Abstract
Background
The effects of neoadjuvant hormonal therapy (NHT) on pathological features and lymphangiogenesis in patients with prostate cancer (PCa) for each pre‐operative risk classification are unclear.
Methods
To clarify the anti‐cancer effects of NHT, we investigated 153 patients (non‐NHT group = 80 and NHT group = 73) who underwent radical prostatectomy (RP) in Nagasaki University Hospital. Lymph vessel density and area (evaluated by D2‐40‐positive vessels), vascular endothelial growth factor (VEGF)‐C and VEGF‐D expressions, and biochemical recurrence (BCR)‐free survival were compared between these two groups for each D'Amico risk classification (low = 33, intermediate = 58, high = 62 patients).
Results
In low‐risk PCa patients, the risks of lymph vessel invasion and BCR were significantly higher in the NHT group than in the non‐NHT group (P = 0.040 and 0.022, respectively). Such significant difference was not seen in the intermediate‐ or high‐risk PCa groups. Lymph vessel density of the peri‐tumoral and intra‐tumoral areas and the lymph vessel area were significantly higher (P < 0.001) in the NHT group than in the non‐NHT group in low‐risk PCa. In regard to the expression of VEGF‐C or VEGF‐D, significant difference was not detected in low‐risk PCa.
Conclusions
NHT stimulated cancer cell progression and BCR via up‐regulation of lymphangiogenesis‐related parameters in patients with low‐risk PCa. Although VEGF‐C and VEGF‐D expressions were not changed by NHT, lymph vessel density and area were increased in low‐risk PCa patients. We suggest that NHT for patients with low‐risk PCa may have a high risk for BCR after RP.
Keywords: biochemical recurrence, lymphangiogenesis, neoadjuvant hormonal therapy, prostate cancer, risk classification
1. INTRODUCTION
Prostate cancer (PCa) is the most common malignancy in men worldwide.1 Androgen deprivation therapy (ADT) is one of the standard tools of care for patients with PCa. Neoadjuvant hormonal therapy (NHT) based on androgen deprivation is administered to improve the successful rate and prognosis of local therapy, including radical prostatectomy (RP) and radiotherapy, in patients with PCa.2, 3 There is general agreement that positive surgical margin or extra‐prostatic extension in RP specimens in patients with low grade and stage PCa is relatively rare, and outcome in these patients is favorable.4 Therefore, NHT is usually selected for patients with high‐risk PCa, as well as some with patients with intermediate‐risk PCa. However, some patients with low‐risk PCa have been administered NHT for a variety of reasons, for example, anxiety due to waiting periods or the patient's wishes. Unfortunately, the anti‐cancer effects of NHT in patients with low‐risk PCa are not fully understood, because such patients are relatively rare. Similarly, the influences of NHT on pathological characteristics and cancer‐related factors according to risk grade have not yet been investigated.
Up‐regulation of lymphangiogenesis leads to increased risk of metastasis and worse prognosis in various types of cancer.5, 6 The most well‐known and strongest regulators of cancer‐related lymphangiogenesis are vascular endothelial growth factor (VEGF)‐C and VEGF‐D.7, 8 The expressions of VEGF‐C and VEGF‐D in human prostate cancer tissues were reported to be positively associated with the metastatic spread of cancer cells.9, 10 On the other hand, several investigators showed that the expressions of VEGF‐C and VEGF‐D in an androgen‐dependent prostate cancer cell line (LNCaP) were up‐regulated by androgen depletion.11, 12, 13 In addition to such in vitro studies, the possibility that ADT may stimulate lymphangiogenesis has been reported in human prostate cancer tissues.13 From these facts, we hypothesized that ADT might stimulate cancer cell dissemination via up‐regulation of lymphangiogenesis in PCa.
In this study, we investigate the differences in pathological features and biochemical recurrence (BCR) between RP tissues from PCa patients who received NHT and those who did not receive NHT according to risk classification. Next, to analyze the detailed anti‐cancer mechanism of NHT, lymphangiogenesis‐related parameters and the expression of VEGF‐C and VEGF‐D were also compared between these two groups.
