Abstract
Background
Oral anticoagulation (OAC) therapy is associated with increased periprocedural risks after cardiac implantable electronic device (CIED) implantation. Patterns of anticoagulation management involving non–vitamin K antagonist oral anticoagulants (NOACs) have not been characterized.
Hypothesis
Anticoagulation strategies and outcomes differ by anticoagulant type in patients undergoing CIED implantation.
Methods
Using the nationwide Outcomes Registry for Better Informed Treatment of Atrial Fibrillation, we assessed how atrial fibrillation (AF) patients undergoing CIED implantation were cared for and their subsequent outcomes. Outcomes were compared by oral anticoagulant therapy (none, warfarin, or NOAC) as well as by anticoagulation interruption status.
Results
Among 9129 AF patients, 416 (5%) underwent CIED implantation during a median follow‐up of 30 months (interquartile range, 24–36). Of these, 60 (14%) had implantation on a NOAC. Relative to warfarin therapy, those on a NOAC were younger (70.5 years [range, 65–77.5 years] vs 77 years [range, 70–82 years]), had less valvular heart disease (15.0% vs 31.3%), higher creatinine clearance (67.3 [range, 59.7–99.0] vs 65.8 [range, 50.0–91.6]), were more likely to have persistent AF (26.7% vs 22.9%), and use concomitant aspirin (51.7% vs 35.2%). OAC therapy was commonly interrupted for CIED in 64% (n = 183 of 284) of warfarin patients and 65% (n = 39 of 60) of NOAC patients. Many interrupted patients received intravenous bridging anticoagulation: 33/183 (18%) interrupted warfarin and 4/39 (10%) interrupted NOAC patients. Thirty‐day periprocedure bleeding and stroke adverse events were infrequent.
Conclusions
Management of anticoagulation among AF patients undergoing CIED implantation is highly variable, with OAC being interrupted in more than half of both warfarin‐ and NOAC‐treated patients. Bleeding and stroke events were infrequent in both warfarin and NOAC‐treated patients.
Keywords: Atrial Fibrillation, Cardiac Implantable Electronic Device, Anticoagulation
1. INTRODUCTION
Cardiac implantable electronic devices (CIEDs) prolong survival and may improve quality of life. Approximately 1 in 5 patients undergoing CIED implantation are chronically treated with oral anticoagulation (OAC) prior to surgery.1, 2 Atrial fibrillation (AF) is the most common indication for OAC in patients undergoing CIED implantation. Perioperative management of OAC in AF patients is challenging and involves several clinical decisions, including whether or not to interrupt therapy. Management of OAC in patients with AF has evolved over the past decade following the results of several important clinical trials. Interruption of warfarin with heparin or low‐molecular‐weight heparin bridging prior to CIED implantation has been shown to lead to significantly increased bleeding and hematoma formation.3, 4, 5 Accordingly, uninterrupted warfarin has been considered the standard of care in patients with AF and moderate to high risk for stroke undergoing CIED implantation. However, the development and introduction of the non–vitamin K antagonist oral anticoagulants (NOACs) into clinical practice has challenged this standard, as many CIED patients are now being treated with NOACs and not warfarin. Despite the growing popularity of NOACs, clinicians have limited data to inform their use in patients undergoing CIED implantation. The objective of this study was to describe patterns of anticoagulation management during CIED implantation and subsequent outcomes in AF patients in US clinical practice.
2. METHODS
2.1. Trial design
We used patient data from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). ORBIT‐AF is a national, multicenter, prospective registry of ambulatory AF patients. The rationale and design of the registry has been previously described.6 Primary care providers, cardiologist, and electrophysiologists enrolled patients in the clinic setting. Patients greater than 18 years of age with electrocardiographic evidence of atrial fibrillation who was not secondary to a reversible cause (eg, postoperative AF) were eligible for inclusion. Patients with a life expectancy less than 6 months and patients participating in a randomized trial of stroke prevention therapy were excluded.
