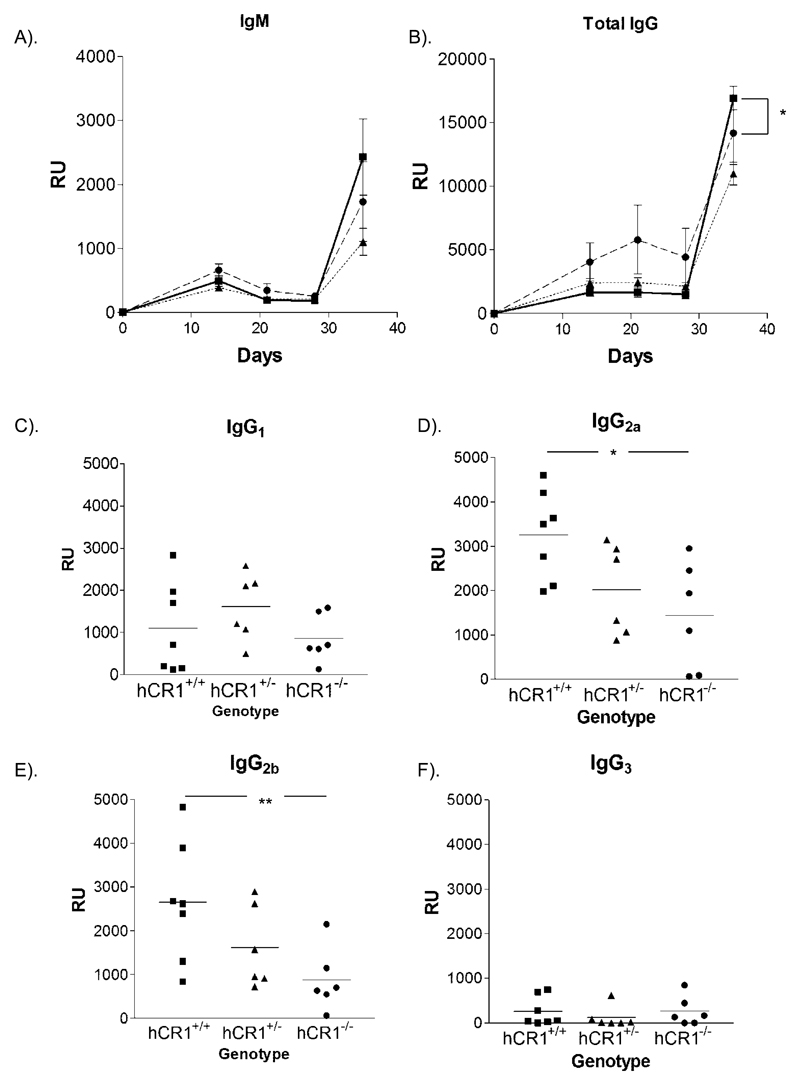
Our previous studies in vλ5 hCR2 transgenic mice have noted that total IgG responses to both T dependent and T independent immunogens is significantly reduced in these mice and surprisingly, demonstrated a poorer humoral response than CR1/2 deficient mice (Marchbank et al, 2002). In light of this and the small changes noted in B cell percentages within the blood, we wished to investigate whether the presence of hCR1 would have any effect on the immune response to SRBC in the presence of endogenous mCR1/2. IgM responses were essential unaltered in the presence of the hCR1 transgene regardless of its level of expression (figure 3a) but in contrast to the hCR2 studies, the IgG response was equal or marginally better than that seen in hCR1 negative littermates after boost (figure 3b). Furthermore, analysis of individual IgG subclass at day 36 demonstrated significant increases in the IgG2a/c and IgG2b isotypes on the hCR1+/+ background compared to hCR1 negative littermates. IgG1 and IgG3 responses were essentially unchanged between the groups of mice immunised. However, we observed lower titre of anti-SRBC titers during the early phase of the immune response in the hCR1 tg mice (figure 3a and 3b). Thus, the presence of hCR1 does not impact the humoral immune response in the same way as seen in hCR2 tg animals, where even the hCR2lo mice showed a significant reduction in Ig responses (Marchbank et al, 2002).
Figure 3. hCR1 mice have an increased response to SRBC boost.
hCR1+/+ (square/solid line); hCR1+/- (triangle/dotted line) and hCR1-/- (circle/dashed line) F4 intercross littermates were immunized with 5x107 SRBC/ml in PBS and subsequently boosted at day 28 with 1x107 SRBC/ml. Mice were bleed weekly by tail nick and serum analysed for the presence of anti-SRBC antibodies with the following isotype specificity (a) IgM, (b) IgG, (c) IgG1, (d) IgG2a/c, (e) IgG2b, and (f) IgG3. Anti-SRBC titre for each mouse shown was established in relative units (RU) using the flow cytometry technique described in materials and methods. Groups of at least 6 mice were used. Mann-Whitney U-tests were used to establish significance. *p<0.05, **p<0.01.