2. MATERIALS AND METHODS
2.1. Patients
We investigated 153 tissues (non‐NHT group = 80 and NHT group = 73) from patients who underwent RP in the Nagasaki University Hospital. To match the clinicopathological features between the two groups, patients who had clinical or pathological invasion into the seminal vesicle or surrounding tissues, presence of metastasis, or a Gleason score (GS) of 10 were excluded. In addition, we also excluded patients with a short duration of NHT (<3 months) or serum prostate‐specific antigen (PSA) levels >90 ng/mL. The study protocol was approved by the Human Ethics Review Committee of the Nagasaki University Hospital, and written informed consent was obtained from each subject. In RP specimens with NHT, necrotic or degenerated area was not evaluated. NHT consisted of an anti‐androgen agent (n = 2, 2.7%), luteinizing hormone‐releasing hormone (LH‐RH) agonists (n = 33, 45.2%), or maximum androgen blockade (n = 38, 52.1%). The median duration of NHT was 7 months (mean, 8.2 months; interquartile range, IQR: 4‐10 months). BCR was defined as serum PSA levels >0.2 ng/mL, as measured on two or more occasions. Risk classification was defined according to the D'Amico risk stratification system.14
2.2. Lymphangiogenesis‐related factors
To evaluate lymphangiogenesis, we measured lymph vessel density (LVD) and lymph vessel area (LVA) by staining for D2‐40‐positive vessels. In addition, the expressions of VEGF‐C and VEGF‐D were also analyzed. These analyses were performed by immunohistochemical staining as described previously.13 Briefly, antigen retrieval was performed at 95°C for 40 min in 0.01 M sodium citrate buffer (pH 6.0). Sections were then immersed in 3% hydrogen peroxide for 30 min to block endogenous peroxidase activity, and then incubated with the primary antibodies (D2‐40: DakoCytomation, Glostrup, Denmark; VEGF‐C: Zymed Laboratories, San Francisco, CA; and VEGF‐D: R&D Systems, Abingdon, UK) at 4°C overnight. The samples were treated with labeled polymer peroxidase from the EnVision+ Peroxidase kit (Dako, Carpinteria, CA) for 60 min. Negative controls consisted of adjacent sections from each sample that were processed without the primary antibody. The positive control for all antibodies was kidney tissue with renal cell carcinoma.
2.3. Evaluation of lymphangiogenesis‐related factors
VEGF expression was semi‐quantitatively analyzed as previously described.15 To determine LVD and LVA, sections labelled with anti‐D2‐40 antibody were examined. For each tumor section, three to five hot spots in the field of view (ie, with the greatest density of positively stained vessels) were evaluated. LVD was defined as the number of positively stained vessels per high‐power field. According to a previous report,5 the terms intra‐ and peri‐tumoral denote within the tumor mass and within an area of 500 µM from the tumor border, respectively. Evaluation and measurements were performed by computer‐aided image analysis (WinROOF version 6.4; Mitani, Fukui, Japan). In similar to pathological features, necrotic or degenerated tissues were not evaluated for lymphangiogenesis‐related factors.
2.4. Statistical analyses
Results are expressed as mean ± SD. The Student's t‐test was applied to continuous variables and the Mann‐Whitney U‐test was used for other data. The χ 2 test and Fisher exact test were used for categorical data comparisons. The Kaplan‐Meier survival curve and log‐rank test along with multivariate analysis using the Cox proportional hazards model were used to assess patient survival. All statistical analyses were performed using the StatView v.5.0 for Windows software (Abacus Concepts, San Francisco, CA).
3. RESULTS
3.1. Pathological features
As shown in Table 1, all pre‐operative parameters, such as serum PSA levels at diagnosis, GS of biopsy specimens, and clinical T stage, were higher in the NHT group than in the non‐NHT group; however, these differences between the two groups were not significant (Table 1). With regard to pathological features of the RP specimens, the frequency of lymph node metastasis in patients was higher in the NHT group (6.8%) than in the non‐NHT group (1.1%); however, the difference did not reach statistical significance (Table 1; P = 0.075). Furthermore, no other pathological parameter showed any statistical difference between the two groups (Table 1).