The electronic medical record at each participating institution served as the primary data source. Data were submitted via a Web‐based form collected in 6‐month intervals after initial enrollment. Maximum follow‐up duration was 3 years. Investigators reported any new medical or surgical therapies at each follow‐up. The specific anticoagulant utilized, monitoring data, bridging data, and outcomes were reported. In patients who underwent cardiac device implantation, providers were queried regarding the type of device implanted (permanent pacemaker, implantable cardioverter‐defibrillator, or cardiac resynchronization therapy defibrillator), periprocedural international normalized ratio, and anticoagulation interruption. Use of bridging anticoagulation was reported and categorized as unfractionated heparin, low‐molecular‐weight heparin, fondaparinux, or other. Adverse events within 30 days of device implantation were also recorded including death, cause‐specific hospitalization (cardiovascular, bleeding, noncardiovascular, nonbleeding), myocardial infarction, stroke, or systemic embolism. Major bleeding events were defined according to the International Society of Thrombosis and Haemostasis criteria,7 which include (1) a fall in hemoglobin level by greater than 2vg/dL, (2) transfusion of 2 or greater units of packed red blood cells, (3) bleeding in a critical site (eg, intracranial), and (4) fatal outcome. Strokes were defined as a sudden new focal neurologic deficit that was not reversible within 24 hours and not secondary to another primary brain pathology (eg, tumor or infection), and were adjudicated using primary source documentation.
2.2. Statistical analysis
Baseline characteristics were compared among AF patients undergoing and not undergoing device implantation. Patients undergoing device implantation were stratified by (1) presence of anticoagulation, (2) anticoagulation strategy (vitamin K antagonist or NOAC), and (3) anticoagulation interruption. For univariate analysis categorical variables are presented as percentages and differences between groups determined by χ2 test. Continuous variables were presented as median (interquartile range) and statistical significance was determined by the Wilcoxon rank sum test.
Unadjusted 30‐day outcomes across clinical subgroups were reported. All statistical analysis were performed using SAS (version 9.3; SAS Institute, Cary, NC). All P values presented are 2‐sided. All ORBIT‐AF participants provided written informed consent prior to study participation. The Duke Institutional Review Board (IRB) approved the ORBIT‐AF Registry, and participating sites obtained approval from local IRBs prior to entering patient data.
3. RESULTS
3.1. Baseline characteristics
Among the overall cohort of 10 137 patients, 388 (3.8%) were excluded due to inadequate follow‐up and 204 due to death within 6 months of baseline. During a median follow‐up of 30 months (interquartile range, 24–36 months), 416 (5.0%) of the 9129 patients included underwent CIED implantation or revision. Patients undergoing device implantation were of similar age and gender to the general AF population, but more likely to have diabetes (34.1% vs 29.0%, P = 0.03), persistent AF (21.9% vs 16.5%, P = 0.008), or prior catheter ablation of AF (P = 0.04) Patients who underwent CIED implant were also more likely to use amiodarone (14.9% vs 9.7%, P < 0.001) or be treated with an oral anticoagulant (82.9% vs 76.2%, P = 0.002) and had higher rates of congestive heart failure (42.6% vs 32.7%, P < 0.0001), intraventricular conduction delay (33.7% vs 29.5%, P < 0.0001), and left atrial enlargement (80.5% vs 75.8%, P = 0.02).
Among those patients who underwent CIED implantation or revision, compared with patients on warfarin, NOAC patients were younger (70.5 years [range, 65–77.5 years] vs 77 years [range, 70–82 years], P = 0.001), less likely to have valvular heart disease (15.0% vs 31.3%, P = 0.01), had higher creatinine clearance (67.3 [range, 59.7–99.0] vs 65.8 [range, 50.0–91.6], P = 0.046), were more likely to have persistent AF (26.7% vs 22.9%, P = 0.02), and use concomitant aspirin (51.7% vs 35.2%, P = 0.01) (Table 1).
Table 1.