An enhanced germinal center response to T-dependent Ags is found in mice expressing hCR1
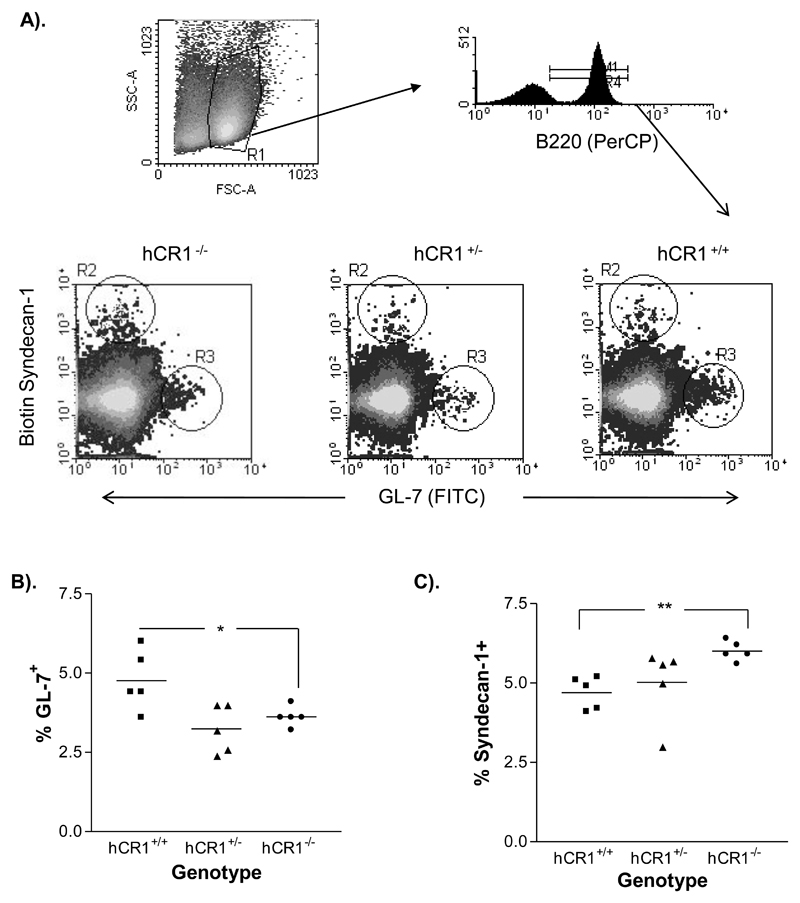
To explore the mechanisms behind the alterations in the humoral immune response we first examined whether normal splenic germinal center formation was evident in the hCR1 transgenic mice using GL-7 as a marker. hCR1+/+ transgenic mice showed a small but significant increase in GC B cell percentages compared with the negative littermates, while the hCR1 heterozygous mice were unaltered (figure 4a and 4b). Using Syndecan-1 as a marker of plasma cells and primary antibody foci, we saw an unexpected reduction in the percentage of cells that were CD138 positive in the spleens of immunized hCR1 tg mice compared with the negative controls (figure 4c), suggesting an altered response to antigen in the hCR1 tg mice. Analysis of spleens using immunohistochemistry did not reveal any gross changes in splenic architecture. Germinal center size and numbers where essentially the same using this analysis (supplemental figure 4).
Figure 4. hCR1 mice have altered B cell responses to antigen.
hCR1+/+ (square); hCR1+/- (triangle) and hCR1-/- (circle) F5 intercross mice were immunized with 5x107 SRBC/ml in PBS. After 10 days, splenocytes were isolated and stained for flow cytometry (a) Illustrates the gating strategy used to delineate the germinal center B cells (B220+ Gl-7+) and plasma cells/antibody forming cells (B220+ CD138+); Representative histographs for GL-7 and CD138 expression on B cells from each genotype of mouse analysed are shown. Scatter plot analysis of (b) GL-7+ B cells and (c) syndecan-1 B cells, as a percentage of total B cells in each mouse of a particular genotype are also shown. 10,000 B cells were collected. Groups of 5 mice were analysed. Mann-Whitney U-tests were used to establish significance. **p<0.01, *p<0.05.
hCR1 can replace part of the B cell developmental role attributed to Cr2 in mice
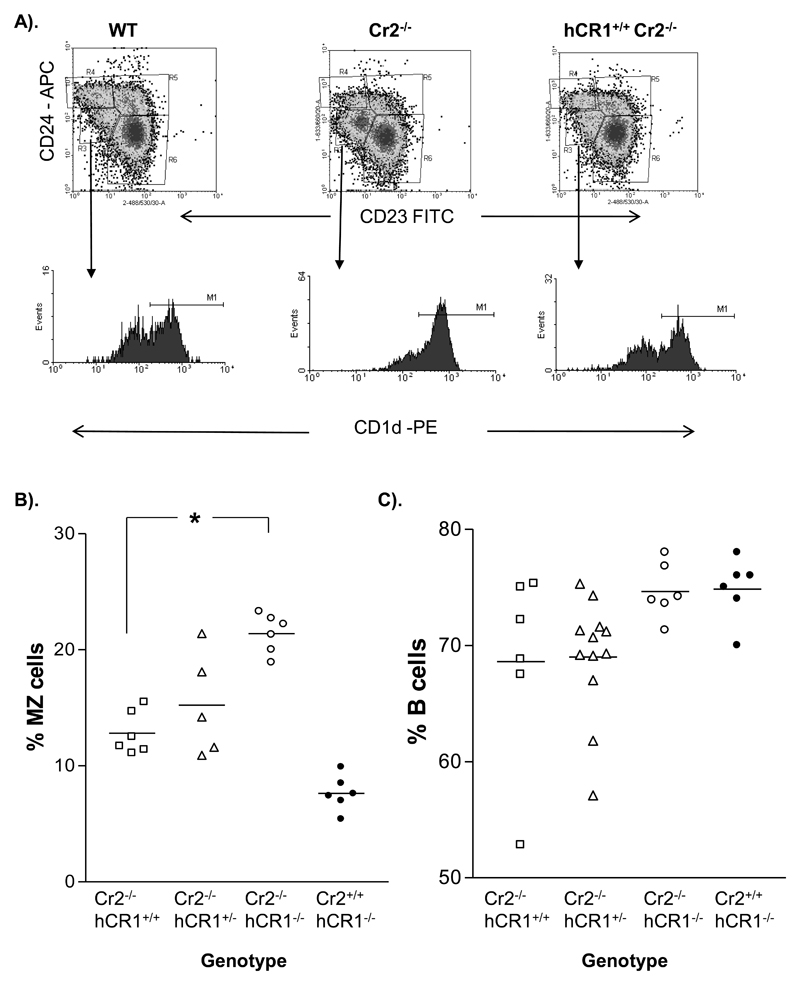
We next wanted to examine the ability of hCR1 to replace the function of mCR1/2 using the CR1/2 knockout mice generated by Molina et al (Molina et al, 1996). We have routinely found an expansion of the marginal zone (MZ) B cell population in our colony of CR1/2 knockout mice, comparable with that published in an independent CR1/2 mouse (Haas et al, 2002) and thus, we were surprised to find during our routine analysis of B cell subpopulations that expression of hCR1 on the mCR1/2 deficient background had returned the MZ B cell population to a level comparable to that seen in wild type mice (figure 5a and 5b). Furthermore, examination of peripheral B cell numbers also indicated that expression of hCR1 had no major impact on B cell percentages compared to controls. This data suggests that hCR1 was having both mCR1/2 dependent and independent effects on B cell development.