Table 1.
Pathological features and status of neoadjuvant hormonal therapy
| Patients, N (%) | Non‐NHT, N = 80 | NHT, N = 73 | P‐value | |
|---|---|---|---|---|
| At diagnosis | ||||
| s‐PSA levels a (ng/mL) | 153 | 11.87/7.72 | 14.35/9.75 | 0.082 |
| Gleason score | 0.624 | |||
| Low: ‐6 | 50 (32.7) | 28 (35.0) | 22 (30.1) | |
| Middle: 7 | 63 (41.2) | 30 (37.5) | 33 (45.2) | |
| High: 8‐ | 40 (26.1) | 22 (27.5) | 18 (24.7) | |
| T stage | 0.083 | |||
| T1 | 61 (39.9) | 38 (47.5) | 23 (31.5) | |
| T2 | 75 (49.0) | 36 (45.0) | 39 (53.4) | |
| T3 | 17 (11.1) | 6 (7.5) | 11 (15.1) | |
| At operation | ||||
| pT stage | 0.274 | |||
| T2 | 98 (64.1) | 48 (60.0) | 50 (68.5) | |
| T3 | 55 (35.9) | 32 (40.0) | 23 (31.5) | |
| pN stage | 0.075 | |||
| N0 | 147 (96.1) | 79 (98.8) | 68 (93.2) | |
| N1 | 6 (3.9) | 1 (1.2) | 5 (6.8) | |
| Lymphatic invasion | 0.284 | |||
| Negative | 79 (51.6) | 38 (47.5) | 41 (56.2) | |
| Positive | 74 (48.4) | 42 (52.5) | 32 (43.8) | |
| Vascular invasion | 0.507 | |||
| Negative | 105 (68.6) | 53 (66.3) | 52 (71.2) | |
| Positive | 48 (31.4) | 27 (33.8) | 21 (28.8) | |
| Neural invasion | 0.674 | |||
| Negative | 76 (49.7) | 38 (47.5) | 38 (52.1) | |
| Positive | 77 (50.3) | 42 (52.5) | 35 (47.9) | |
NHT, neoadjuvant hormonal therapy; s‐PSA, serum prostate‐specific antigen.
Data were showed as mean/SD.
Relationships between pathological features and NHT in RP specimens according to D'Amico risk classification are shown in Table 2. There was no significant difference in pT stage or lymph node metastasis between the non‐NHT and NHT groups across all D'Amico risk classifications. Similar results were also found for venous invasion and nerve invasion (Table 2). In the non‐NHT group, lymphatic invasion was more frequent with increasing risk grade (low‐risk = 26.3%, intermediate‐risk = 51.6%, high‐risk = 70.0%). However, in the NHT group, the rate of lymphatic invasion in patients with low‐risk disease (64.3%) was higher compared to that in patients with intermediate‐ (29.7%) and high‐risk disease (46.9%). In addition, in patients with low‐risk prostate cancer, the frequency of lymphatic invasion was significantly higher in the NHT group (64.3%) than in the non‐NHT group (26.3%; P = 0.029) (Table 2). Although a similar trend was observed in the intermediate‐ and high‐risk patients, this difference did not reach statistical significance (P = 0.090 and 0.065, respectively).
Table 2.
