Summary
While angiosperm clocks can be described as an intricate network of interlocked transcriptional feedback loops, clocks of green algae have been modelled as a loop of only two genes. To investigate the transition from a simple clock in algae to a complex one in angiosperms, we performed an inventory of circadian clock genes in bryophytes and charophytes. Additionally, we performed functional characterization of putative core clock genes in the liverwort Marchantia polymorpha and the hornwort Anthoceros agrestis.
Phylogenetic construction was combined with studies of spatiotemporal expression patterns and analysis of M. polymorpha clock gene mutants.
Homologues to core clock genes identified in Arabidopsis were found not only in bryophytes but also in charophytes, albeit in fewer copies. Circadian rhythms were detected for most identified genes in M. polymorpha and A. agrestis, and mutant analysis supports a role for putative clock genes in M. polymorpha.
Our data are in line with a recent hypothesis that adaptation to terrestrial life occurred earlier than previously expected in the evolutionary history of charophyte algae. Both gene duplication and acquisition of new genes was important in the evolution of the plant circadian clock, but gene loss has also contributed to shaping the clock of bryophytes.
Keywords: bryophyte, circadian clock, Marchantia polymorpha, evolution, transcription factor
Introduction
Adaptation to changing environments is critical to all life. Some of these changes are predictable, such as day–night cycles and the ever‐changing seasons. Accordingly, organisms from all kingdoms of life have developed mechanisms to anticipate such predictable changes. Intrinsic clocks that generate circadian rhythms are present in most organisms, from cyanobacteria to land plants and animals. Although the overall architecture is generally conserved, the key genes involved are generally not, suggesting multiple independent origins of circadian clocks (Dunlap, 1999; Young & Kay, 2001; McClung, 2013).
The circadian clock is a self‐sustaining oscillator and the c. 24 h rhythm results mainly from transcriptional and translational feedback loops (Harmer, 2009). The clock must interact with a fluctuating environment and needs to be adjusted daily. The major environmental cues for this entrainment are light and temperature (Johnson et al., 2003). The clock also has to cope with unpredictable variations in sunlight and temperature and is generally thought to have evolved towards a more complex and thereby flexible and robust architecture (Rand et al., 2004; Tsai et al., 2008).
Circadian regulation is important in the control of a number of diverse processes. In vascular plants, well‐studied examples include stomatal opening, leaf movement, growth, metabolism, induction of flowering and response to stress (Greenham & McClung, 2015). Correspondingly, thousands of genes are under circadian control (Covington et al., 2008; Michael et al., 2008). Disruption of the circadian clock has also been shown to confer fitness costs, implicating its importance (Yerushalmi & Green, 2009).
The plant circadian clock has been intensely studied in a few model species, mainly the angiosperm Arabidopsis thaliana, and the green algae Chlamydomonas reinhardtii and Ostreococcus tauri. Recent models of the Arabidopsis clock describe it as an intricate network of interlocked transcriptional feedback loops (Pokhilko et al., 2013; Fogelmark & Troein, 2014; De Caluwé et al., 2016). The main components of those models include a set of single MYB domain transcription factors, a family of PSEUDO‐RESPONSE REGULATORs (PRRs), and a few plant‐specific genes with unknown biochemical function (Table 1).
Table 1.
Clock gene homologues in Chlamydomonas reinhardtii (Cr), Ostreococcus tauri (Ot), Klebsormidium flaccidum (Kf), Marchantia polymorpha (Mp), Anthoceros agrestis (Aa), Physcomitrella patens (Pp), Selaginella moellendorffii (Sm), Picea abies (Pa), Oryza sativa (Os) and Arabidopsis thaliana (At)
| Speciesa | RVE | PRR | ELF3 | ELF4 | LUX | GI | ZTL | ||
|---|---|---|---|---|---|---|---|---|---|
| CCA1‐clade | LCL‐clade | TOC1‐clade | PRR‐clade | ||||||
| Cr | 1b | 2c | 0 | 1e | 2e | 0 | 0 | ||
| Ot | 1b | 1c | 0 | 0 | 0 | 0 | 0 | ||
| Kf | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 0d | 1 |
| Mp | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Aa | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 1 |
| Pp | 2 | 3 | 0 | 4 | 3 | 1 | 3 | 0 | 0 |
| Sm | 0 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 |
| Pa | 3 | 0 | 1 | 2 | 0 | 5 | 2 | 1 | 1 |
| Os | 1 | 6 | 1 | 4 | 2 | 3 | 1 | 1 | 3 |
| At | 6 | 5 | 1 | 4 | 2 | 5 | 2 | 1 | 3 |
Corresponding locus names and accession numbers are found in Supporting Information Table S1.
These genes could not be assigned to either the CCA1/LHY or the LCL clade.
These genes could not be assigned to either the PRR or the TOC1 clade.
GI was not found in K. flaccidum, but was identified in other charophytes.
Potential homologue.
The components of the Arabidopsis clock can be classified according to their phase of expression. The morning‐phased genes CIRCADIAN CLOCK‐ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) are two MYB‐like transcription factors that function mainly as repressors of day‐ and evening‐phased genes (Wang et al., 1997; Schaffer et al., 1998; Mizoguchi et al., 2002; Fogelmark & Troein, 2014; Kamioka et al., 2016). They bind to a motif coined the evening element (EE; Harmer et al., 2000) that is present in the promoters of several day‐ and evening‐phased genes, as well as in a large number of putative output genes.
The evening‐phased component includes three proteins that form the evening complex (EC). The complex contains the MYB transcription factor LUX ARRHYTHMO (LUX), and the two proteins EARLY FLOWERING 3 (ELF3), and ELF4 (Hicks et al., 2001; Doyle et al., 2002; Hazen et al., 2005; Nusinow et al., 2011). The EC functions as a repressor of at least two PRR genes as well as LUX itself (Nusinow et al., 2011; Chow et al., 2012).
The family of PRR genes comprise five members in Arabidopsis: PRR1, PRR3, PRR5, PRR7, and PRR9. PRR1 is also known as TIMING OF CAB EXPRESSION 1 (TOC1), which, together with CCA1, constituted the first conceptual model of the Arabidopsis clock (Alabadí et al., 2001). The expression of PRR genes ranges from morning to evening, with PRR9 peaking in the morning, PRR5 and PRR7 around noon, and PRR3 and TOC1 around dusk (Matsushika et al., 2000). PRR proteins are in recent models incorporated as transcriptional repressors of CCA1/LHY and other PRR genes (Fogelmark & Troein, 2014).
The proteins described are reported to function as repressors. However, recently a family of MYB transcription factors related to CCA1/LHY was identified (Rawat et al., 2011; Hsu et al., 2013), some of which were reported to work as activators in the circadian clock. These REVEILLE (RVE) genes are transcribed mainly in the morning, but the protein amounts of at least some members peak in the afternoon. RVE8 and probably RVE4 and RVE6 bind to the same EE as CCA1/LHY and induce evening‐phased genes. Conversely, one or more PRR proteins repress RVE8 and possibly other RVE genes.
Additionally, ZEITLUPE (ZTL), an F‐box protein, and GIGANTEA (GI), encoding a large protein with unclear biochemical function, are implicated to function in the circadian clock (Fowler et al., 1999; Somers et al., 2000). GI interacts with ZTL which, in turn, regulates the stability of at least TOC1 and PRR5 (Más et al., 2003; Kim et al., 2007). The activity of GI is under clock control, including repression by EC and CCA1/LHY and probably also activation by RVE8 (Berns et al., 2014). GI is also important for flowering time regulation through its interaction with the ZTL paralogue FLAVIN‐BINDING, KELCH REPEAT, F‐BOX1 (FKF1; Sawa et al., 2007). The GI–FKF1 complex controls the degradation of CYCLING DOF FACTOR proteins, which in turn are repressors of the flowering time gene CONSTANS (Fornara et al., 2009).
The clocks in green algae differ considerably from the angiosperm clock (Matsuo et al., 2008; Corellou et al., 2009). In both C. reinhardtii and O. tauri, putative homologues to CCA1 and TOC1 have been identified, however, no obvious ELF3, ELF4, GI or ZTL homologues could be found (Matsuo et al., 2008; Corellou et al., 2009). Even though the identification of clock components in these algae might be incomplete, comparatively simple models can be built that reproduce the dynamics of their circadian clocks. For example, the O. tauri clock was successfully modelled as a feedback loop between a CCA1 and a TOC1 homologue (Troein et al., 2011).
Comparisons of circadian clock genes in green algae and angiosperms suggest that additional genes have been recruited during plant evolution, resulting in successively more complex clocks with multiple feedback loops. However, when and how these genes were recruited is currently unknown. Land plants form a monophyletic group, which nests within a freshwater Charophycean algal clade, implying that land plants evolved from an ancestral freshwater or terrestrial alga (Bowman, 2013; and references therein; Harholt et al., 2016). Liverworts, mosses and hornworts are gametophyte‐dominant land plants collectively known as bryophytes, and comprise the closest extant relatives to the first embryophytic land plants (Shaw et al., 2011). Their phylogenetic position makes them crucial for studying the evolution of biological processes from freshwater plants to land plants, and from relatively simple basal land plants to more complex forms. A few studies investigating clock genes in the moss Physcomitrella patens have concluded that although multiple PRR genes and homologues to CCA1 and the EC genes were identified, orthologues to important clock genes such as GI, TOC1 and ZTL were not (Okada et al., 2009; Holm et al., 2010; Satbhai et al., 2011).
Even though the circadian clock has been studied in a few plant model species over the last decades, little is known of clock function in the bryophytes. Because of their key phylogenetic position, bryophytes have the potential to reveal the mechanisms regulating the ancestral clock present in the first plants that colonized land. This study gives a comparative overview of charophyte and bryophyte clock gene families, spanning over all three major clades of bryophytes – mosses, liverworts and hornworts – and suggests that most components present in angiosperm circadian clocks were already recruited in the ancestors of land plants (the charophytes). Gene duplication has generally resulted in an increased complexity of the plant circadian clock, but independent gene losses in various lineages have shaped the architecture of the individual clock networks.
Materials and Methods
Plant material and growth conditions
Marchantia polymorpha ssp. ruderalis from Uppsala, Sweden, referred to as Upp, was grown aseptically on agar solidified Gamborg's B5 (Gamborg et al., 1968; PhytoTechnology Laboratories, Lenexa, KS, USA), pH 5.5. Strains Takaragaike (Tak) ‐1 and Tak‐2 were grown in a similar way in the Kyoto laboratory, and used as indicated in the text. Plants were grown under cool white fluorescent light (50–60 μmol photons m−2 s−1) in 16 : 8 h, light : dark cycles at 20°C (Uppsala), or in continuous light at 22°C (Kyoto).