Baseline characteristics based on oral anticoagulation strategy
| No Implantation, n = 9129 | Implantation | ||||
|---|---|---|---|---|---|
| Warfarin, n = 284 | NOAC, n = 60 | P (Implant vs No Implant) | P (NOAC vs Warfarin) | ||
| Age, y | 75.0 (67.0, 82.0) | 77 (70.0, 82.0) | 70.5 (65.0, 77.5) | 0.115 | 0.001 |
| Hypertension | 7575 (83.0%) | 246 (86.6%) | 52 (86.7%) | 0.259 | 0.992 |
| Diabetes mellitus | 2651 (29.0%) | 101 (35.6%) | 21 (35.0%) | 0.026 | 0.934 |
| Congestive heart failure | 6236 (68.3%) | 127 (44.7%) | 21 (35.0%) | <0.0001 | 0.522 |
| Valvular disease | 2299 (25.2%) | 89 (31.3%) | 9 (15.0%) | 0.074 | 0.011 |
| Coronary artery disease | 3274 (35.9%) | 113 (39.8%) | 19 (31.7%) | 0.168 | 0.241 |
| COPD | 1454 (15.9%) | 47 (16.5%) | 8 (13.3%) | 0.253 | 0.537 |
| OSA | 1661 (18.2%) | 61 (21.5%) | 9 (15.0%) | 0.161 | 0.258 |
| CrCl, Cockcroft‐Gault | 70.1 (50.6, 97.2) | 65.8 (50.0, 91.6) | 67.3 (59.7, 99.0) | 0.009 | 0.046 |
| Type of AF | 0.017 | ||||
| Paroxysmal | 4674 (51.2%) | 132 (46.5%) | 20 (33.3%) | ||
| Persistent | 1508 (16.5%) | 65 (22.9%) | 16 (26.7%) | ||
| Permanent | 2541 (27.8%) | 76 (26.8%) | 16 (26.7%) | ||
| Medications | |||||
| β‐Blocker | 5875 (64.4%) | 186 (65.5%) | 34 (57.7%) | 0.499 | 0.196 |
| ACE inhibitor | 3232 (35.4%) | 113 (39.8%) | 29 (48.3%) | 0.023 | 0.223 |
| Aspirin | 4046 (44.3%) | 100 (35.2%) | 31 (51.7%) | 0.538 | 0.017 |
| P2Y12 inhibitor | 653 (7.2%) | 21 (7.4%) | 5 (8.3%) | 0.331 | 0.803 |
| Amiodarone | 882 (9.7%) | 41 (14.4%) | 5 (8.3%) | 0.0005 | 0.208 |
| CHA2DS2VASc | 4 (3.0, 5.0) | 4 (3.0, 5.0) | 4 (2.5, 5.0) | 0.001 | 0.006 |
| ATRIA | 3.0 (1.0, 4.0) | 3 (1.0, 5.0) | 3 (1.0, 5.0) | 0.035 | 0.025 |
Abbreviations: ACE, angiotensin‐converting enzyme; AF, atrial fibrillation, ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; NOAC, non–vitamin K antagonist oral anticoagulants; OSA, obstructive sleep apnea.
Patients with baseline implant devices who do not undergo a revision procedure (eg, change out) are included as the nonimplantation group
CHA2DS2‐VASc, Congestive Heart Failure, Hypertension, Age ≥75 Years, Diabetes Mellitus, Stroke or Transient Ischemic Attack, Vascular Disease, Age 65‐74 Years, Sex
3.2. Management of anticoagulation during device implantation and 30‐day adverse events
The majority of patients undergoing CIED implant (n = 284, 64%) were managed with warfarin, whereas 14% (n = 60) were treated with NOACs and 17% (n = 72) with no oral anticoagulant. Among patients who underwent device implantation while on warfarin, anticoagulation was interrupted in 64% (n = 183). Among patients managed with interrupted warfarin, 18% (n = 33) received bridging anticoagulation. Bridged warfarin patients received either low‐molecular‐weight heparin (n = 26/31, 84%) or unfractionated heparin (n = 5/31, 16%). Patients managed on NOACs (n = 60) had an oral anticoagulant held prior to device surgery in 65% of cases (n = 39).