Pathological features in radical surgical specimens according to D'Amico risk classification
| Low risk | Intermediate risk | High risk | ||||
|---|---|---|---|---|---|---|
| Non‐NHT, N = 19 | NHT, N = 14 | Non‐NHT, N = 31 | NHT, N = 27 | Non‐NHT, N = 30 | NHT, N = 32 | |
| pT stage | ||||||
| T2 | 14 (73.7) | 12 (85.7) | 20 (64.5) | 18 (66.7) | 14 (46.7) | 20 (62.5) |
| T3 | 5 (26.3) | 2 (14.3) | 11 (35.5) | 9 (33.3) | 16 (53.3) | 12 (37.5) |
| P‐value | 0.404 | 0.864 | 0.211 | |||
| pN stage | ||||||
| N0 | 19 (100) | 14 (100) | 31 (100) | 26 (96.3) | 29 (96.7) | 28 (87.5) |
| N1 | 0 (0) | 0 (0) | 0 (0) | 1 (3.7) | 1 (0.3) | 4 (12.5) |
| P‐value | – | 0.280 | 0.185 | |||
| Lymphatic invasion | ||||||
| Negative | 14 (73.7) | 5 (35.7) | 15 (48.4) | 19 (70.3) | 9 (30.0) | 17 (53.1) |
| Positive | 5 (26.3) | 9 (64.3) | 16 (51.6) | 8 (29.7) | 21 (70.0) | 15 (46.9) |
| P‐value | 0.029 | 0.090 | 0.065 | |||
| Vascular invasion | ||||||
| Negative | 15 (78.9) | 10 (71.4) | 20 (64.5) | 22 (81.5) | 18 (60.0) | 20 (62.5) |
| Positive | 4 (21.1) | 4 (28.6) | 11 (35.5) | 5 (18.5) | 12 (40.0) | 12 (27.5) |
| P‐value | 0.618 | 0.149 | 0.840 | |||
| Neural invasion | ||||||
| Negative | 12 (63.2) | 8 (57.1) | 12 (38.7) | 16 (59.3) | 14 (46.7) | 14 (43.8) |
| Positive | 7 (36.8) | 6 (42.9) | 19 (51.3) | 11 (40.7) | 16 (53.3) | 18 (56.2) |
| P‐value | 0.727 | 0.118 | 0.818 | |||
| NHT | ||||||
| Anti‐androgen | – | 1 (7.1) | – | 1 (3.7) | – | 0 (0.0) |
| LH‐RH agonist | – | 11 (78.6) | – | 14 (51.9) | – | 8 (25.0) |
| MAB | – | 2 (14.3) | – | 12 (44.4) | – | 24 (75.0) |
NHT, neoadjuvant hormonal therapy; LH‐RH, luteinizing hormone‐releasing hormone; MAB, maximum androgen blockage.
3.2. Biochemical recurrence
Kaplan‐Meier survival curves showed that the BCR‐free survival rate in the NHT group was significantly worse compared to the non‐NHT group in patients with low‐risk disease (P = 0.022; Figure 1A). There was no significant difference between the non‐NHT and NHT groups in patients with intermediate‐ (P = 0.713; Figure 1B) and high‐risk disease (P = 0.732; Figure 1C). A multivariate analysis model including D'Amico risk classification and NHT showed that NHT was not an independent predictive factor for BCR‐free survival (hazard ratio = 1.45, 95% confidence interval = 0.85‐2.49; P = 0.174).
Figure 1.
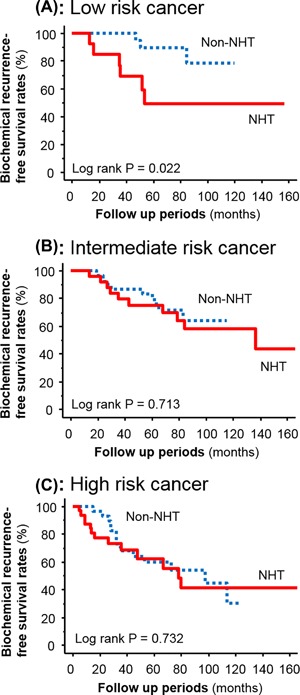
Kaplan‐Meier survival curves showing biochemical recurrence‐free survival in patients receiving neoadjuvant hormonal therapy (NHT) versus patients not receiving NHT (non‐NHT) in low‐risk prostate cancer (A), intermediate‐risk prostate cancer (B) and high‐risk prostate cancer (C)
3.3. Lymphangiogenesis
Representative images of D2‐40‐positive lymph vessels in PCa tissues are shown in Figure 2. In the non‐NHT group, nearly all of the D2‐40‐positive vessels were relapsed and the intraluminal space was narrow (Figure 2A). In particular, there were few lymph vessels with a lumen in the intra‐tumoral area of samples from patients in the non‐NHT group. In fact, we could not measure the LVA in the intra‐tumoral area. In contrast, D2‐40‐positive lymph vessels in tissues from patients in the NHT group had a wider inner cavity compared to the non‐NHT group (Figure 2B). In addition, freed from pressure by tumor mass and contained cancer cells were detected in non‐NHT group (Figure 2C). The level of peri‐LVD was significantly higher in the NHT group (10.3 ± 3.1) than in the non‐NHT group (7.9 ± 4.0; P < 0.001). When similar analyses were performed according to D'Amico risk classification, a significant difference was detected in low‐risk patients (P < 0.001), but not in intermediate‐ or high‐risk patients (P = 0.079 and P = 0.108, respectively) (Figure 3A). In regard to the relationship between intra‐LVD and D'Amico risk classification, a significant difference was detected in low‐risk patients (P < 0.001), but not in intermediate‐ or high‐risk patients (P = 0.199 and 0.754, respectively) (Figure 3B). Peri‐LVA was significantly higher in the NHT group (481.8 ± 133.5) than in the non‐NHT group (230.3 ± 104.9; P < 0.001), and this difference was significant across all D'Amico risk classifications (Figure 3C). However, the degree of difference in peri‐LVA between the non‐NHT and NHT groups decreased according to D'Amico risk classification (Figure 3C).