RNA isolation and quantitative reverse transcription polymerase chain reaction (qRT‐PCR)
Marchantia polymorpha (Upp) and Anthoceros agrestis were grown on half‐strength Gamborg's B5 with 1% sucrose in growth cabinets (MLR‐350; Sanyo, Osaka, Japan) with fluorescent tubes producing 40–50 μmol m−2 s−1, red : far red ≈ 7.0 at 18°C. Plants were entrained in neutral day (ND; 12 : 12 h, light : dark cycles), and then transferred to constant darkness (DD), constant light (LL) or ND. Total RNA extraction and qRT‐PCR were performed as previously described (Holm et al., 2010), with PCR primers listed in Supporting Information Table S2. Primers targeting Mpβ‐tubulin2 (MpTUB2; Mapoly0158s0010.1; Buschmann et al., 2016), MpACTIN (MpACT) and MpADENINE PHOSPHORIBOSYL TRANSFERASE were utilized for normalization of gene expression (Saint‐Marcoux et al., 2015). Corresponding primers for A. agrestis targeted AaACT and AaTUB. Normalization of gene expression was performed using analysis of covariance (ANCOVA) in R (v.3.0.2; R Core Team, 2016), where the response variable was the Ct value of the target gene and explanatory variables Ct values from the reference genes plus time. Residuals from the ANCOVA analyses were used for plotting and analysis of rhythmic expression using JTK_CYCLE (Hughes et al., 2010).
RNA isolation and gene expression analyses in M. polymorpha (Tak) were performed as described previously (Kubota et al., 2014), with at least two technical replicates for each cDNA sample. Primers are shown in Table S2. Expression data were normalized against ELONGATION FACTOR 1α (MpEF1). Relative expression values for each gene and sample were then calculated as the average of three biological replicates.
Cloning and construction of plasmids
To generate targeting vectors for MpPRR, MpRVE, and MpTOC1, c. 3 kb flanking regions were PCR‐amplified from Tak‐1 genomic DNA. Primers are listed in Table S2. The PCR‐amplified fragments were cloned into the PacI and AscI sites of pJHY‐TMp1 (Ishizaki et al., 2013), using the In‐Fusion HD cloning kit (Clontech, Kusatsu, Japan). The resulting plasmids were introduced into sporelings derived from crosses between Tak‐1 and Tak‐2 (Ishizaki et al., 2008). Screening for correctly targeted lines was performed by genomic PCR as described previously (Ishizaki et al., 2013; see later Figs S8–S10). For the complementation test of Mprve ko and Mpprr ko, genomic fragments including entire regions of MpRVE or MpPRR were PCR‐amplified from Tak‐1 genomic DNA and subsequently cloned into pENTR/D‐TOPO (Life Technologies, Yokohama, Japan). Fragments were subcloned into pMpGWB301 (Ishizaki et al., 2015) in order to generate binary plasmids harbouring MpRVE pro :gMpRVE or MpPRR pro :gMpPRR. The resulting plasmids were introduced into corresponding knockout mutants, Mprve ko or Mpprr ko, as described previously (Kubota et al., 2013).
Binary promoter:LUC plasmids were built by PCR‐amplifying regulatory regions from genomic DNA (Upp) extracted using the DNeasy Plant Mini Kit (Qiagen). Fragments were subcloned into pMpGWB431 (Nakajima et al., 2010; Ishizaki et al., 2015), via pENTR/D‐TOPO (Invitrogen). These plasmids were transformed into sporelings (Upp) as previously described (Ishizaki et al., 2008). To generate the 35S pro :LUC construct, a HindIII/SmaI fragment from pBI221 (Takara Bio, Kusatsu, Japan) was blunt‐ended and inserted into the DraI/EcoRV site of pENTR1a (Life technologies). This fragment was then subcloned into pMpGWB331 (Ishizaki et al., 2015). 35S pro :LUC was introduced into Tak‐1, Mptoc1 ko , Mprve ko or Mpprr ko as described previously (Kubota et al., 2013).
Luciferase expression analysis
Gemmalings entrained in ND at 20°C on medium containing 1% sucrose (Uppsala) or 22°C (Kyoto) for 4–7 d were sprayed with a 1 mM solution of D‐Luciferin (Pierce D‐Luciferin; Fisher Scientific, Göteborg, Sweden). After an additional 1–2 d in ND, bioluminescence imaging was performed. In Kyoto, a dish monitoring system with photomultiplier tubes (R329P; Hamamatsu Photonics K.K., Hamatsu City, Japan) was used, as described in Miwa et al. (2006). To reduce fluorescent signals form Chl, a short‐pass filter (VS0630; Asahi Spectra Co. Ltd, Tokyo, Japan) was used. Each dish was subjected to 30s measurement of bioluminescence every 20 min. In Uppsala, plates were imaged with an ImagEM charge‐coupled device (CCD) camera (Hamamatsu Photonics K.K.) in a light contained box equipped with blue and red LEDs. Light intensity was set to equal amounts of blue and red light with a total intensity of 50 μmol m−2 s−1. Ten‐minute exposures were taken every hour with the light turned off starting 3 min before exposure during light periods. Intensity data were extracted with ImageJ (Abramoff et al., 2004), and analysed with Spectrum Resampling (Costa et al., 2013). For 12 h T‐cycle experiments, light intensity was reduced to 5 μmol m−2 s−1 to increase the possibility of revealing frequency demultiplication.
To visualize spatial LUC expression, patterns plants were sprayed with 1 mM D‐Luciferin and incubated for 12 h in continuous light. Plants were then placed in darkness for 5 min, and imaged with the CCD camera for 10 min.
Results
Identification of putative bryophyte and charophyte clock genes
To trace the origin of the plant circadian clock, we performed an inventory of homologues to known plant circadian clock genes in available bryophyte and charophyte genomes (Table 1; Methods S1). To identify orthologues and examine the evolutionary relationship of plant circadian clock genes, phylogenetic trees were constructed based on alignments shown in Fig. S1. The results are presented below on a gene family basis.
REVEILLE
The 11 members of the RVE family in Arabidopsis – CCA1, LHY and nine RVE genes – all encode proteins with a single highly conserved MYB/SANT domain (SHAQKYF class; Rawat et al., 2011; Farinas & Mas, 2011). Two subgroups have been named in this family, one comprising CCA1, LHY, RVE1, 2, 7 and RVE7‐like and the other RVE3, 4, 5, 6 and 8. The second group, referred to as the LCL subfamily, shares an additional conserved region outside the MYB domain (Farinas & Mas, 2011).
As previously reported, the green algae C. reinhardtii and O. tauri contain single homologues that are similar to genes in the CCA1/LHY clade (Matsuo et al., 2008; Corellou et al., 2009). However, in the charophyte Klebsormidium flaccidum two homologues were found, one in the CCA1/LHY clade (KfCCA1) and the other in the LCL clade (KfRVE; Fig. 1; Table 1). Likewise, A. agrestis and P. patens contain genes from both groups (Fig. 1; Table 1).
Figure 1.
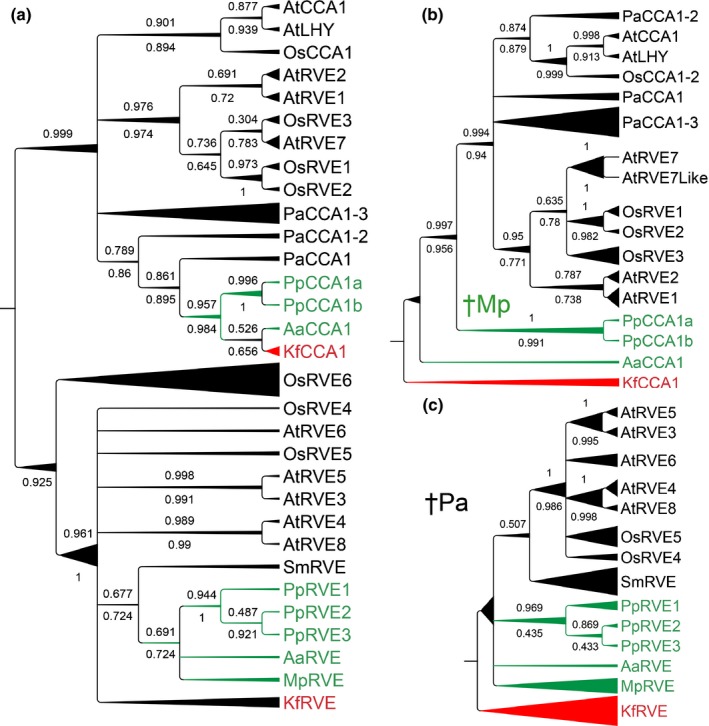
Inferred phylogeny of homologues to the CCA/LHY/RVE gene family. Separate trees were constructed from the complete alignment (a), the CCA1 subfamily (b), and the LCL subfamily (c). Trees were constructed using MrBayes and PhyML on an amino acid alignment of proteins retrieved from Arabidopsis thaliana (At), Oryza sativa (Os), Picea abies (Pa), Selaginella moellendorffii (Sm), Physcomitrella patens (Pp), Marchantia polymorpha (Mp), Anthoceros agrestis (Aa) and Klebsormidium flaccidum (Kf). Bayesian trees are shown with posterior probabilities (above) and bootstrap proportions from PhyML analysis (below) for each node. Nodes with conflicting support from the two methods were collapsed. Branch length is relative to the thickness of individual branches: the shortest branches have a straight line and the longest are increasingly triangular. †Mp indicates loss of a CCA1/LHY homologue in Mp (as well as all surveyed liverworts); †Pa indicates loss of LCL‐like genes in Pa. The MYB/SANT domain includes, in the LCL group, a SHAQKYF‐version of the SHAQKYF‐motif, while the CCA1 subclade shares SHAQKFF.
In the liverwort M. polymorpha only one homologue was identified, MpRVE, which clustered with the LCL clade in a phylogenetic analysis (Fig. 1; Table 1), suggesting that M. polymorpha has lost the CCA1/LHY‐type gene. Based on data from the oneKP database, the presence of one LCL gene and no CCA1/LHY gene is shared among all 28 deposited liverwort species, while mosses and hornworts contain both LCL‐like and CCA1/LHY‐like homologues.
PSEUDO‐RESPONSE REGULATORS
PSEUDO‐RESPONSE REGULATOR proteins contain a response regulator receiver (REC) domain and a CONSTANS, CONSTANS‐like and TOC1 (CCT) domain (Strayer et al., 2000). This family consists of five members in Arabidopsis: TOC1/PRR1, PRR3, PRR5, PRR7 and PRR9 (Matsushika et al., 2000).