Figure 5. Marginal Zone B cells in hCR1 mice resemble wild type.
(A) Splenocytes were isolated, counted and incubated with Fcblock (BD Pharmingen). Marginal zone B cells were then identified by a surface marker profile of B220+CD23- CD24loCD1dhi. (B) The MZ sub-population of each genotype is shown in scatter plot and is expressed as a percentage of total B cells in the lymphoid gate. (c) Shows percentage of B cell (B220+) events collected as a percentage of all cells in the lymphoid gate. 10, 000 B220+ events were collected and at least 5 mice were used per group. Mann-Whitney U-test was used to establish significance. ***p<0.0005, **p<0.005, *p<0.05.
Human CR1, in the presence or absence of hCR2, cannot replace mCR1/2 or reverse the negative effects of premature hCR2 expression on the humoral response to SRBC
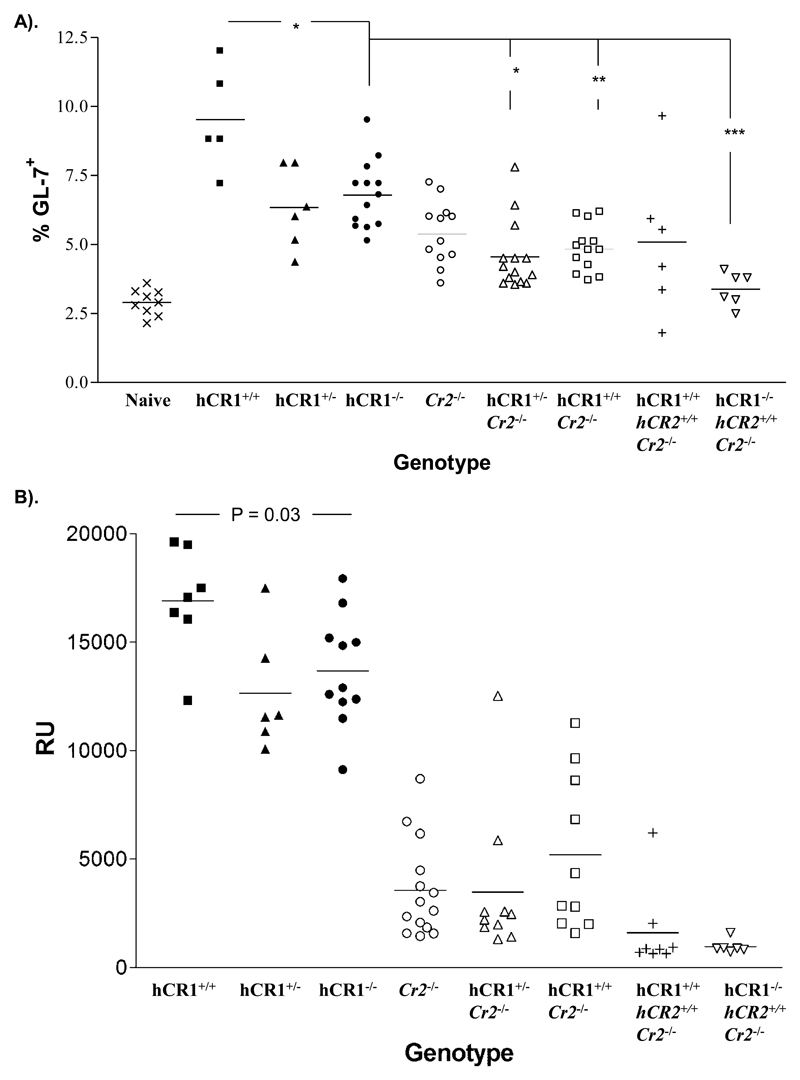
Considering the results above, we wondered whether hCR1 was able to replace the function of mCR1/2 in driving the immune response to SRBC. Since one important role for hCR1 in the process is the breakdown of C3b to C3dg (Holers et al, 1986). We also wanted to look at the effects of hCR1 in combination with hCR2 expression in the absence of the endogenous receptors. Both results from the day 10 germinal center experiments and day 36 post boost analysis of anti-SRBC Ig revealed that hCR1 could not provide any enhancement in the immune response to SRBC in absence of endogenous mCR1/2 (figure 6a and 6b). Furthermore, the backcross to the hCR2.mCR1/2-/- background did not yield any significant change in this finding (figure 6a and 6b). Furthermore, analysis of splenic and BM B cell populations of mCR1/2-/- versus hCR1.hCR2.mCR1/2-/- mice revealed that expression of hCR1 could not reverse the marked changes previously identified with expression of the hCR2 tg ((Marchbank et al, 2002); Supplemental figures 5 and 6).
Figure 6. Immune response to SRBC.