Figure 2.
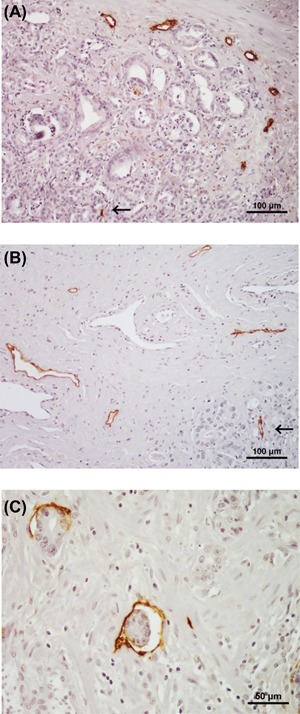
Representative examples of D2‐40‐positive lymph vessels in prostate cancer tissue from patients who did not received neoadjuvant hormonal therapy (non‐NHT) (A) and received NHT (B). As shown in A, most lymph vessels are relapsed and the intraluminal space is narrow in the non‐NHT sample (Magnification ×200). On the other hand, lymph vessels in the NHT group had a relatively wide inner cavity (B: Magnification ×200). In regard to lymph vessel in intra‐tumoral area (allow), intraluminal space in NHT group (B) is wider compared to that in non‐NHT group (A). In addition, vessels freed from pressure by tumor mass and contained some cells were found in the NHT specimen (C: magnification ×400)
Figure 3.
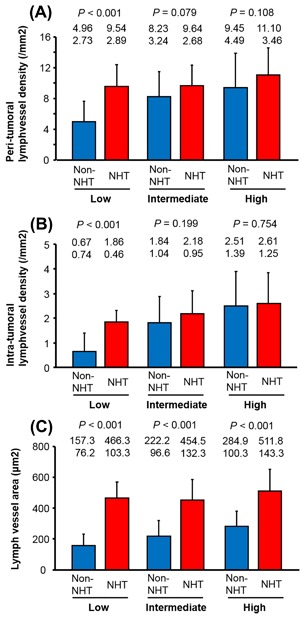
Peri‐tumoral (A) and intra‐tumoral (B) lymph vessel density in prostate cancer patients receiving neoadjuvant hormonal therapy (NHT) and those not receiving NHT (non‐NHT) in low‐, intermediate‐, and high‐risk prostate cancer. Lymph vessel area in patients receiving NHT and those not receiving NHT (non‐NHT) in low‐, intermediate‐, and high‐risk prostate cancer (C). Data are shown as mean (upper row) and standard deviation (lower row)
3.4. Lymphangiogenesis‐related factors
Across all risk groups, the IRS of VEGF‐C expression was significantly higher in the NHT group (2.8 ± 1.3) than in the non‐NHT group (2.3 ± 1.0; P = 0.013). However, in contrast to LVD and LVA, the difference between the two groups in low‐risk patients was not significant (P = 0.205; Figure 4). As with VEGF‐C, there was no significant difference in VEGF‐D expression between the two groups in low‐risk patients (P = 0.108; Figure 4), although the difference was significant in intermediate‐ and high‐risk patients (P = 0.011 and 0.004, respectively; data not shown).