One and two genes from this family are found in O. tauri and C. reinhardtii, respectively (Table 1; Matsuo et al., 2008; Corellou et al., 2009), but it is unclear whether these are more closely related to TOC1 or to the other PRRs. K. flaccidum contains two PRR family genes, one in a clade with AtTOC1 and the other one in a clade with AtPRR3/7 (Fig. 2; Table 1). In A. agrestis, only one PRR gene was found, belonging to the PRR3/7 clade (AaPRR; Fig. 2), while in P. patens, four PRR genes, all belonging to the PRR3/7 clade, were identified (Fig. 2; Table 1). In oneKP database searches, TOC1‐related genes were not found in moss or hornwort transcriptomes (41 and eight species, respectively).
Figure 2.
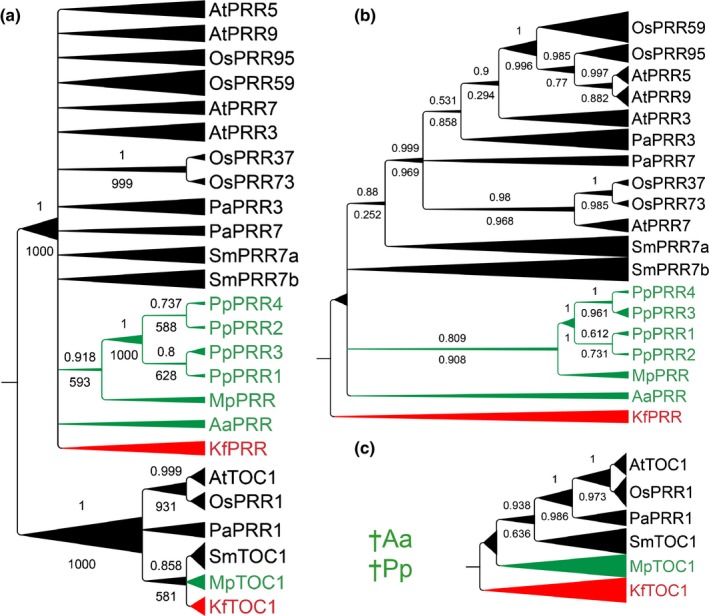
Inferred phylogeny of homologues to the PSEUDO‐RESPONSE REGULATOR (PRR) gene family. Separate trees were constructed from the complete alignment (a), the PRR subfamily (b), and the TOC1 subfamily (c). Trees were constructed using MrBayes and PhyML on an amino acid alignment of proteins retrieved from Arabidopsis thaliana (At), Oryza sativa (Os), Picea abies (Pa), Selaginella moellendorffii (Sm), Physcomitrella patens (Pp), Marchantia polymorpha (Mp), Anthoceros agrestis (Aa), and Klebsormidium flaccidum (Kf). The Bayesian tree is shown with posterior probabilities (above) and bootstrap proportions from PhyML analysis (below) for each node. Nodes with conflicting support from the two methods were collapsed. Branch length is relative to the thickness of individual branches: the shortest branches have a straight line and the longest are increasingly triangular. †Aa and †Pp indicate loss of TOC1 in Aa and Pp, respectively (as well as all surveyed hornworts and mosses).
In M. polymorpha two PRR genes were identified of which one clustered in a clade with AtTOC1 (MpTOC1), and one with the AtPRR3/7 clade (MpPRR; Fig. 2; Table 1). We conclude that a first duplication event resulting in separate TOC1 and PRR3/7 paralogues occurred before or during the evolution of charophytes. Furthermore, TOC1 paralogues were lost in both hornworts and mosses.
EARLY FLOWERING 3
ELF3 is predicted to encode a soluble protein that is particularly rich in serine, proline, and glutamine (Hicks et al., 2001). No ELF3 homologues have been detected in green algae, but a single homologue was found in K. flaccidum, M. polymorpha, A. agrestis, and Selaginella moellendorffii, and three homologues were detected in P. patens (Fig. S2; Table 1). As previously reported, no homologue was detected in Picea abies, but two homologues were found in Arabidopsis and Oryza sativa (Fig. S2; Table 1). Thus, data suggest that ELF3 arose in charophytes.
EARLY FLOWERING 4
ELF4 is a small protein with a conserved domain of unknown function (DUF1313; Li et al., 2016). A putative protein with a DUF1313 domain has been reported in C. reinhardtii, but no ELF4 homologue was found in the early diverging green alga O. tauri (Tables 1, S1). Two homologues were found in K. flaccidum, A. agrestis and S. moellendorffii, while a single ELF4 homologue was identified in each of M. polymorpha and P. patens (Fig. S3; Table 1). P. abies, Arabidopsis and O. sativa contain five, five and three homologues, respectively (Fig. S3; Table 1). Previous phylogenetic analyses in angiosperms have identified two clusters of ELF4 or ELF4‐like (from now on referred to as EFL) proteins, one corresponding to the AtELF4/AtEFL1 clade in our analyses, and the other corresponding to the clade including AtEFL2, 3, 4. It should be noted that only proteins in the first clade (AtELF4 and AtEFL1) have been shown to possess circadian clock function (Kolmos et al., 2009).
LUX ARRHYTHMO
LUX and its homologue BROTHER OF LUX (BOA) are MYB‐like transcription factors, belonging to the SANT superfamily (Dai et al., 2011). Proteins with similarity to the LUX MYB‐domain are found in green algae (Tables 1, S1). In K. flaccidum two proteins were identified with BLAST. One of them had a high e‐value, but as reciprocal BLAST against Arabidopsis identified AtBOA as the top hit, this protein was included in the phylogeny. Single homologues to AtLUX were identified in A. agrestis and M. polymorpha (Fig. S4; Table 1). Three LUX homologues were identified in P. patens, one in S. moelendorffii and O. sativa, whereas two were identified in P. abies (Fig. S4; Table 1).
GIGANTEA
GIGANTEA is a large well‐conserved plant‐specific protein with no known domains and unknown biochemical function. Remarkably, a single GI copy is found in most land plant species, including Arabidopsis, S. moelendorffii, O. sativa and P. abies. No trace of GI‐like sequences were found in green algae or in K. flaccidum; however, GI homologues were detected in the more recently diverged charophytes Coleochaete irregularis (Coleochaetales) and Cylindrocystis cushleckae (Zygnematales). Searches in the oneKP database confirmed that charophytes, belonging to Zygnematales and Coleochaetales, do contain single GI homologues. A single GI gene is found also in M. polymorpha and A. agrestis, but no gene with detectable homology to GI was found in P. patens (Holm et al., 2010; Fig. S5; Table 1). According to BLAST searches in oneKP databases, the only moss, out of 41 species in 31 genera, that contains a homologue to GI is the early diverging moss Takakia lepidozioides. These data indicate that GI first occurred in charophytes suggested as sisters to land plants, but was uniquely lost within the moss lineage.
ZEITLUPE
In Arabidopsis there are three ZTL‐family F‐box proteins which are involved in blue‐light regulated protein degradation: ZTL, FKF1 and LOV KELCH PROTEIN2 (LKP2; Schultz et al., 2001). The genes of this family have three main domains; a Light, oxygen or voltage domain (LOV; belongs to the Per‐ARNT‐Sim (PAS) superfamily), a central F‐box and multiple Kelch‐repeats in the C‐terminus. The LOV domain confers blue‐light sensing, while F‐box and Kelch‐repeats strongly suggest a role in ubiquitin‐mediated protein degradation (Ito et al., 2012).
As for ELF3, homologues to the ZTL family are first observed in charophytes. One homologue was identified in each of K. flaccidum, M. polymorpha, A. agrestis, and S. moelendorffii (Fig. S6; Table 1). Previously, it has been shown that the P. patens genome does not contain any ZTL homologue (Holm et al., 2010). As for GI, a BLAST search against oneKP revealed that the only moss, among the 41 species in the database, that expresses a ZTL/FKF1 homologue is T. lepidozioides.
In summary, homologues to most of the core clock genes and important clock‐associated genes identified in Arabidopsis were not only found in bryophytes but also in charophytes, suggesting that the circadian clockwork of land plants may have arisen earlier than previously assumed, perhaps in land‐living charophyte algae (Table 1). Our data also suggest that besides gene duplication resulting in redundancy and functional diversification, gene loss has been important in shaping circadian clocks in hornworts, liverworts and mosses. The three genes that previously have been shown to be missing in P. patens (GI, ZTL and TOC1; Holm et al., 2010) were not found in any moss except the basal moss species T. lepidozioides. GI and ZTL were identified in liverworts, hornworts and charophytes, but TOC1 was also absent from hornworts. Furthermore, our phylogenetic analyses suggest that liverworts have lost their CCA1 orthologue.
Knockout mutants support a role for MpPRR, MpRVE and MpTOC1 in the M. polymorpha circadian clock
The identification of orthologues of most angiosperm circadian clock genes in early land plants prompted us to start probing their role as circadian clock genes. As M. polymorpha has emerged as a model species with well‐developed molecular genetic tools, we investigated if in particular MpPRR, MpTOC1, and MpRVE are required for circadian rhythmicity. First, using qRT‐PCR we found that a majority of putative M. polymorpha clock genes, including MpPRR, MpTOC1 and MpRVE, showed diel expression patterns in mature thalli grown in ND conditions (Table 2; Fig. S7). The rhythmic expression of most of these genes also persisted after the transition to LL and DD conditions (Table 2; Fig. S7).
Table 2.
Rhythms in Marchantia polymorpha clock gene transcript abundance identified by quantitative reverse transcription polymerase chain reaction (qRT‐PCR) and JTK_CYCLE analysis
| Gene | Conditiona | P‐valueb | Period |
|---|---|---|---|
| MpPRR | ND | < 0.001 | 24 |
| DD | 0.001 | 28 | |
| LL | < 0.001 | 28 | |
| MpTOC1 | ND | < 0.001 | 24 |
| DD | < 0.001 | 28 | |
| LL | 0.001 | 28 | |
| MpRVE | ND | 0.003 | 24 |
| DD | 0.020 | 28 | |
| LL | 0.061 | 28 | |
| MpELF3 | ND | 0.005 | 24 |
| DD | 0.073 | 28 | |
| LL | 0.008 | 28 | |
| MpGI | ND | 0.035 | 20 |
| DD | < 0.001 | 28 | |
| LL | 0.080 | 28 | |
| MpLUX | ND | 0.073 | 20 |
| DD | 0.007 | 28 | |
| LL | 0.149 | 28 | |
| MpEFL | ND | 1 | 20 |
| DD | 1 | 28 | |
| LL | 1 | 28 | |
| MpFKF | ND | 1 | 28 |
| DD | 0.121 | 28 | |
| LL | 0.225 | 24 |
Light condition: neutral day (ND; 12 : 12 h, light : dark cycles), constant darkness (DD) or constant light (LL).