(a) Mice were immunized with 5x107 SRBC/ml in PBS or PBS as control and after 10 days the germinal center reaction in the spleen was assessed by flow cytometry using forward and side scatter profiles, B220 and GL-7 expression to identify the germinal center B cells.10,000 B220+ events were collected flow cytometry. (b) Blood was collected one week after boost (day 36) and allowed to clot. Total IgG anti-SRBC titre was then established by flow cytometry as described in the Methods section. Experiments were carried out in small blocks with groups of 3-4 mice, using wild type and Cr2-/- mice as central controls to which all data were standardized. Groups of between 4-10 mice were analysed. Mann-Whitney U-test was used to establish significance between individual groups. **p<0.005, *p<0.05.
Discussion
Here, we report the development of mice expressing the common CR1*1 (F or A allotype) of human CR1 predominately restricted to the B cell population. Our aim was to further explore the roles of CR1 and CR2 in B cell development and function. As in our previous studies which focused on hCR2 (Marchbank et al, 2002), we used a murine Vλ2 promoter/Vλ2-4 enhancer or lambda minigene to express hCR1. In our hands, this transgene has been found to be largely B cell specific and can produce high expression of proteins, dependent on copy number of transgene incorporated into the genome (Marchbank et al, 2002). Here, we found that expression of CR1 was as predicted, in that its expression stage was identical to that seen with the hCR2 tg and was within the range noted for the lambda hCR2 tg lines (Marchbank et al, 2002). Using semi-quantitative flow cytometric methods, the expression level of hCR1 on mouse B cells was found to be several fold that of hCR2lo mice but less than hCR2int mice (figure 2) and PCR data was indicative of there being between 1-3 copies of the transgene in the mouse genome.
The hCR1 transgenic line represents the 4th fully studied line of mice using the lambda mini-gene construct and is the first where we found any expression in the T cell compartment. However, as T cells have been found to express CR1/2 under some circumstances (Kaya et al, 2005) and expression levels herein were found to be very low, this is not a major concern with this model and is likely due to the insertion point of the lambda construct into the mouse genome in this occasion. The use of the lambda minigene in the generation of hCR1 transgenic mice was calculated to shed some light on whether the expression of any protein on the B cell surface using this transgene would interfere with normal B cell function. Here our results were mixed, heterozygous expression of hCR1 had no marked effect on B cell numbers and general development (figure 2; supplemental 1) but a small and consistent reduction in peripheral B cell percentages was noted in mice that were bred to homozygous hCR1 tg expression. This is in contrast to hCR2int mice, where even heterozygous expression of hCR2 i.e. broadly similar CR2 expression levels have a more substantial defect in B cell development and immune responses than seen in the homozygous hCR1 mice (figure 2b & 3, (Marchbank et al, 2002)). Potentially this indicates that level of receptor and the signaling generated regardless of whether it is hCR1 or hCR2 has the ability to interfere with normal B cell development in the bone marrow or that the increased copy number of the lambda transgene has an impact on normal BCR selection/generation but the mechanism for such an effect is unclear. From the examination of hCR2hi mice, we have concluded that surface expression of the pre-BCR after expression of hCR2 is linked with the initial loss of B cells and that this intrinsically alters normal B cell signaling pathways (Kulik et al, 2007). Our data in the hCR1 mice does not demonstrate loss B cells in the bone marrow compartment (supplemental figure 2b), even with homozygous hCR1 expression, and indicate that either expression level or the ligand interactions are critical in this setting. It is also clear from our analysis of hCR2hi mice that the signaling events during this phase of B cell development overrides or renders subsequent signals through murine CR1/2 irrelevant and thus, represents a dominant negative phenotype with respect to normal signaling via mCR1/2 (Birrell et al, 2005; Kulik et al, 2007; Pappworth et al, 2009; Twohig et al, 2007; Twohig et al, 2009). Our analysis of the hCR1 mice would suggest that this situation is not the case in the hCR1 mice as mice expressing both hCR1 and endogenous mCR1/2 have a marginally better immune response and suggests some co-operation between the function of hCR1 and mCR1/2. This should be considered in the context of the fact that the presence of hCR1 and/or hCR2 in mice will modify the ability of endogenous receptors to bind to C3 ligands and will likely directly influence the generation and stability of the murine C3, C5 convertases and thus the production of C3a and C5a. These changes could have an impact on normal immune response and handling of IC.