Among patients treated with interrupted NOAC therapy, 10% (n = 4) received bridging anticoagulation. Bridged NOAC patients received either low‐molecular‐weight heparin (n = 2/4, 50%) or unfractionated heparin (n = 2/4, 50%). Management of anticoagulation differed based on stroke risk as determined by CHA2DS2‐VASc score (Figure 2). Anticoagulation strategy did not differ between patients with valvular and nonvalvular AF (P = 0.087) (Figure 2). There was no statistically significant difference in utilization of bridging anticoagulation in patients who underwent device implantation after publication of the Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial (BRUISE‐CONTROL) trial (6% vs 11%, P = 0.24), a prospective trial that demonstrated 4‐fold increased rates of pocket hematoma with bridging anticoagulation. Adverse events were rare in the 30‐day period following CIED implantation, regardless of anticoagulation strategy. In the 284 patients treated with warfarin, there was 1 International Society on Thrombosis and Haemostasis major bleeding episode (0.3%), 3 stroke/transient ischemic attack (TIA) events (1%), 18 all‐cause hospitalizations (6%), 12 cardiovascular hospitalizations (4.2%), and no bleeding hospitalizations (Table 2). Rates of all‐cause hospitalization did not differ between patients treated with interrupted vs uninterrupted oral anticoagulation (7% vs 4%, P = 0.25). Among 3 patients who suffered from stroke or TIA, 1 was managed with uninterrupted warfarin, 1 with interruption and bridging, and 1 interrupted without bridging. Patients who suffered stroke/TIA in the peri‐implant setting had an average CHA2DS2‐VASc of 5, and 2 of 3 (66%) had valvular atrial fibrillation. In the 60 patients treated with a NOAC, there were no major bleeding episodes, no stroke/TIA, 3 all‐cause hospitalizations (5%), 1 cardiovascular hospitalization (2%), and 1 bleeding hospitalization (2%).
Figure 2.
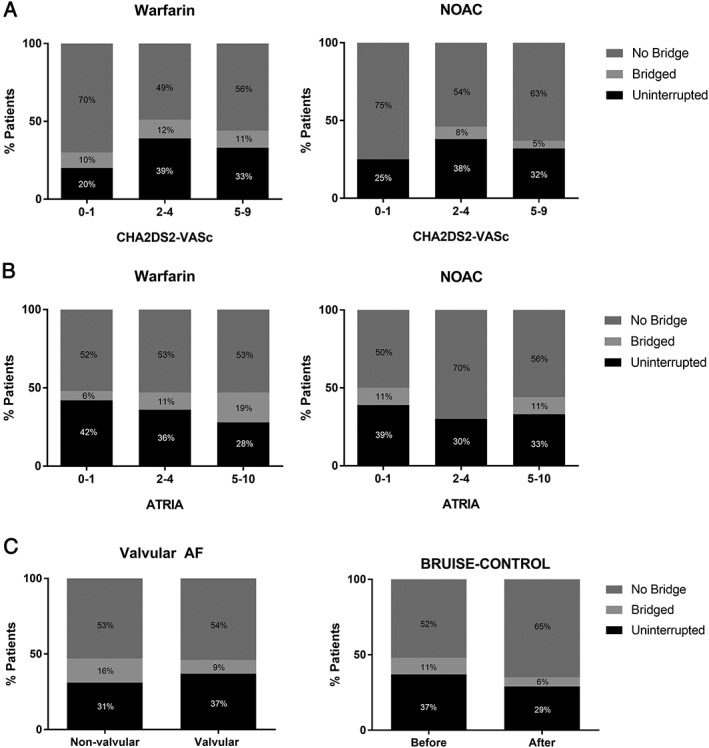
Anticoagulation strategies in patients undergoing device implantation by (A) stroke risk, (B) bleeding risk, (C) valvular disease, and (D) timing relative to publication of BRUISE‐CONTROL. Abbreviations: AF, atrial fibrillation; ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; BRUISE‐CONTROL, Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial; NOAC, non–vitamin K antagonist oral anticoagulant. CHA2DS2‐VASc, Congestive Heart Failure, Hypertension, Age ≥75 Years, Diabetes Mellitus, Stroke or Transient Ischemic Attack, Vascular Disease, Age 65‐74 Years, Sex.
Table 2.