Adjusted P‐values < 0.05 are presented in bold.
Next, we generated knockout lines of MpPRR, MpTOC1 and MpRVE in F1 sporelings derived from crosses between Tak‐1 and Tak‐2 (Figs S8–S10), and utilized a luciferase reporter controlled by a CaMV35S promoter (35Spro :LUC) to assay bioluminescence in transgenic lines. As previously observed in the angiosperm Lemna gibba (Muranaka et al., 2014), 35Spro :LUC lines showed diel oscillation under ND and persistent rhythm in LL for several days (Fig. 3). In white light, relative amplitude error (RAE)‐weighted period mean for three independent 35Spro :LUC lines was 28.9 h, with RAE < 0.07 for each line. Analysis of mRNA levels of LUC under equivalent conditions revealed a diel rhythm under ND that markedly dampened upon transfer to LL (Fig. S11). Thus, it is not obvious that the bioluminescence rhythm seen with 35Spro :LUC in LL is caused primarily by a transcriptional rhythm. Irrespective of the cause of the circadian rhythm in 35Spro :LUC bioluminescence, a perturbation of this rhythm in knockout lines of putative clock genes would suggest a role for these genes in the generation of circadian rhythmicity. Therefore, we introduced 35Spro :LUC into knockout lines of MpPRR, MpRVE and MpTOC1. Several reporter lines were obtained for Mpprr ko and Mprve ko, but only one line for Mptoc1 ko. Bioluminescence rhythm was strongly affected in all knockout lines. Under ND conditions, the 35Spro :LUC Mpprr ko and 35Spro :LUC Mptoc1 ko lines showed a pattern with an almost linear increase and decrease in light and dark, respectively, while 35Spro :LUC Mprve ko showed a pattern more similar to the 35Spro :LUC lines (Fig. 3). In LL, bioluminescence rhythm was abolished in all knockout lines (Fig. 3). Obtained period estimates consequently varied widely and RAEs were high (Fig. 3). These results show that MpPRR, MpRVE and MpTOC1 do have a role in controlling the bioluminescence rhythm observed in 35Spro :LUC, and suggest that the M. polymorpha circadian clock generates this rhythm.
Figure 3.
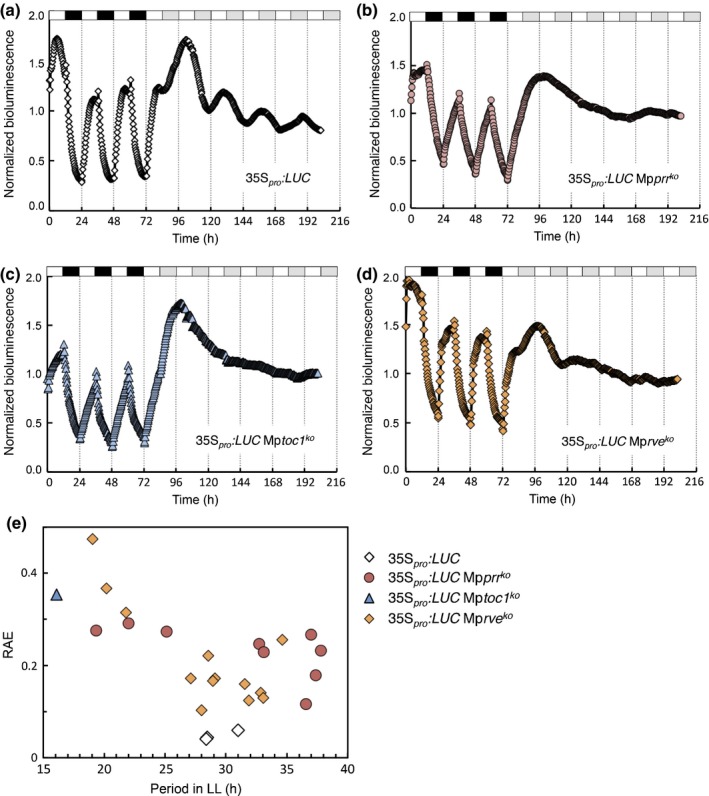
Bioluminescence rhythm of 35Spro :LUC is abolished in Marchantia polymorpha knockout mutants of MpPRR, MpRVE and MpTOC1. Bioluminescence was recorded under neutral‐day (ND; 12 : 12 h, light : dark cycles) and constant‐light (LL) conditions in wild‐type (Tak‐1) and clock mutants. Normalized bioluminescence for representative lines are shown, as follows: (a) 35Spro :LUC; (b) 35Spro :LUC Mpprr ko; (c) 35Spro :LUC Mptoc1 ko; (d) 35Spro :LUC Mprve ko. (e) Estimates of period plotted against relative amplitude error (RAE). Each plotted symbol in (e) represents an independent line.
To further test these conclusions, we studied MpPRR expression in Mprve ko and Mptoc1 ko knockout lines with qRT‐PCR. If these genes are part of transcriptional feedback loops typical of circadian clocks, we should expect changes in their temporal expression patterns. In Mprve ko lines, reduced peak levels of MpPRR expression were observed in both ND and LL (Fig. 4a,b). The deviant expression patterns in ND were restored by the introduction of a genomic fragment of MpRVE into the mutant (Figs 4a, S12a), suggesting that MpRVE positively regulates MpPRR, similar to the role of AtRVE4, 6, 8 in promoting expression of evening‐phased genes in Arabidopsis (Rawat et al., 2011; Hsu et al., 2013).
Figure 4.
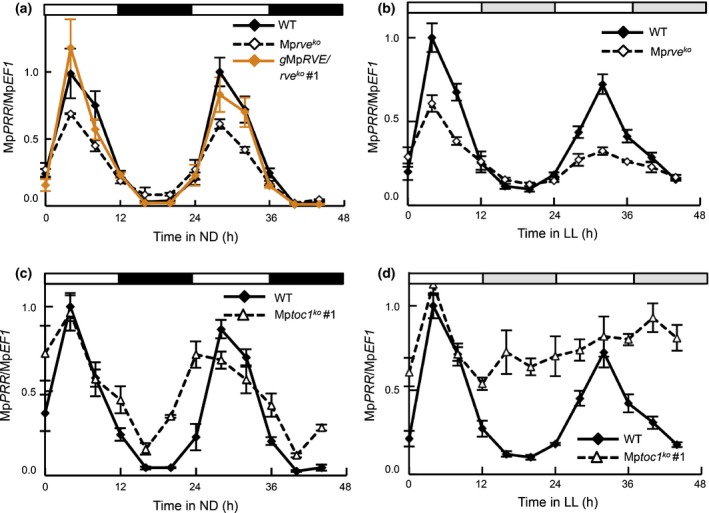
Temporal expression of MpPRR in neutral‐day (ND; 12 : 12 h, light : dark cycles) and constant‐light (LL) conditions is affected in knockout mutants of putative Marchantia polymorpha circadian clock genes. (a, b) Expression of MpPRR in wild‐type (WT; Tak‐1, solid line), Mprve ko mutant (broken line) and restored mutant (orange line) under ND conditions (a) and in WT and mutant under LL conditions (b). (c, d) Expression of MpPRR in WT (Tak‐1, solid line) and Mptoc1 ko mutant (broken line) under ND (c) and LL conditions (d). Mean expression values measured using quantitative reverse transcription polymerase chain reaction are shown with standard errors based on three biological replicates. Expression was normalized using MpEF1.
In Mptoc1 ko lines grown under ND conditions, night‐time decrease in MpPRR expression was slightly reduced (Figs 4c, S12b). In LL, expression of MpPRR remained at peak levels, resulting in arrhythmicity (Figs 4d, S12c). Thus, these data support the idea that MpTOC1 directly or indirectly represses the expression of MpPRR, supporting a conserved role for TOC1 in land plants.
Collectively, these data support the idea that MpPRR, MpRVE and MpTOC1 have central roles in the M. polymorpha circadian clock and that these roles are at least partially conserved in plants.
Characterization of circadian rhythms in M. polymorpha
To further study circadian rhythms of M. polymorpha clock genes, promoter activities of MpELF3, MpGI, MpLUX, MpPRR and MpRVE were analysed using transgenic promoter:LUC reporter lines. All lines showed distinct diurnal bioluminescence rhythms in ND (Fig. 5a–e). Significant rhythms were detected for all genes in LL, but rapid dampening of the bioluminescence signal was observed in DD (Fig. 5a–f). This rapid dampening of the luciferase signal in DD seems at odds with evidence for rhythmic expression of several of these genes in DD in qRT‐PCR assays, and suggests that the reduced bioluminescence signal may be a result of an attenuated luciferase activity in DD (e.g. owing to low concentrations of ATP) rather than a reduction in transcription of clock genes. Still, no increase in signal was observed after the addition of sucrose to culture media. With one exception (MpPRR), the amplitudes in ND and LL were also modest. In these conditions, amplitudes in qRT‐PCR assays and bioluminescence assays were generally in agreement, indicating that MpPRR is an exception in exhibiting a strong transcriptional rhythm in both light driven and free‐running conditions.
Figure 5.
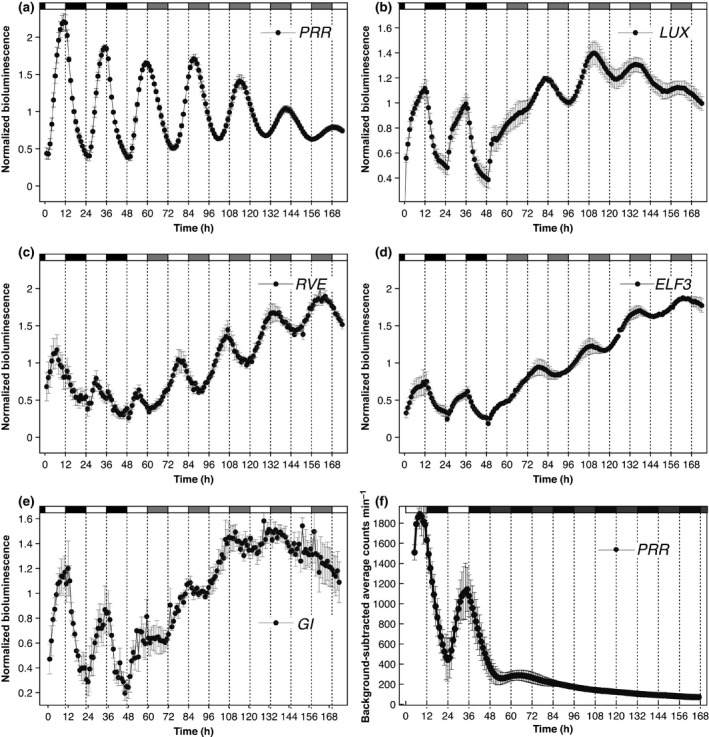
Luciferase reporter lines driven by Marchantia polymorpha clock gene promoters show rhythmic expression under neutral‐day (ND; 12 : 12 h, light : dark) and constant‐light (LL) cycles. (a, f) MpPRR pro :LUC; (b) MpLUX pro :LUC; (c) MpRVE pro :LUC; (d) MpELF3 pro :LUC; (e) MpGI pro :LUC. Transcriptional reporter lines were entrained under ND conditions and transferred to LL (a–e) or constant darkness (DD; f). Means of triplicates of representative reporter lines are shown with standard errors. Light periods are represented by white areas and dark periods by black areas on top of the graphs. In (a–e), grey areas represent subjective night in LL conditions. In (f), grey areas represent subjective day in DD conditions. (a–e) Normalized luminescence; (f) background‐subtracted average counts min–1.