CR1 binds to both C3b and C4b and can help inactivate classical, alternative, and lectin pathway C3/C5 convertases, indeed soluble human recombinant CR1 (sCR1) has been developed as a therapeutic reagent to control unwanted C activation in vivo (Mulligan et al, 1992; Ramaglia et al, 2008; Weisman et al, 1990a; Weisman et al, 1990b). Studies in mice with sCR1 have demonstrated that hCR1 can serve as a viable C regulatory protein in the murine system (Pemberton et al, 1993). There is further evidence that hCR1 can bind to mouse C3b and demonstrate co-factor function (against hC4b) with mouse factor I (Alexander et al, 2010; Kai et al, 1980). The role of CR1 in the generation of C3d in the human system is long established (Fearon, 1980; Law et al, 1979), thus based on all these facts we would predict mice expressing additional CR1 should have an increased capacity to control C activation and quickly generate C3dg coated surfaces or IC. This should lead to increased CR2 binding sites which could potentiate the well established BCR co-receptor function of CR2 (Bradbury et al, 1992; Lee et al, 2005; Matsumoto et al, 1991; Matsumoto et al, 1993; Smith et al, 2006). Indeed, our data suggest that mice expressing hCR1 in the presence of mCR1/2 increases antibody titers to the T dependent antigen SRBC (figure 3) and can alter B cell differentiation pathways (figure 4). In the SRBC immunisation experiments carried out by Molina et al on the CR1/2 deficient mice, IgG1 and IgG2a/c isotype production was particularly affected (Molina et al., 1996). Therefore, we expected to see the opposite affect with hCR1 expression in the presence of endogenous mCR1/2 and indeed, an increased IgG2a/c response was evident. However, we saw no enhanced IgG1 response but instead noted a significant increase in IgG2b isotype anti-SRBC antibody levels. Together this suggests a bias towards IFN-γ, Th1 and TGF-β, regulatory cytokine profiles (Janeway, 2001) and may represent a skewing of the Th populations in these mice. These skews could have impact on the ability of mice to defend against influenza (Huber et al, 2006) or parasite infection (Takehara et al, 1981) and would likely have an impact on disease progression in autoimmune models such as collagen induced arthritis and the B6.lpr (Kulik et al, 2007; Pappworth et al, 2009). These possibilities and the responses to hapten antigens, such as the T dependent antigen, NP- KLH and the T independent antigen NP-Ficoll will be explored in subsequent studies. It is important to note that, the potentiated immune response in hCR1 tg mice is only evident in mCR1/2 sufficient animals, which do up regulate endogenous mCR1/2 (figure 2). This suggests that these effects could be indicative of a need for appropriate CR2 signaling/function. The fact that mCR1/2 deficient and hCR2hi mice continue to display defective responses to SRBC when hCR1 is introduced enforces that conclusion. Additionally, too much signaling via CR2 can be inhibitory (Lee et al, 2005). Conceivably, additional ligand production and/or signals generated by increases in CR1 and endogenous mCR1/2 may be inhibitory at the ‘top end’ of what was normal signaling, but would have a positive effect on previously sub-optimal signaling. In this scenario, we would not visualize a huge change in the aggregate response and we would not see massive increases in B cell activation as a new balance between responding and non responding B cells would be set. It is difficult to find clear evidence for this scenario within our current models although the changes in Ig isotype usage, GC cell and CD138+ cell percentages could be a marker of such a redefined balance between activation and tolerance mechanisms.
Human CR1 has been shown to possess a phagocytic role (Holers et al, 1992) and this might be important for antigen processing in man and thus, may be transferred to mouse B cells expressing hCR1. Furthermore, hCR1 has been shown to bind and transport mC3b coated particles (Alexander et al, 2010; Li et al, 2010). Thus, hCR1 expressing B cells may have a great propensity to transport IC to the lymph node/macrophage to drive additional antigen presentation in that microenvironment (Phan et al, 2007), altering the kinetics of IC transfer and handling, which in aggregate would provide an improved environment to drive the humoral immune response through achieving improved T cell help or accessory cell function (such as Follicular dendritic cells, FDC). The alteration in GC and CD138 plasma cells percentages in hCR1 tg mice could be interpreted as a marker of such changes (figure 4). Of course, the B cell restricted expression of hCR1 we have created using the lambda minigene in mice is close to the normal murine expression pattern for CR1 except for the lack of CR1 on FDC (Fearon, 1980). This absence could be particularly important for the immune response because ICs trapped by CR1/2 and displayed on FDC are thought to play an important role in providing a constant source of antigen to B cells to help drive for affinity maturation in the germinal center response and possibly also to maintain B memory cells (Fang et al, 1998; Prodeus et al, 1998; Wu et al, 2000). Thus, any effect that hCR1 expression might have on IC transfer may be tempered on the mCR1/2 deficient background because of the lack of mCR1/2 on the FDC; indeed our hCR2 tg mice are also likely to suffer from this issue. Recently, a new hCR2 BAC tg mouse became available (Kulik et al, 2011) and we are currently arranging transfer of the hCR1 tg to this mouse to allow this question to be addressed more thoroughly.
It has also been recently proposed that many of the changes noted in the mCR1/2 deficient mice are a result of an increase in C activation that disrupts normal lymphoid tissues/cell profiles in the follicles and around the FDC (Jacobson et al, 2008). As hCR1 in the mouse is a potent regulator of C activation (Pemberton et al, 1993) and the fact we found a restoration to normal marginal zone cell percentages in hCR1.CR1/2 deficient mice (figure 4) could indicate that the expansion of marginal zone B cells in CR1/2 deficient mice is a result of the presence of increased anaphylotoxins levels in the splenic microenvironment. It would be interesting to directly test that through backcross to C3aR and C5aR receptor deficient animals or use other approaches to limit the effects of anaphylotoxins in mCR1/2 deficient mice. As it stands, our studies do not allow us to directly address what effect hCR1 may have on any resultant C activation imbalance due to loss of endogenous mCR1/2 (Jacobson et al, 2008) but the development of hCR1 transgenic mice do offer a way to tease out the effects of loss of mCR1/2 that can be attributed to loss of B cell signaling versus a general increase in the levels of pro-inflammatory complement components due to the loss of murine CR1 function complement regulatory function in the lymphoid compartment. Further to that, murine CR1/2 has also been shown to have a role in the transport of IC from macrophages in the subcapsular sinus, via follicular B cells (expressing CR1/2), to FDCs (Phan et al, 2007). Indeed, in vivo tracking using CR1/2 mAb has also provided evidence of a transfer mechanism between marginal zone B cells and FDCs reliant on mCR1/2 (Whipple et al, 2004). Thus, the fact that hCR1 can bind to murine C3b (Alexander et al, 2010; Kai et al, 1980) and transport IC suggest the hCR1 tg mice will be an excellent tool to study IC transfer reactions recently described in mice (Batista & Harwood, 2009; Cyster, 2010; Harwood & Batista, 2009; Phan et al, 2007; Whipple et al, 2004) and help establish whether CR1 or CR2 ligands are key in this important stage of the immune response to particulate antigens.