Figure 4.
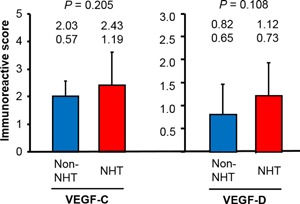
Vascular endothelial growth factor (VEGF)‐C and VEGF‐D expression in low‐risk prostate cancer patients receiving neoadjuvant hormonal therapy (NHT) and those not receiving NHT (non‐NHT). Data are shown as mean (upper row) and SD (lower row)
4. DISCUSSION
Several clinical trials have shown that NHT can significantly decrease the rate of positive surgical margin and the risk of extra‐prostatic disease extension in PCa patients treated with RP.2, 16, 17, 18 On the other hand, studies have also shown that NHT has no impact on down‐staging or improvement of outcome in these patients.19 Speculated reasons for this discrepancy include differences in study population, pathological backgrounds, and methods of NHT. We also believe that inclusion of prostate cancer patients with low‐risk disease influenced our results on the anti‐cancer effects of NHT, because the clinical merits of NHT in low‐risk disease are guessed to be minimum at best, and potentially none. However, surprisingly, our results showed that NHT led to cancer progression and a shortening of the BCR‐free survival period in low‐risk PCa patients, but not in intermediate‐ or high‐risk patients.
In order to explain the mechanisms behind these findings, we investigated lymphangiogenesis‐related parameters according to risk classification. Our results showed that LVA was significantly higher in the NHT group than in the non‐NHT group in all risk classifications. Intra‐ and peri‐LVD were also significantly different between the non‐NHT group and the NHT group, although only in patients with low‐risk PCa. In short, all lymphangiogenesis‐related parameters were higher in RP tissues from the NHT group compared to those from the non‐NHT group in low‐risk PCa patients only. Increased LVA and LVD are known to be positively associated with tumor development and worse prognosis in patients with PCa.20, 21 From these facts, we speculated that up‐regulation of lymphangiogenesis by NHT was one of the reasons for this association. There is a question as to why increased LVA was not associated with progression in patients with intermediate‐ and high‐risk PCa. Although we did not determine the reason for this phenomenon in this study, we postulate that the malignant behavior of prostate cancer cells in high grade and stage disease is regulated by more varied and stronger cancer‐related factors.
Next, we analyzed the detailed mechanisms of lymphangiogenesis under NHT in tissues from patients with low‐risk PCa. At first, we hypothesized that VEGF‐C and/or VEGF‐D would be up‐regulated by NHT, because VEGF‐C and VEGF‐D are strong stimulators of lymphangiogenesis in PCa,8, 9, 10 and their expressions were increased by androgen deprivation in vivo and in vitro.12, 13 However, contrary to our expectation, there was no significant difference in the expressions of these factors between the non‐NHT group and the NHT group in patients with low‐risk PCa. From these results, we speculated that up‐regulated lymphangiogenesis played a minimal role for such phenomena in patients with low‐risk PCa, although, unfortunately, we were unable to further pursue this question with our study design. However, we paid special attention to the pressure from interstitial fluid and mechanical compression in the intra‐ and peri‐tumoral areas. In short, several previous reports and this study have shown that lymph vessels in PCa specimens from patients who did not receive ADT were small and collapsed compared to those from patients who did receive ADT, as well as normal prostate tissues.13, 20, 21 Additionally, pressure from interstitial fluid and mechanical compression in the intra‐ and peri‐tumoral areas have been proposed to explain these observations.22, 23 In fact, our finding that LVA was higher in the NHT group than in the non‐NHT group could be explained by the decrease in pressure and mechanical compression by NHT within and around the tumor mass. Furthermore, we also speculated that increased LVD in the NHT group could be explained through a similar mechanism. That is, extremely occluded lymph vessels within the intra‐tumoral area in the non‐NHT group could not be counted as LVD; however, obstructed lymph vessels by NHT were counted as LVD. Thus, the anti‐cancer effects of NHT create a favorable microenvironment for lymph vessel invasion and cancer cell dissemination via cancer‐related lymph vessels.