The apparently weak amplitudes of circadian rhythms and the rapid dampening of rhythms in DD prompted us to investigate further the relative importance of light‐driven vs circadian control on clock gene expression in M. polymorpha. In environmental cycles with time periods close to half the circadian period, circadian rhythms tend to frequency demultiply, meaning that every other environmental cycle is skipped (Bruce, 1960; Thines & Harmon, 2010). Luciferase reporter lines driven by M. polymorpha clock gene promoters were entrained in ND, and transferred to a T‐cycle of 12 h (6 : 6 h, light : dark). Even for the MpPRR pro :LUC lines displaying the strongest rhythm in LL, an immediate adoption of a 12 h period was evident and no tendency for frequency demultiplication could be observed (Fig. 6). The same result was obtained with MpELF3, MpGI, MpLUX and MpRVE, suggesting that the M. polymorpha clock is strongly driven by light changes and that the circadian rhythms are generally weak (Fig. S13).
Figure 6.
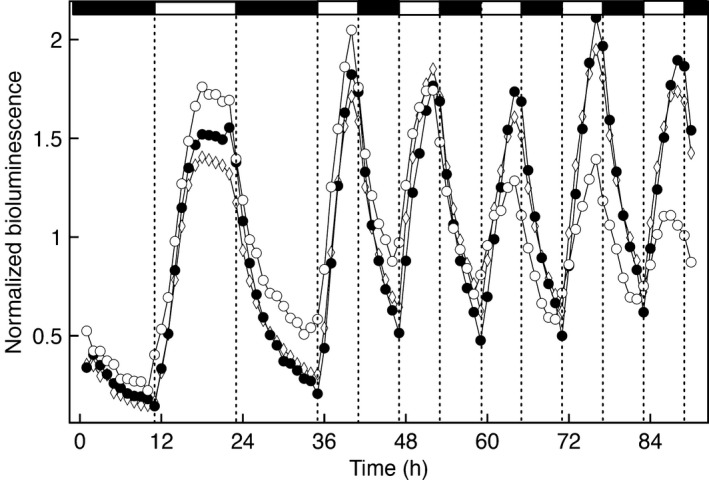
Expression of Marchantia polymorpha circadian clock genes is strongly driven by light. MpPRR pro :LUC bioluminescence of plants entrained in a 12 : 12 h, light : dark photoperiod and transferred to T = 12 photocycles. Light intensity was set to 5 μmol m−2 s−1. Data from three independent transformants are shown. Expression patterns were readily adjusted to a T = 12 photocycle without frequency demultiplication.
To further characterize and directly compare circadian rhythms observed in M. polymorpha with those of Arabidopsis, bioluminescence rhythms for both species were simultaneously compared under different light qualities and intensities. After entrainment in blue plus red light, plants were released into different intensities of red light. As expected, AtCCA1 pro :LUC, AtPRR9 pro :LUC, AtTOC1 pro :LUC and AtCAB pro :LUC displayed a clear shortening of period with increasing light intensity (Fig. 7; Somers et al., 1998). By contrast, all M. polymorpha lines, including 35Spro :LUC, showed a slight increase in period with increased light intensity (Fig. 7). These results suggest that the role of light in entrainment of the M. polymorpha clock is different from that of Arabidopsis. One striking difference between rhythms observed in Arabidopsis and M. polymorpha was the absence of early‐phased genes in the latter. In Arabidopsis, both CCA1/LHY and PRR9 have an early morning phase with peaks at and shortly after dawn, respectively. A homologue to CCA1/LHY is lacking in M. polymorpha and the closest relative, MpRVE, showed a broad expression peak throughout the light period (Fig. 8a). Additionally, the closest homologue to PRR9, MpPRR, showed an afternoon peak of MpPRR pro :LUC activity, although MpPRR mRNA levels peaks earlier during the day (Figs 4, 5a, 8b, S11a, S12). Both CCA1/LHY and PRR9 are also acutely induced by light, and CCA1/LHY have been suggested to be important for entrainment at dawn (Wang & Tobin, 1998); however, no such acute induction was evident for our assayed M. polymorpha genes (Figs 5, 8). This apparent lack of dawn‐phased genes with acute light response might explain the lack of period shortening with increased light intensity observed for M. polymorpha.
Figure 7.
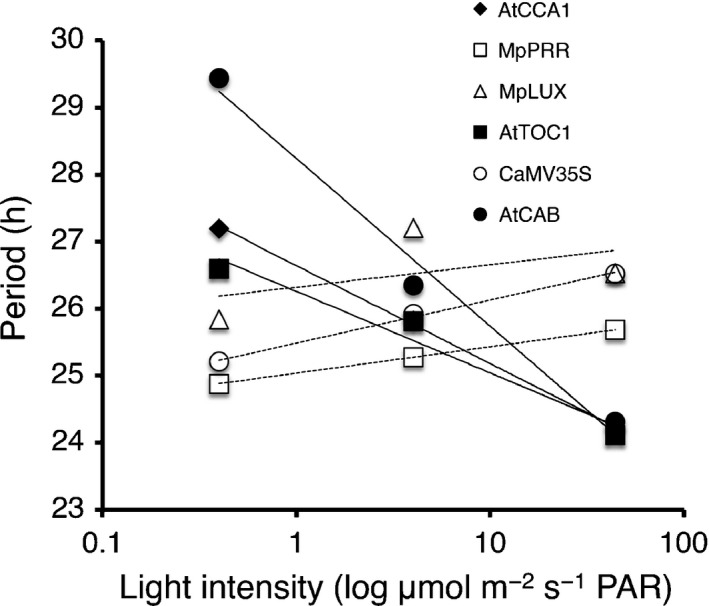
Marchantia polymorpha circadian clock genes do not follow Aschoff's rule. Periods of circadian rhythm for Arabidopsis (closed symbols) and M. polymorpha (open symbols) promoter:LUC lines were estimated in different intensities of continuous red light. Plants harbouring promoter:LUC constructs as indicated in the figure were first entrained in red and blue light, and then released into 0.4, 4 or 44 μmol m−2 s−1 of red light. Period in constant light (LL) was estimated with Spectrum Resampling (Costa et al., 2013) and plotted against logged light intensity. PAR, photosynthetically active radiation.
Figure 8.
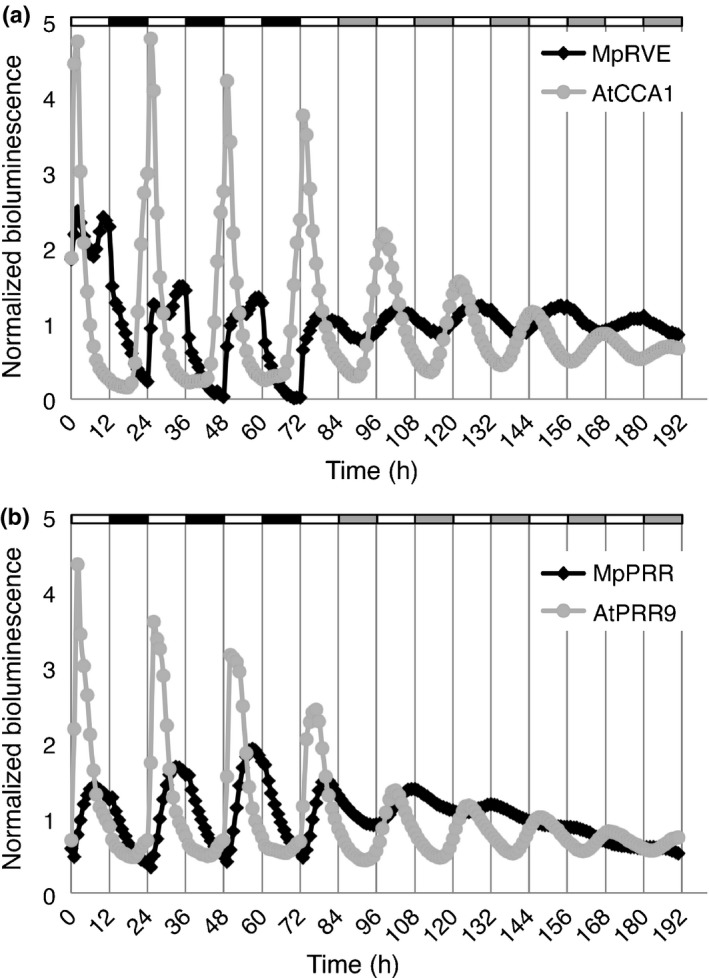
Comparison of bioluminescence rhythm for Arabidopsis and Marchantia polymorpha promoter:LUC reporter lines. (a, b) Bioluminescence of MpRVE pro :LUC, AtCCA1pro :LUC (a), and MpPRR pro :LUC and AtPRR9 pro :LUC (b). Plants were entrained in neutral‐day conditions (ND; 12 : 12 h, light : dark cycles), monitored for 3 d and released into constant light (LL) and monitored for an additional 5 d.
Marchantia polymorpha clock genes are highly expressed in meristematic regions
To investigate the spatial expression pattern of M. polymorpha clock gene homologues, we assayed bioluminescence on different developmental stages of M. polymorpha using luciferase reporter lines for MpELF3, MpGI, MpLUX, MpPRR and MpRVE. We also generated transgenic plants harboring MpPRR pro :GUS to better assay patterns of weak expression. For all LUC‐lines, bioluminescence was detected in the apical thallus in and/or around the apical cell (Figs 9, S14). In gemmalings, expression was confined to the apical notches (Figs 9a,b, S14). Expression was also seen in the developing gemma cups, but as the cups matured, this expression markedly decreased and was confined to the walls of the cup (Figs 9c–e, S14). After extended exposure, a weak signal could sometimes be detected along the midrib in older thalli (Figs 9e, S14). MpPRR pro :GUS lines support a strong expression in meristematic regions, but prolonged staining also revealed expression in chlorenchyma cells within air chambers (Fig. S15). These observations suggest that the M. polymorpha circadian clock is highly active in meristematic or rapidly dividing cells and tissues, but also in photosynthetically active tissues.