We conclude that hCR1 alone cannot replace the role of CR2 in mice. It is also likely that the changes in humoral immune response noted in the wild type mice expressing hCR1 could be a result of the increase in C3 convertase activity/C3b breakdown and/or the increase in endogenous protein mCR1/2 proteins brought about by hCR1 expression. Interestingly, the effects of premature hCR2 expression during BCR development are not significantly altered by the addition of hCR1 at that developmental stage or beyond. This data further confirms the assertion that hCR2 expression and its effects on signaling in the mouse B cell remain dominant over subsequent input from either hCR1 or endogenous receptors.
Supplementary Material
Acknowledgements
This work was supported by the Wellcome Trust (IYP, KJM; WT068541), the Northern Counties Kidney Research Fund and Newcastle Joint health care charities. The authors thank the assistance of the members of staff at the animal and flow cytometry facilities in both Cardiff and Newcastle Universities.
These studies were supported by a Wellcome Trust Career Development Award (KJM; WT068541), Newcastle Joint health care charities and the Northern counties kidney research fund.
Abbreviations
- CR
Complement receptor
- hCR1
human Complement receptor 1
- mCR1/2
mouse complement receptor 1 and complement receptor 2
- SCR
short consensus repeat
- HRPO
horse-radish peroxidase
- SRBC
sheep red blood cells
- BM
bone marrow
- GC
germinal center
- MZ
marginal Zone
- FO
follicular
- SLE
systemic lupus erythematosus
- Ig
Immunoglobulin
- B6 or WT
C57BL/6 mice
- RT-PCR
reverse transcriptase polymerase chain reaction
- IC
immune complex
- FDC
follicular dendritic cells
References
- Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4(3):251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Hack BK, Cunningham PN, Quigg RJ. A protein with characteristics of factor H is present on rodent platelets and functions as the immune adherence receptor. J Biol Chem. 2001;276(34):32129–32135. doi: 10.1074/jbc.M101299200. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Hack BK, Jacob A, Chang A, Haas M, Finberg RW, Quigg RJ. Abnormal immune complex processing and spontaneous glomerulonephritis in complement factor H-deficient mice with human complement receptor 1 on erythrocytes. J Immunol. 2010;185(6):3759–3767. doi: 10.4049/jimmunol.1000683. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Quigg RJ. The simple design of complement factor H: Looks can be deceiving. Mol Immunol. 2007;44(1–3):123–132. doi: 10.1016/j.molimm.2006.07.287. [DOI] [PubMed] [Google Scholar]
- Bacle F, Haeffner-Cavaillon N, Laude M, Couturier C, Kazatchkine MD. Induction of IL-1 release through stimulation of the C3b/C4b complement receptor type one (CR1, CD35) on human monocytes. J Immunol. 1990;144(1):147–152. [PubMed] [Google Scholar]
- Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9(1):15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- Birrell L, Kulik L, Morgan BP, Holers VM, Marchbank KJ. B cells from mice prematurely expressing human complement receptor type 2 are unresponsive to T-dependent antigens. J Immunol. 2005;174(11):6974–6982. doi: 10.4049/jimmunol.174.11.6974. [DOI] [PubMed] [Google Scholar]
- Bradbury LE, Kansas GS, Levy S, Evans RL, Tedder TF. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149(9):2841–2850. [PubMed] [Google Scholar]
- Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11(11):989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271(5247):348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160(11):5273–5279. [PubMed] [Google Scholar]
- Fearon DT. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthridge JM, Young K, Gipson MG, Sarrias MR, Szakonyi G, Chen XS, Malaspina A, Donoghue E, James JA, Lambris JD, Moir SA, et al. Epitope mapping using the X-ray crystallographic structure of complement receptor type 2 (CR2)/CD21: identification of a highly inhibitory monoclonal antibody that directly recognizes the CR2-C3d interface. J Immunol. 2001;167(10):5758–5766. doi: 10.4049/jimmunol.167.10.5758. [DOI] [PubMed] [Google Scholar]
- Haas KM, Hasegawa M, Steeber DA, Poe JC, Zabel MD, Bock CB, Karp DR, Briles DE, Weis JH, Tedder TF. Complement receptors CD21/35 link innate and protective immunity during Streptococcus pneumoniae infection by regulating IgG3 antibody responses. Immunity. 2002;17(6):713–723. doi: 10.1016/s1074-7613(02)00483-1. [DOI] [PubMed] [Google Scholar]
- Harwood NE, Batista FD. The antigen expressway: follicular conduits carry antigen to B cells. Immunity. 2009;30(2):177–179. doi: 10.1016/j.immuni.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Hivroz C, Fischer E, Kazatchkine MD, Grillot-Courvalin C. Differential effects of the stimulation of complement receptors CR1 (CD35) and CR2 (CD21) on cell proliferation and intracellular Ca2+ mobilization of chronic lymphocytic leukemia B cells. J Immunol. 1991;146(6):1766–1772. [PubMed] [Google Scholar]
- Holers VM, Kinoshita T, Molina H. The evolution of mouse and human complement C3-binding proteins: divergence of form but conservation of function. Immunol Today. 1992;13(6):231–236. doi: 10.1016/0167-5699(92)90160-9. [DOI] [PubMed] [Google Scholar]
- Holers VM, Seya T, Brown E, O'Shea JJ, Atkinson JP. Structural and functional studies on the human C3b/C4b receptor(CR1) purified by affinity chromatography using a monoclonal antibody. Complement. 1986;3(2):63–78. doi: 10.1159/000467882. [DOI] [PubMed] [Google Scholar]
- Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, Makarova N, Perez DR, Macdonald GH, McCullers JA. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006;13(9):981–990. doi: 10.1128/CVI.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iking-Konert C, Stocks S, Weinsberg F, Engelbrecht R, Bleck E, Perniok A, Fischer-Betz R, Pincus S, Nardone L, Schneider M. First clinical trials of a new heteropolymer technology agent in normal healthy volunteers and patients with systemic lupus erythematosus: safety and proof of principle of the antigen-heteropolymer ETI-104. Ann Rheum Dis. 2004;63(9):1104–1112. doi: 10.1136/ard.2003.016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson AC, Weis JJ, Weis JH. Complement receptors 1 and 2 influence the immune environment in a B cell receptor-independent manner. J Immunol. 2008;180(7):5057–5066. doi: 10.4049/jimmunol.180.7.5057. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. edn. Garland; 2001. [Google Scholar]
- Jozsi M, Prechl J, Bajtay Z, Erdei A. Complement receptor type 1 (CD35) mediates inhibitory signals in human B lymphocytes. J Immunol. 2002;168(6):2782–2788. doi: 10.4049/jimmunol.168.6.2782. [DOI] [PubMed] [Google Scholar]
- Kai S, Fujita T, Gigli I, Nussenzweig V. Mouse C3b/C4b inactivator: purification and properties. J Immunol. 1980;125(6):2409–2415. [PubMed] [Google Scholar]
- Kaya Z, Tretter T, Schlichting J, Leuschner F, Afanasyeva M, Katus HA, Rose NR. Complement receptors regulate lipopolysaccharide-induced T-cell stimulation. Immunology. 2005;114(4):493–498. doi: 10.1111/j.1365-2567.2004.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Takeda J, Hong K, Kozono H, Sakai H, Inoue K. Monoclonal antibodies to mouse complement receptor type 1 (CR1). Their use in a distribution study showing that mouse erythrocytes and platelets are CR1-negative. J Immunol. 1988;140(9):3066–3072. [PubMed] [Google Scholar]
- Krych-Goldberg M, Atkinson JP. Structure-function relationships of complement receptor type 1. Immunol Rev. 2001;180:112–122. doi: 10.1034/j.1600-065x.2001.1800110.x. [DOI] [PubMed] [Google Scholar]
- Kulik L, Chen K, Huber BT, Holers VM. Human complement receptor type 2 (CR2/CD21) transgenic mice provide an in vivo model to study immunoregulatory effects of receptor antagonists. Mol Immunol. 2011;48(6–7):883–894. doi: 10.1016/j.molimm.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Kulik L, Marchbank KJ, Lyubchenko T, Kuhn KA, Liubchenko GA, Haluszczak C, Gibson MG, Boackle SA, Holers VM. Intrinsic B cell hypo-responsiveness in mice prematurely expressing human CR2/CD21 during B cell development. Eur J Immunol. 2007;37(3):623–633. doi: 10.1002/eji.200636248. [DOI] [PubMed] [Google Scholar]
- Kurtz CB, O'Toole E, Christensen SM, Weis JH. The murine complement receptor gene family. IV. Alternative splicing of Cr2 gene transcripts predicts two distinct gene products that share homologous domains with both human CR2 and CR1. J Immunol. 1990;144(9):3581–3591. [PubMed] [Google Scholar]
- Law SK, Fearon DT, Levine RP. Action of the C3b-inactivator on the cell-bound C3b. J Immunol. 1979;122(3):759–765. [PubMed] [Google Scholar]
- Lee Y, Haas KM, Gor DO, Ding X, Karp DR, Greenspan NS, Poe JC, Tedder TF. Complement component C3d-antigen complexes can either augment or inhibit B lymphocyte activation and humoral immunity in mice depending on the degree of CD21/CD19 complex engagement. J Immunol. 2005;175(12):8011–8023. doi: 10.4049/jimmunol.175.12.8011. [DOI] [PubMed] [Google Scholar]
- Li J, Wang JP, Ghiran I, Cerny A, Szalai AJ, Briles DE, Finberg RW. Complement receptor 1 expression on mouse erythrocytes mediates clearance of Streptococcus pneumoniae by immune adherence. Infect Immun. 2010;78(7):3129–3135. doi: 10.1128/IAI.01263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbank KJ, Kulik L, Gipson MG, Morgan BP, Holers VM. Expression of human complement receptor type 2 (CD21) in mice during early B cell development results in a reduction in mature B cells and hypogammaglobulinemia. J Immunol. 2002;169(7):3526–3535. doi: 10.4049/jimmunol.169.7.3526. [DOI] [PubMed] [Google Scholar]
- Marchbank KJ, Watson CC, Ritsema DF, Holers VM. Expression of human complement receptor 2 (CR2, CD21) in Cr2-/- mice restores humoral immune function. J Immunol. 2000;165(5):2354–2361. doi: 10.4049/jimmunol.165.5.2354. [DOI] [PubMed] [Google Scholar]
- Matsumoto AK, Kopicky-Burd J, Carter RH, Tuveson DA, Tedder TF, Fearon DT. Intersection of the complement and immune systems: a signal transduction complex of the B lymphocyte-containing complement receptor type 2 and CD19. J Exp Med. 1991;173(1):55–64. doi: 10.1084/jem.173.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto AK, Martin DR, Carter RH, Klickstein LB, Ahearn JM, Fearon DT. Functional dissection of the CD21/CD19/TAPA-1/Leu-13 complex of B lymphocytes. J Exp Med. 1993;178(4):1407–1417. doi: 10.1084/jem.178.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci U S A. 1996;93(8):3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina H, Kinoshita T, Inoue K, Carel JC, Holers VM. A molecular and immunochemical characterization of mouse CR2. Evidence for a single gene model of mouse complement receptors 1 and 2. J Immunol. 1990;145(9):2974–2983. [PubMed] [Google Scholar]
- Mongini PK, Vilensky MA, Highet PF, Inman JK. The affinity threshold for human B cell activation via the antigen receptor complex is reduced upon co-ligation of the antigen receptor with CD21 (CR2) J Immunol. 1997;159(8):3782–3791. [PubMed] [Google Scholar]