Unadjusted outcomes 30 days after cardiac implantable electronic device implantation by oral anticoagulation strategy
| Warfarin | NOAC | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Uninterrupted | Interrupted | Overall | Uninterrupted | Interrupted | |||
| Bridging | No Bridging | Bridging | No Bridging | |||||
| No. | 284 | 101 | 33 | 150 | 60 | 21 | 4 | 35 |
| Major bleeding | 1 (0.4%) | 0 (0%) | 1 (3.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Stroke/TIA | 3 (1.1%) | 1 (1.0%) | 1 (3.0%) | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| All‐cause hospitalization | 18 (6.3%) | 4 (4.0%) | 4 (12.1%) | 10 (6.7%) | 3 (5.0%) | 1 (4.7%) | 1 (25.0%) | 1 (2.9%) |
| Cardiovascular hospitalization | 12 (4.2%) | 2 (2.0%) | 3 (9.1%) | 7 (4.7%) | 1 (1.6%) | 0 (0.0%) | 0 (0.0%) | 1 (2.9%) |
| Bleeding hospitalization | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 0 (0.0%) | 0 (0.0%) | 1 (2.9%) |
Abbreviations: NOAC, non–vitamin K antagonist oral anticoagulants; TIA, transient ischemic attack.
Values are reported as number with percentage of total patients experiencing adverse event in parenthesis.
4. DISCUSSION
We evaluated patterns of perioperative anticoagulation use during CIED implantation in a nationwide cohort of patients with AF. There are 4 major findings in our analysis. First, anticoagulation use and management surrounding CIED implantation was highly variable. Second, a substantial number of patients on warfarin received bridging anticoagulation. Third, we found considerable variation in perioperative NOAC management. Finally, the occurrence of serious thrombotic or bleeding events was rare, regardless of treatment with warfarin or NOAC therapy.
Periprocedural anticoagulation management was highly variable. We found that 35% of patients treated with a NOAC undergoing device implantation were managed with uninterrupted anticoagulation. Surprisingly, the rate of uninterrupted NOAC therapy was similar to the 36% rate observed in patients treated with warfarin. Previous data from a large Canadian survey of 22 centers showed that 18% of implanting physicians continued a NOAC during CIED implantation.8 These data are contemporaneous with our findings, and may be attributable to variation in practice patterns between countries (Figure 1).
Figure 1.
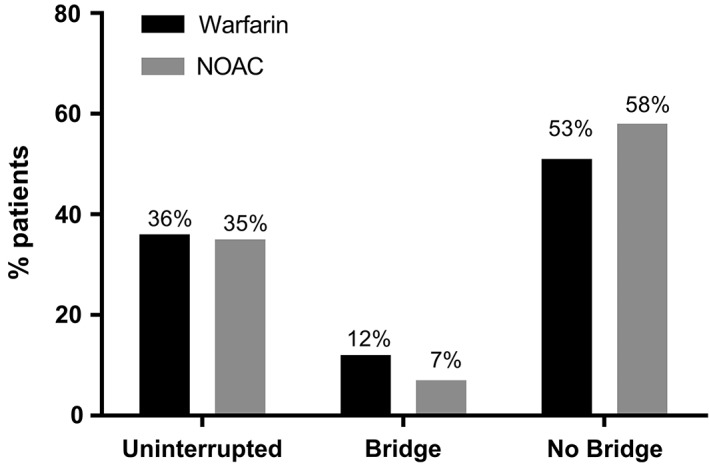
Anticoagulation strategies in patients undergoing device implantation. Abbreviations: NOAC = non–vitamin K antagonist oral anticoagulant.