Figure 9.
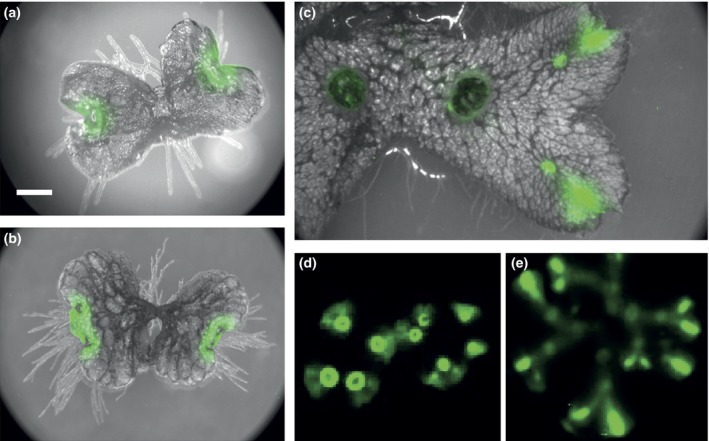
The MpPRR promoter drives luciferase expression in meristematic regions. Luciferase imaging in transgenic Marchantia polymorpha plants expressing luciferase under the control of the MpPRR promoter. (a–e) MpPRR pro :LUC: (a) 4‐d‐old gemmaling showing expression at the apical notches; (b) 7‐d‐old gemmaling with expression at the recently split apical notches; (c) 4‐wk‐old thallus showing strong expression in apical regions and young gemma cups; (d) 3‐wk‐old thallus grown on 2% sucrose with high expression in young gemma cups; (e) 5‐wk‐old thallus showing weak expression along the midrib. Bioluminescense is pseudocolored in green. Illuminated images are shown in grey in (a–c), but omitted from (d) and (e) to reveal weak signals.
Temporal expression patterns support a role also for hornwort clock gene homologues in a circadian clock
Quantitative RT‐PCR was also used to study endogenous transcript abundance of putative A. agrestis clock genes identified earlier. These data suggested that a majority of the assayed genes showed a clear diurnal rhythm under ND. Significant P‐values from analysis of rhythmicity with JTK‐cycle were obtained for all genes except AaRVE (Table 3; Fig. S16). Most genes also displayed a significant free‐running circadian rhythm under at least one constant condition (LL or DD). The exception was AaRVE, which lacked evidence of significant rhythmic expression under all conditions (Table 3; Fig. S16). Data suggest an early morning phased expression of AaCCA1, and a near dusk expression of AaELF3, AaEFL‐1, 2, AaGI, AaLUX and AaPRR. Low amplitude of AaRVE expression precluded estimation of phase for this gene. qRT‐PCR data also support rhythmic expression under day–night cycles or free‐running conditions for additional putative circadian clock genes in M. polymorpha (Table 2; Fig. S7).
Table 3.
Circadian rhythms in Anthoceros agrestis clock gene transcript abundance identified by JTK_CYCLE
| Gene | Conditiona | P‐valueb | Period |
|---|---|---|---|
| AaCCA1 | ND | < 0.001 | 24 |
| LL | 0.001 | 24 | |
| DD | 1 | 28 | |
| AaRVE | ND | 0.225 | 24 |
| LL | 1 | 20 | |
| DD | 1 | 28 | |
| AaPRR | ND | < 0.001 | 24 |
| LL | 0.598 | 20 | |
| DD | 0.261 | 24 | |
| AaELF3 | ND | 0.002 | 20 |
| LL | 0.225 | 20 | |
| DD | 0.029 | 24 | |
| AaGI | ND | 0.004 | 24 |
| LL | 1 | 28 | |
| DD | 0.225 | 20 | |
| AaLUX | ND | < 0.001 | 24 |
| LL | 0.035 | 28 | |
| DD | 0.010 | 24 | |
| AaZTL | ND | < 0.001 | 20 |
| LL | 0.121 | 20 | |
| DD | 1 | 24 | |
| AaEFL‐1 | ND | < 0.001 | 24 |
| LL | 0.024 | 28 | |
| DD | 0.020 | 24 | |
| AaEFL‐2 | ND | < 0.001 | 24 |
| LL | 0.013 | 28 | |
| DD | 0.073 | 24 |
Light condition: neutral day (ND; 12 : 12 h, light : dark cycles), constant darkness (DD) or constant light (LL).
Adjusted P‐values < 0.05 are presented in bold.
In conclusion, temporal expression patterns are consistent with a role in the circadian clock for a majority of homologues to Arabidopsis clock genes identified in the hornwort A. agrestis and the liverwort M. polymorpha. Estimates of phase are also in agreement with an A. agrestis clock model consisting of a dawn‐phased CCA1/LHY homologue and evening‐phased genes including those belonging to the EC (ELF3, ELF4 and LUX homologues). While Arabidopsis contains several PRR‐family genes with phases ranging from morning to evening, one and two homologues were detected in A. agrestis and M. polymorpha, none of which showed a dawn‐phased expression. The apparent lack of dawn‐phased components in M. polymorpha might explain some of the differences in clock behaviour observed between M. polymorpha and Arabidopsis.
Discussion
Transition through a shallow fresh water environment to a terrestrial environment probably had a profound impact on the plant circadian clock and the processes it controls. In marine algae, the circadian clock is important for control of, for example, phototaxis, chemotaxis and cell division (Bruce, 1970; Byrne et al., 1992; Goto & Johnson, 1995; Moulager et al., 2007). In angiosperms the circadian clock regulates a wide range of processes, including those affecting metabolism, growth, abiotic and biotic stress, and various photoperiodic responses (Greenham & McClung, 2015, and references therein).
A majority of plant circadian clock genes are present in charophytes
Evolution of the plant circadian clock is characterized by an increase in gene number and interactions through additional feedback loops. This has partly been accomplished through gene duplication and functional divergence but also through the acquisition of new genes. While green algae clocks can be modelled as a simple two‐gene, one‐loop circuit, angiosperm clocks seem to consist of a complex multi‐loop network comprising many genes.
Our data suggest that all the known major core clock components present in angiosperms were actually present already in charophytes. Novel components in charophytes, which are crucial in angiosperm clocks, are the EC genes ELF3, ELF4 and LUX, but also GI and genes of the ZTL family. Charophytes also contain both TOC1 and PRR3/7 homologues. The function of the EC genes in charophytes or bryophytes is, to our knowledge, unknown. Besides an important function in control of the circadian rhythm, the EC in Arabidopsis is also implicated in output processes such as circadian and temperature control of hypocotyl growth (Nusinow et al., 2011; Box et al., 2015).
In angiosperms, the GI protein interacts with several proteins, including ZTL family proteins, ELF3 and ELF4, to regulate protein stability and localization (references in Kim et al., 2013). In M. polymorpha, Kubota et al. (2014) showed that MpGI interacts with the single ZTL family member MpFKF and affects photoperiodic induction of reproduction, similar to the role of AtFKF1 in flower induction. A role for nonangiosperm GI and ZTL family proteins in clock function has, to our knowledge, not been reported. The GI protein is a large protein without known protein domains. Its protein sequence is also highly conserved, and remarkably the gene occurs in most species as a single copy. No homologous proteins are detected outside Streptophyta, and within this group homologous proteins can be found in Embryophytes, Zygnematales and Coleochaetales, but not in, for example, Klebsormidiales. This pattern supports the notion that Zygnematales or Coleochaetales is sister to land plants, and that GI arose de novo in parallel with the transition to a terrestrial environment.
Gene loss has been important in shaping early land plant circadian clocks
Although gene numbers and complexity have generally increased during plant circadian clock evolution, several examples of gene loss are also evident. It was previously suggested that the reduced circadian clock of P. patens without GI, TOC1 and ZTL homologues might represent an ancestral, less complex, state (Holm et al., 2010). It is now clear that the lack of these three components is the result of gene loss. Interestingly, homologues to GI, TOC1 and ZTL are present in the basal moss T. lepidozioides. This was originally described as a liverwort, but retrieval and analysis of reproductive structures resulted in reclassification of the genus Takakia as a basal moss (Smith, 1993). Following this reclassification, this set of clock genes was lost very early after the branching of mosses. In Arabidopsis these three genes show close interaction, as physical interaction between GI and ZTL proteins regulate TOC1 stability (Más et al., 2003; Kim et al., 2009). It is thus intriguing that these three genes were all lost early in the moss lineage. In hornworts, on the other hand, our data suggest that homologues to GI and ZTL are retained and only TOC1 was lost. An additional intriguing gene loss is CCA1 in liverworts. CCA1 has until now been considered a fundamental component of all plant circadian clocks, from algae to angiosperms. Functional studies of circadian clock genes in bryophytes will shed light on the effects of these gene losses and give a better understanding of the evolution of the plant circadian clock.
Are circadian clocks of early land plants less robust and do they control fewer processes?
Analysis of circadian rhythms of putative clock genes in M. polymorpha suggests that these rhythms are weak compared with those in angiosperms. With one exception (MpPRR), significant but weak rhythms were detected for M. polymorpha genes in constant conditions. In P. patens, clear rhythms are seen in DD but not in LL conditions, while in the gymnosperm P. abies a rapid dampening of rhythms was observed for clock gene homologues in both LL and DD conditions (Holm et al., 2010; Gyllenstrand et al., 2014). Even though a free‐running clock in constant conditions is often considered a hallmark of circadian rhythm, the advantage of such a property is not entirely obvious, as plants occur in a geophysical rhythmic environment. One advantage of a complex free‐running oscillator could be increased resilience to external noise (Brown et al., 2012). It has been suggested that a less complex clock with fewer interlocked feedback loops and less control of degradation of its component results in a more rapidly dampened timer (Brown et al., 2012). If circadian clocks of nonangiosperm plants are less complex or less resilience to external noise awaits further study.
Analysis of LUC and GUS reporter lines driven by promoters of putative M. polymorpha clock genes suggested that these genes were predominantly expressed in meristematic regions, but weaker expression was also detected in chlorenchyma cells within air chambers. This suggests that basic metabolic processes such as photosynthesis and starch metabolism may also be under circadian control in M. polymorpha. Processes confined to meristematic regions that are probably under circadian control include photoperiodic induction of reproduction. Gametangia production in M. polymorpha is induced by long photoperiods and requires MpGI and MpFKF (Voth & Hamner, 1940; Kubota et al., 2014).