- Mulligan MS, Yeh CG, Rudolph AR, Ward PA. Protective effects of soluble CR1 in complement- and neutrophil-mediated tissue injury. J Immunol. 1992;148(5):1479–1485. [PubMed] [Google Scholar]
- Pappworth IY, Kulik L, Haluszczak C, Reuter JW, Holers VM, Marchbank KJ. Increased B cell deletion and significantly reduced auto-antibody titre due to premature expression of human complement receptor 2 (CR2, CD21) Mol Immunol. 2009 doi: 10.1016/j.molimm.2008.08.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton M, Anderson G, Vetvicka V, Justus DE, Ross GD. Microvascular effects of complement blockade with soluble recombinant CR1 on ischemia/reperfusion injury of skeletal muscle. J Immunol. 1993;150(11):5104–5113. [PubMed] [Google Scholar]
- Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8(9):992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- Prodeus AP, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot EM, Goodnow CC, Carroll MC. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9(5):721–731. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- Ramaglia V, Wolterman R, de Kok M, Vigar MA, Wagenaar-Bos I, King RH, Morgan BP, Baas F. Soluble complement receptor 1 protects the peripheral nerve from early axon loss after injury. Am J Pathol. 2008;172(4):1043–1052. doi: 10.2353/ajpath.2008.070660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Young J, Weis JJ, Weis JH. Expression of the mouse fragilis gene products in immune cells and association with receptor signaling complexes. Genes Immun. 2006;7(2):113–121. doi: 10.1038/sj.gene.6364278. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kozono Y, Waldschmidt TJ, Berthiaume D, Quigg RJ, Baron A, Holers VM. Mouse complement receptors type 1 (CR1;CD35) and type 2 (CR2;CD21): expression on normal B cell subpopulations and decreased levels during the development of autoimmunity in MRL/lpr mice. J Immunol. 1997;159(3):1557–1569. [PubMed] [Google Scholar]
- Takehara HA, Perini A, da Silva MH, Mota I. Trypanosoma cruzi: role of different antibody classes in protection against infection in the mouse. Exp Parasitol. 1981;52(1):137–146. doi: 10.1016/0014-4894(81)90069-2. [DOI] [PubMed] [Google Scholar]
- Thieblemont N, Haeffner-Cavaillon N, Ledur A, L'Age-Stehr J, Ziegler-Heitbrock HW, Kazatchkine MD. CR1 (CD35) and CR3 (CD11b/CD18) mediate infection of human monocytes and monocytic cell lines with complement-opsonized HIV independently of CD4. Clin Exp Immunol. 1993;92(1):106–113. doi: 10.1111/j.1365-2249.1993.tb05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokos GC, Berger M, Balow JE. Modulation of human B cell immunoglobulin secretion by the C3b component of complement. J Immunol. 1984;132(2):622–626. [PubMed] [Google Scholar]
- Tuveson DA, Ahearn JM, Matsumoto AK, Fearon DT. Molecular interactions of complement receptors on B lymphocytes: a CR1/CR2 complex distinct from the CR2/CD19 complex. J Exp Med. 1991;173(5):1083–1089. doi: 10.1084/jem.173.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twohig J, Kulik L, Haluszczak C, Reuter J, Rossbach A, Bull M, Holers VM, Marchbank KJ. Defective B cell ontogeny and immune response in human complement receptor 2 (CR2, CD21) transgenic mice is partially recovered in the absence of C3. Mol Immunol. 2007;44(13):3434–3444. doi: 10.1016/j.molimm.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twohig JP, Pappworth IY, Sivasankar B, Kulik L, Bull M, Holers VM, Wang EC, Marchbank KJ. Defective B cell ontogeny and humoral immune response in mice prematurely expressing human complement receptor 2 (CR2, CD21) is similar to that seen in aging wild type mice. Mol Immunol. 2009;46(10):2002–2013. doi: 10.1016/j.molimm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynova E, Tchorbanov A, Prechl J, Nikolova M, Baleva M, Erdei A, Vassilev T. An antibody-based construct carrying DNA-mimotope and targeting CR1(CD35) selectively suppresses human autoreactive B-lymphocytes. Immunol Lett. 2008;116(2):168–173. doi: 10.1016/j.imlet.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Weisman HF, Bartow T, Leppo MK, Boyle MP, Marsh HC, Jr, Carson GR, Roux KH, Weisfeldt ML, Fearon DT. Recombinant soluble CR1 suppressed complement activation, inflammation, and necrosis associated with reperfusion of ischemic myocardium. Trans Assoc Am Physicians. 1990a;103:64–72. [PubMed] [Google Scholar]
- Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990b;249(4965):146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- Whipple EC, Shanahan RS, Ditto AH, Taylor RP, Lindorfer MA. Analyses of the in vivo trafficking of stoichiometric doses of an anti-complement receptor 1/2 monoclonal antibody infused intravenously in mice. J Immunol. 2004;173(4):2297–2306. doi: 10.4049/jimmunol.173.4.2297. [DOI] [PubMed] [Google Scholar]
- Wu X, Jiang N, Deppong C, Singh J, Dolecki G, Mao D, Morel L, Molina HD. A role for the Cr2 gene in modifying autoantibody production in systemic lupus erythematosus. J Immunol. 2002;169(3):1587–1592. doi: 10.4049/jimmunol.169.3.1587. [DOI] [PubMed] [Google Scholar]
- Wu X, Jiang N, Fang YF, Xu C, Mao D, Singh J, Fu YX, Molina H. Impaired affinity maturation in Cr2-/- mice is rescued by adjuvants without improvement in germinal center development. J Immunol. 2000;165(6):3119–3127. doi: 10.4049/jimmunol.165.6.3119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.