The major limitation of this study was the small sample size. Another limitation is the inherent potential of bias due to its retrospective nature. However, the frequency of patients with low‐risk PCa treated with RP after NHT is relatively rare. In addition, a prospective randomized trial of patients with low‐risk PCa to confirm the anti‐cancer effects of NHT, including changes to cancer‐related factors, is difficult because low‐risk PCa patients are usually treated with local therapy, including RP and radiotherapy, without NHT or active surveillance. Furthermore, a retrospective multi‐center study would be inadequate for our study design, owing to differences in pathological diagnosis and methods of RP.
NHT is mainly performed in patients with intermediate‐ and high‐risk PCa. Therefore, the clinical usefulness of our results might be limited. However, in addition to RP, NHT has been performed in patients receiving radiotherapy and cryotherapy.3, 24 Furthermore, ADT administration was selected for patients with high age and severe complicated diseases in spite of low‐risk PCa. Based on these facts, we believe that the results of this study provide key information in understanding the pathological changes precipitated by ADT and in discussing treatment strategies in PCa patients with low‐risk disease.
5. CONCLUSIONS
Our results demonstrated that NHT increased cancer progression and decreased BCR‐free survival via up‐regulation of lymphangiogenesis‐related parameters, such as LVD and LVA in patients with low‐risk PCa. On the other hand, the expressions of VEGF‐C and VEGF‐D in the NHT group were not different from the non‐NHT group. Our results suggest that NHT for patients with low‐risk PCa may increase the risk for BCR after RP.
ACKNOWLEDGMENTS
Parts of this study were funded by a grant‐in‐aid from the Japan Society for the Promotion of Science (No: 16K15690 to Y Miyata). No private funding was received for this study.
CONFLICTS OF INTEREST
The authors declare no potential conflicts of interest.
Miyata Y, Nakamura Y, Yasuda T, et al. Neoadjuvant hormonal therapy for low‐risk prostate cancer induces biochemical recurrence after radical prostatectomy via increased lymphangiogenesis‐related parameters. The Prostate. 2017;77: 1408–1415. https://doi.org/10.1002/pros.23402
REFERENCES
- 1. Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- 2. Gleave ME, Goldenberg SL, Jones EC, et al. Biochemical and pathological effects of 8 months of neoadjuvant androgen withdrawal therapy before radical prostatectomy in patients with clinically confined prostate cancer. J Urol. 1996; 155:213–219. [PubMed] [Google Scholar]
- 3. Zilli T, Dal Pra A, Kountouri M, et al. Prognostic value of biochemical response to neoadjuvant androgen deprivation before external beam radiotherapy for prostate cancer: A systematic review of the literature. Cancer Treat Rev. 2016; 46:35–41. [DOI] [PubMed] [Google Scholar]
- 4. Anderson BB, Oberlin DT, Razmaria AA, et al. Extraprostatic extension is extremely rare for contemporary Gleason score 6 prostate cancer. Eur Urol. 2016; in press. [DOI] [PubMed] [Google Scholar]
- 5. Miyata Y, Kanda S, Ohba K, et al. Tumor lymphangiogenesis in transitional cell carcinoma of the upper urinary tract: association with clinicopathological features and prognosis. J Urol. 2006; 176:348–353. [DOI] [PubMed] [Google Scholar]
- 6. Datta K, Muders M, Zhang H, et al. Mechanism of lymph node metastasis in prostate cancer. Future Oncol. 2010; 6:823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyata Y, Kanda S, Ohba K, et al. Lymphangiogenesis and angiogenesis in bladder cancer: prognostic implications and regulation by vascular endothelial growth factors‐A, ‐C, and ‐D. Clin Cancer Res. 2006; 12:800–806. [DOI] [PubMed] [Google Scholar]