Our inventory of circadian clock gene homologues in charophytes and bryophytes suggests an early acquisition of a complex circadian network, with all known main components already present before or concurrent with the occurrence of land plants, although in lower copy numbers. It has been suggested that physiological adaptations to land had already evolved in early terrestrial charophytes (Delwiche & Cooper, 2015; Harholt et al., 2016). Work on cell wall evolution (Mikkelsen et al., 2014) suggests that key adaptations to terrestrial habitats had already occurred in Klebsormidium, which is in line with our observations of an early complexity of the circadian network. Our data provide good grounds for pursuing functional studies of circadian clocks not only in bryophytes but also in charophytes. Our preliminary observations of circadian rhythms in M. polymorpha, suggesting weak rhythms, lack of morning‐phased genes that show acute light response, and deviations from predictions of Aschoff's rule for diurnal organisms, point to significant differences in the wiring of the M. polymorpha circadian network.
Author contributions
A‐M.L., A.K., K.H., N.C., N.G., R.N., T.K. and U.L. conceived the project; A‐M.L. and U.L. performed the sequence retrieval and phylogenetic analyses; A‐M.L., A.K., D.M.E., E.R.A.P., K.H., N.G. and U.L. performed the qRT‐PCR experiments; A‐M.L. and A.K. analysed qRT‐PCR data. A.K., D.M.E., E.R.A.P., T.M., T.O. and U.L. performed the luciferase experiments; A‐M.L., A.K., T.M., T.O. and U.L. analysed the luciferase data. N.C. provided new materials. A‐M.L., D.M.E. and U.L. wrote the article with contributions of all the authors.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Alignments used for phylogenetic construction.
Fig. S2 Inferred phylogeny of homologues to the ELF3 family.
Fig. S3 Inferred phylogeny of homologues to the ELF4 family.
Fig. S4 Inferred phylogeny of homologues to the LUX family.
Fig. S5 Inferred phylogeny of homologues to the GI gene family.
Fig. S6 Inferred phylogeny of homologues to the ZTL gene family.
Fig. S7 Temporal expression patterns of putative circadian clock genes in Marchantia polymorpha (Mp) under ND, LL and DD conditions.
Fig. S8 Generation of MpRVE knockout mutant.
Fig. S9 Generation of MpPRR knockout mutant.
Fig. S10 Generation of MpTOC1 knockout mutants.
Fig. S11 Temporal expression pattern of MpPRR and 35Spro :LUC under ND and LL conditions.
Fig. S12 Temporal expression pattern of MpPRR in WT, Mprve ko, Mptoc1 ko and restored lines of Mprve ko.
Fig. S13 pro:LUC bioluminescence for MpELF3, MpGI, MpLUX and MpRVE.
Fig. S14 Luciferase imaging in transgenic Marchantia polymorpha plants expressing luciferase under the control of Marchantia polymorpha promoters.
Fig. S15 MpPRR pro :GUS expression in mature thallus.
Fig. S16 Temporal expression patterns of putative circadian clock genes in Anthoceros agrestis (Aa) under ND, LL and DD conditions.
Table S1 Gene names, family/subclade, gene ID or accession number
Table S2 Oligonucleotides used in this study
Methods S1 Supplemental materials and methods describing sequence retrieval, sequence analysis and phylogenetic reconstruction.
Acknowledgements
We are grateful for the technical assistance of Kerstin Jeppsson. This project was supported by the Swedish Research Council VR, grant nos 2011‐5609 and 2014‐522 to U.L. and by JSPS KAKENHI, grants nos 25113009 and 26291059 to T.K. A.K. was supported by JSPS KAKENHI Grants‐in‐Aid for JSPS fellows (no. 24‐6987).
References
- Abramoff MD, Magalhaes PJ, Ram SJ. 2004. Image processing with image j. Biophotonics International 11: 7. [Google Scholar]
- Alabadí D, Oyama T, Yanovsky MJ, Harmon FG, Más P, Kay SA. 2001. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293: 880–883. [DOI] [PubMed] [Google Scholar]
- Berns MC, Nordström K, Cremer F, Tóth R, Hartke M, Simon S, Klasen JR, Bürstel I, Coupland G. 2014. Evening expression of Arabidopsis GIGANTEA is controlled by combinatorial interactions among evolutionarily conserved regulatory motifs. Plant Cell 26: 3999–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL. 2013. Walkabout on the long branches of plant evolution. Current Opinion in Plant Biology 16: 70–77. [DOI] [PubMed] [Google Scholar]
- Box MS, Huang BE, Domijan M, Jaeger KE, Khattak AK, Yoo SJ, Sedivy EL, Jones DM, Hearn TJ, Webb AAR et al 2015. ELF3 controls thermoresponsive growth in Arabidopsis . Current Biology 25: 194–199. [DOI] [PubMed] [Google Scholar]
- Brown SA, Kowalska E, Dallmann R. 2012. (Re)inventing the circadian feedback loop. Developmental Cell 22: 477–487. [DOI] [PubMed] [Google Scholar]
- Bruce VG. 1960. Environmental entrainment of circadian rhythms. Cold Spring Harbor Symposia on Quantitative Biology 25: 29–48. [DOI] [PubMed] [Google Scholar]
- Bruce VG. 1970. The biological clock in Chlamydomonas reinhardi . Journal of Protozoology 17: 328–334. [Google Scholar]
- Buschmann H, Holtmannspötter M, Borchers A, O'Donoghue M‐T, Zachgo S. 2016. Microtubule dynamics of the centrosome‐like polar organizers from the basal land plant Marchantia polymorpha . New Phytologist 209: 1469–8137. [DOI] [PubMed] [Google Scholar]
- Byrne TE, Wells MR, Johnson CH. 1992. Circadian rhythms of chemotaxis to ammonium and of methylammonium uptake in Chlamydomonas . Plant Physiology 98: 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, Helfer A, Nusinow DA, Kay SA. 2012. ELF3 recruitment to the PRR9 promoter requires other Evening Complex members in the Arabidopsis circadian clock. Plant Signaling & Behavior 7: 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corellou F, Schwartz C, Motta J‐P, Djouani‐Tahri EB, Sanchez F, Bouget F‐Y. 2009. Clocks in the green lineage: comparative functional analysis of the circadian architecture of the Picoeukaryote Ostreococcus . Plant Cell 21: 3436–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MJ, Finkenstädt B, Roche V, Lévi F, Gould PD, Foreman J, Halliday K, Hall A, Rand DA. 2013. Inference on periodicity of circadian time series. Biostatistics (Oxford, England) 14: 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington MF, Maloof JN, Straume M, Kay SA, Harmer SL. 2008. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biology 9: R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Wei X, Pei L, Thompson RL, Liu Y, Heard JE, Ruff TG, Beachy RN. 2011. BROTHER OF LUX ARRHYTHMO is a component of the Arabidopsis circadian clock. Plant Cell 23: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caluwé J, Xiao Q, Hermans C, Verbruggen N, Leloup J‐C, Gonze D. 2016. A compact model for the complex plant circadian clock. Frontiers in Plant Science 7: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche CF, Cooper ED. 2015. The evolutionary origin of a terrestrial flora. Current Biology 25: R899–R910. [DOI] [PubMed] [Google Scholar]
- Doyle MR, Davis SJ, Bastow RM, McWatters HG, Kozma‐Bognár L, Nagy F, Millar AJ, Amasino RM. 2002. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana . Nature 419: 74–77. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. 1999. Molecular bases for circadian clocks. Cell 96: 271–290. [DOI] [PubMed] [Google Scholar]
- Farinas B, Mas P. 2011. Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. Plant Journal 66: 318–329. [DOI] [PubMed] [Google Scholar]
- Fogelmark K, Troein C. 2014. Rethinking transcriptional activation in the Arabidopsis circadian clock. PLoS Computational Biology 10: e1003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornara F, Panigrahi KCS, Gissot L, Sauerbrunn N, Rühl M, Jarillo JA, Coupland G. 2009. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Developmental Cell 17: 75–86. [DOI] [PubMed] [Google Scholar]
- Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, Coupland G, Putterill J. 1999. GIGANTEA: a circadian clock‐controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane‐spanning domains. EMBO Journal 18: 4679–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. 1968. Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research 50: 151–158. [DOI] [PubMed] [Google Scholar]
- Goto K, Johnson CH. 1995. Is the cell division cycle gated by a circadian clock? The case of Chlamydomonas reinhardtii . Journal of Cell Biology 129: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenham K, McClung CR. 2015. Integrating circadian dynamics with physiological processes in plants. Nature Reviews Genetics 16: 598–610. [DOI] [PubMed] [Google Scholar]
- Gyllenstrand N, Karlgren A, Clapham D, Holm K, Hall A, Gould PD, Källman T, Lagercrantz U. 2014. No time for spruce: rapid dampening of circadian rhythms in Picea abies (L. Karst). Plant and Cell Physiology 55: 535–550. [DOI] [PubMed] [Google Scholar]
- Harholt J, Moestrup Ø, Ulvskov P. 2016. Why plants were terrestrial from the beginning. Trends in Plant Science 21: 96–101. [DOI] [PubMed] [Google Scholar]
- Harmer SL. 2009. The circadian system in higher plants. Annual Review of Plant Biology 60: 357–377. [DOI] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA. 2000. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science (New York, N.Y.) 290: 2110–2113. [DOI] [PubMed] [Google Scholar]
- Hazen SP, Schultz TF, Pruneda‐Paz JL, Borevitz JO, Ecker JR, Kay SA. 2005. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proceedings of the National Academy of Sciences, USA 102: 10387–10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks KA, Albertson TM, Wagner DR. 2001. EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell 13: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm K, Källman T, Gyllenstrand N, Hedman H, Lagercrantz U. 2010. Does the core circadian clock in the moss Physcomitrella patens (Bryophyta) comprise a single loop? BMC Plant Biology 10: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PY, Devisetty UK, Harmer SL. 2013. Accurate timekeeping is controlled by a cycling activator in Arabidopsis. eLife 2: e00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Hogenesch JB, Kornacker K. 2010. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome‐scale data sets. Journal of Biological Rhythms 25: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K, Chiyoda S, Yamato KT, Kohchi T. 2008. Agrobacterium‐mediated transformation of the haploid liverwort Marchantia polymorpha L., an emerging model for plant biology. Plant & Cell Physiology 49: 1084–1091. [DOI] [PubMed] [Google Scholar]