- 8. Tuomela J, Valta M, Seppänen J, et al. Overexpression of vascular endothelial growth factor C increases growth and alters the metastatic pattern of orthotopic PC‐3 prostate tumors. BMC Cancer. 2009; 9:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsurusaki T, Kanda S, Sakai H, et al. Vascular endothelial growth factor‐C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer. 1999; 80:309–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woollard DJ, Opeskin K, Coso S, et al. Differential expression of VEGF ligands and receptors in prostate cancer. Prostate. 2013; 73:563–572. [DOI] [PubMed] [Google Scholar]
- 11. Jinping Li, Wang E, Rinaldo F, et al. Upregulation of VEGF‐C by androgen depletion: the involvement of IGF‐1R‐FOXO pathway. Oncogene. 2005; 24:5510–5520. [DOI] [PubMed] [Google Scholar]
- 12. Rinaldo F, Li J, Wang E, et al. RalA regulates vascular endothelial growth factor‐C (VEGF‐C) synthesis in prostate cancer cells during androgen ablation. Oncogene. 2007; 26:1731–1738. [DOI] [PubMed] [Google Scholar]
- 13. Asai A, Miyata Y, Matsuo T, et al. Changes in lymphangiogenesis and vascular endothelial growth factor expression by neo‐adjuvant hormonal therapy in prostate cancer patients. Prostate. 2017; 77:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280:969–274. [DOI] [PubMed] [Google Scholar]
- 15. Miyata Y, Kanda S, Sakai H, et al. Relationship between changes in prostate cancer cell proliferation, apoptotic index, and expression of apoptosis‐related proteins by neoadjuvant hormonal therapy and duration of such treatment. Urology. 2005; 65:1238–1243. [DOI] [PubMed] [Google Scholar]
- 16. Labrie F, Cusan L, Gomez JL, et al. Downstaging by combination therapy with flutamide and an LHRH agonist before radical prostatectomy. Cancer Surv. 1995; 23:149–156. [PubMed] [Google Scholar]
- 17. Soloway MS, Sharifi R, Wajsman Z, et al. Randomized prospective study comparing radical prostatectomy alone versus radical prostatectomy preceded by androgen blockade in clinical stage B2 (T2bNxM0) prostate cancer. The Lupron Depot Neoadjuvant Prostate Cancer Study Group. J Urol. 1995; 154:424–428. [PubMed] [Google Scholar]
- 18. Narita T, Koie T, Ookubo T, et al. The impact of extended lymph node dissection versus neoadjuvant therapy with limited lymph node dissection on biochemical recurrence in high‐risk prostate cancer patients treated with radical prostatectomy: a multi‐institutional analysis. Med Oncol. 2017; 34:1. [DOI] [PubMed] [Google Scholar]
- 19. Soloway MS, Pareek K, Sharifi R, et al. Lupron Depot Neoadjuvant Prostate Cancer Study Group. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5‐year results. J Urol. 2002; 167:112–116. [PubMed] [Google Scholar]
- 20. Zeng Y, Opeskin K, Baldwin ME, et al. Expression of vascular endothelial growth factor receptor‐3 by lymphatic endothelial cell is associated with lymph node metastasis in prostate cancer. Clin Cancer Res. 2004; 10:5137–5144. [DOI] [PubMed] [Google Scholar]
- 21. Ambrosio MR, Rocca BJ, Barone A, et al. Lymphatic vascularization in prostate adenocarcinoma: correlation with tumor grade, androgen withdrawal and prognosis. Anticancer Res. 2015; 35:5595–5600. [PubMed] [Google Scholar]
- 22. Padera TP, Kadambi A, di Tomaso E, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002; 296:1883–1886. [DOI] [PubMed] [Google Scholar]
- 23. Kim H‐S, Sung W, Lee S, et al. Lymphatic vessel densities of lymph node‐negative prostate adenocarcinoma in Korea. Pathol Res Pract. 2009; 205:249–254. [DOI] [PubMed] [Google Scholar]
- 24. Tay KJ, Polascik TJ, Elshafei A, et al. Primary cryotherapy for high‐grade clinically localized prostate cancer: oncologic and functional outcomes from the COLD registry. J Endourol. 2016; 30:43–48. [DOI] [PubMed] [Google Scholar]