- Ishizaki K, Johzuka‐Hisatomi Y, Ishida S, Iida S, Kohchi T. 2013. Homologous recombination‐mediated gene targeting in the liverwort Marchantia polymorpha L. Scientific Reports 3: 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K, Nishihama R, Ueda M, Inoue K, Ishida S, Nishimura Y, Shikanai T, Kohchi T. 2015. Development of gateway binary vector series with four different selection markers for the liverwort Marchantia polymorpha . PLoS ONE 10: e0138876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Song YH, Imaizumi T. 2012. LOV domain‐containing F‐box proteins: light‐dependent protein degradation modules in Arabidopsis . Molecular Plant 5: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Elliott JA, Foster R. 2003. Entrainment of circadian programs. Chronobiology International 20: 741–774. [DOI] [PubMed] [Google Scholar]
- Kamioka M, Takao S, Suzuki T, Taki K, Higashiyama T, Kinoshita T, Nakamichi N. 2016. Direct repression of evening genes by CIRCADIAN CLOCK‐ASSOCIATED1 in the Arabidopsis circadian clock. Plant Cell 28: 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W‐Y, Fujiwara S, Suh S‐S, Kim J, Kim Y, Han L, David K, Putterill J, Nam HG, Somers DE. 2007. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 449: 356–360. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lim J, Yeom M, Kim H, Kim J, Wang L, Kim WY, Somers DE, Nam HG. 2013. ELF4 regulates GIGANTEA chromatin access through subnuclear sequestration. Cell Reports 3: 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmos E, Nowak M, Werner M, Fischer K, Schwarz G, Mathews S, Schoof H, Nagy F, Bujnicki JM, Davis SJ. 2009. Integrating ELF4 into the circadian system through combined structural and functional studies. HFSP Journal 3: 350–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota A, Ishizaki K, Hosaka M, Kohchi T. 2013. Efficient Agrobacterium‐mediated transformation of the liverwort Marchantia polymorpha using regenerating thalli. Bioscience, Biotechnology, and Biochemistry 77: 167–172. [DOI] [PubMed] [Google Scholar]
- Kubota A, Kita S, Ishizaki K, Nishihama R, Yamato KT, Kohchi T. 2014. Co‐option of a photoperiodic growth‐phase transition system during land plant evolution. Nature Communications 5: 3668. [DOI] [PubMed] [Google Scholar]
- Li J, Hu E, Chen X, Xu J, Lan H, Li C, Hu Y, Lu Y. 2016. Evolution of DUF1313 family members across plant species and their association with maize photoperiod sensitivity. Genomics 107: 199–207. [DOI] [PubMed] [Google Scholar]
- Más P, Kim W‐Y, Somers DE, Kay SA. 2003. Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana . Nature 426: 567–570. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Okamoto K, Onai K, Niwa Y, Shimogawara K, Ishiura M. 2008. A systematic forward genetic analysis identified components of the Chlamydomonas circadian system. Genes & Development 22: 918–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushika A, Makino S, Kojima M, Mizuno T. 2000. Circadian waves of expression of the APRR1/TOC1 family of pseudo‐response regulators in Arabidopsis thaliana: insight into the plant circadian clock. Plant and Cell Physiology 41: 1002–1012. [DOI] [PubMed] [Google Scholar]
- McClung CR. 2013. Beyond Arabidopsis: the circadian clock in non‐model plant species. Seminars in Cell & Developmental Biology 24: 430–436. [DOI] [PubMed] [Google Scholar]
- Michael TP, Mockler TC, Breton G, McEntee C, Byer A, Trout JD, Hazen SP, Shen R, Priest HD, Sullivan CM et al 2008. Network discovery pipeline elucidates conserved time‐of‐day–specific cis‐regulatory modules. PLoS Genetics 4: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen MD, Harholt J, Ulvskov P, Johansen IE, Fangel JU, Doblin MS, Bacic A, Willats WG. 2014. Evidence for land plant cell wall biosynthetic mechanisms in charophyte green algae. Annals of Botany 114: 1217–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Serikawa M, Suzuki S, Kondo T, Oyama T. 2006. Conserved expression profiles of circadian clock‐related genes in two Lemna species showing long‐day and short‐day photoperiodic flowering responses. Plant and Cell Physiology 47: 601–612. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Wheatley K, Hanzawa Y, Wright L, Mizoguchi M, Song H‐R, Carré IA, Coupland G. 2002. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis . Developmental Cell 2: 629–641. [DOI] [PubMed] [Google Scholar]
- Moulager M, Monnier A, Jesson B, Bouvet R, Mosser J, Schwartz C, Garnier L, Corellou F, Bouget F‐Y. 2007. Light‐dependent regulation of cell division in Ostreococcus: evidence for a major transcriptional input. Plant Physiology 144: 1360–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranaka T, Okada M, Yomo J, Kubota S, Oyama T. 2014. Characterisation of circadian rhythms of various duckweeds. Plant Biology 17: 66–74. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Yamazaki T, Nishii S, Noguchi T, Hoshino H, Niwa K, Viviani VR, Ohmiya Y. 2010. Enhanced beetle luciferase for high‐resolution bioluminescence imaging. PLoS ONE 5: e10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusinow DA, Helfer A, Hamilton EE, King JJ, Imaizumi T, Schultz TF, Farré EM, Kay SA. 2011. The ELF4‐ELF3‐LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475: 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada R, Kondo S, Satbhai SB, Yamaguchi N, Tsukuda M, Aoki S. 2009. Functional characterization of CCA1/LHY homolog genes, PpCCA1a and PpCCA1b, in the moss Physcomitrella patens . Plant Journal 60: 551–563. [DOI] [PubMed] [Google Scholar]
- Pokhilko A, Mas P, Millar AJ. 2013. Modelling the widespread effects of TOC1 signalling on the plant circadian clock and its outputs. BMC Systems Biology 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rand DA, Shulgin BV, Salazar D, Millar AJ. 2004. Design principles underlying circadian clocks. Journal of the Royal Society Interface 1: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat R, Takahashi N, Hsu PY, Jones MA, Schwartz J, Salemi MR, Phinney BS, Harmer SL. 2011. REVEILLE8 and PSEUDO‐REPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock. PLoS Genetics 7: e1001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint‐Marcoux D, Proust H, Dolan L, Langdale JA. 2015. Identification of reference genes for real‐time quantitative PCR experiments in the liverwort Marchantia polymorpha . PLoS ONE 10: e0118678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satbhai SB, Yamashino T, Okada R, Nomoto Y, Mizuno T, Tezuka Y, Itoh T, Tomita M, Otsuki S, Aoki S. 2011. Pseudo‐response regulator (PRR) homologues of the moss Physcomitrella patens: insights into the evolution of the PRR family in Land plants. DNA Research 18: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T. 2007. FKF1 and GIGANTEA complex formation is required for day‐length measurement in Arabidopsis . Science 318: 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carré IA, Coupland G. 1998. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 93: 1219–1229. [DOI] [PubMed] [Google Scholar]
- Schultz TF, Kiyosue T, Yanovsky M, Wada M, Kay SA. 2001. A role for LKP2 in the circadian clock of Arabidopsis. Plant Cell 13: 2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AJ, Szövényi P, Shaw B. 2011. Bryophyte diversity and evolution: windows into the early evolution of land plants. American Journal of Botany 98: 352–369. [DOI] [PubMed] [Google Scholar]
- Smith D. 1993. Antheridia and sporophytes in Takakia ceratophylla (Mitt.) Grolle: evidence for reclassification among the mosses. Journal of the Hattori Botanical Laboratory 73: 263–271. [Google Scholar]
- Somers DE, Devlin PF, Kay SA. 1998. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282: 1488–1490. [DOI] [PubMed] [Google Scholar]
- Somers DE, Schultz TF, Milnamow M, Kay SA. 2000. ZEITLUPE encodes a novel clock‐associated PAS protein from Arabidopsis . Cell 101: 319–329. [DOI] [PubMed] [Google Scholar]
- Strayer C, Oyama T, Schultz TF, Raman R, Somers DE, Más P, Panda S, Kreps JA, Kay SA. 2000. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 289: 768–771. [DOI] [PubMed] [Google Scholar]
- Thines B, Harmon FG. 2010. Ambient temperature response establishes ELF3 as a required component of the core Arabidopsis circadian clock. Proceedings of the National Academy of Sciences 107: 3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troein C, Corellou F, Dixon LE, van Ooijen G, O'Neill JS, Bouget F‐Y, Millar AJ. 2011. Multiple light inputs to a simple clock circuit allow complex biological rhythms. Plant Journal 66: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TY‐C, Choi YS, Ma W, Pomerening JR, Tang C, Ferrell JE. 2008. Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science 321: 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voth PD, Hamner KC. 1940. Responses of Marchantia polymorpha to nutrient supply and photoperiod. Botanical Gazette 102: 169–205. [Google Scholar]
- Wang ZY, Kenigsbuch D, Sun L, Harel E, Ong MS, Tobin EM. 1997. A Myb‐related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell 9: 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z‐Y, Tobin EM. 1998. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93: 1207–1217. [DOI] [PubMed] [Google Scholar]
- Yerushalmi S, Green RM. 2009. Evidence for the adaptive significance of circadian rhythms. Ecology Letters 12: 970–981. [DOI] [PubMed] [Google Scholar]
- Young MW, Kay SA. 2001. Time zones: a comparative genetics of circadian clocks. Nature Reviews Genetics 2: 702–715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Alignments used for phylogenetic construction.
Fig. S2 Inferred phylogeny of homologues to the ELF3 family.
Fig. S3 Inferred phylogeny of homologues to the ELF4 family.
Fig. S4 Inferred phylogeny of homologues to the LUX family.
Fig. S5 Inferred phylogeny of homologues to the GI gene family.
Fig. S6 Inferred phylogeny of homologues to the ZTL gene family.
Fig. S7 Temporal expression patterns of putative circadian clock genes in Marchantia polymorpha (Mp) under ND, LL and DD conditions.
Fig. S8 Generation of MpRVE knockout mutant.
Fig. S9 Generation of MpPRR knockout mutant.
Fig. S10 Generation of MpTOC1 knockout mutants.
Fig. S11 Temporal expression pattern of MpPRR and 35Spro :LUC under ND and LL conditions.
Fig. S12 Temporal expression pattern of MpPRR in WT, Mprve ko, Mptoc1 ko and restored lines of Mprve ko.
Fig. S13 pro:LUC bioluminescence for MpELF3, MpGI, MpLUX and MpRVE.
Fig. S14 Luciferase imaging in transgenic Marchantia polymorpha plants expressing luciferase under the control of Marchantia polymorpha promoters.
Fig. S15 MpPRR pro :GUS expression in mature thallus.
Fig. S16 Temporal expression patterns of putative circadian clock genes in Anthoceros agrestis (Aa) under ND, LL and DD conditions.
Table S1 Gene names, family/subclade, gene ID or accession number
Table S2 Oligonucleotides used in this study
Methods S1 Supplemental materials and methods describing sequence retrieval, sequence analysis and phylogenetic reconstruction.