Although the BRUISE‐CONTROL trial demonstrated improved safety (less bleeding) with an uninterrupted vitamin K antagonist therapy, there are no randomized data with NOACs. Direct NOACs possess unique pharmacokinetic and pharmacodynamics properties that are relevant to their management in the setting of device implantation. Brief interruptions of therapy are relatively easy to coordinate owing to their short elimination half‐lives and rapid onset of action. A retrospective study comparing rivaroxaban and dabigatran in 176 patients demonstrated no difference in 30‐day bleeding complications between groups, but only analyzed interrupted therapy.9 Another small prospective observational study of 25 patients treated with periprocedural dabigatran revealed no bleeding episodes at 30 days, but many patients were off therapy for more than 1 to 2 half‐lives at the time of CIED implantation.10 A case‐control series comparing uninterrupted dabigatran to warfarin during CIED implantation demonstrated no significant differences in hemorrhagic complications, but was also limited by small size and a limited number of events.11
Based upon the available evidence, the European Heart Rhythm Association (EHRA) recommends cessation of NOAC therapy 12 to 24 hours prior to any invasive procedure associated with low bleeding risk (such as CIED implant) in patients with normal renal function. The EHRA also recommends that NOACs can be restarted within 6 to 8 hours of surgery if hemostasis is obtained.12 Based on the available body of evidence, one might expect providers to more frequently interrupt OAC therapy in patients undergoing device implantation on NOACs, as reflected in the Canadian survey. Variability in perioperative NOAC management may reflect the absence of high‐quality prospective data, which will soon be addressed by BRUISE‐CONTROL 2 (NCT01675076), an ongoing randomized clinical trial comparing uninterrupted vs interrupted NOACs.
In this nationwide cohort, a substantial number of patients undergoing device implantation received parenteral bridging anticoagulation, including 12% of those treated with warfarin and 7% of patients treated with a NOAC. The lower rate of bridging anticoagulation in NOAC patients likely reflects providers’ comfort with the rapid onset and offset of these agents. Comparing our data to similar experiences in Canada and Europe, we found that intravenous bridging anticoagulation was used less frequently in US patients. Reported rates of bridging anticoagulation appear to be higher in Canada, where 27% of sites surveyed in 2014 continued to bridge patients with heparin.8
It is of interest to compare the patterns of anticoagulation management we observed in our large North American AF registry with recent observational data from Europe. The European Snapshot Survey on Procedural Routines for Electronic Device Implantation examined patterns of anticoagulation management in 723 patients undergoing CIED implantation in 2015.13 European providers were more likely to continue oral anticoagulation (67% vs 35% in ORBIT‐AF). However, patients managed with uninterrupted OACs in Europe were more likely to receive bridging anticoagulation with intravenous heparin (35.8% of those receiving warfarin and 25.6% of those receiving a NOAC).
Continued use of bridging therapy is somewhat unexpected given the evidence of increased bleeding complications and worse outcomes with heparinoid bridging. The Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial demonstrated that interrupted warfarin without bridging is noninferior to low‐molecular‐weight heparin bridging in patients undergoing elective surgery with respect to prevention of arterial thromboembolism, with lower rates of major bleeding complications.14 In 2012, Gold and colleagues conducted a large meta‐analysis across 13 studies in 5978 patients undergoing device implantation while on warfarin, 72% of which were anticoagulated due to AF.15 Uninterrupted OAC during device implantation did not result in excess bleeding relative to no therapy, whereas patients managed with a heparin‐bridging strategy had over 5‐fold increased bleeding compared to continued OAC.10 Another meta‐analysis by Ghanbari and colleagues in 2007 demonstrated reduced bleeding risk in patients treated with uninterrupted warfarin relative to heparin bridging, without increased risk of thromboembolic stroke.16 In early 2013, BRUISE‐CONTROL demonstrated that uninterrupted warfarin reduces pocket hematoma formation 4‐fold compared to heparin bridging, with no difference in thromboembolic complications.5 However, our findings suggest that these studies may not have resulted in immediate, dramatic changes in device implantation anticoagulation management. For instance, there was no evidence that perioperative anticoagulant management changed after publication of BRUISE‐CONTROL (Figure 2).
The risk of a major adverse event in the first 30‐days after device implantation was low regardless of anticoagulation management strategy. Two of 3 patients (66%) who suffered a stroke/TIA had valvular AF, although perioperative management of anticoagulation management did not differ in this subgroup (Figure 2). The unadjusted rate of adverse events in those who received bridging were higher compared to the other treatment groups; however, some caution is required given the relatively small sample.
4.1. Limitations
This study has several important limitations. The ORBIT‐AF registry was a prospective observational registry and patients were not randomized to treatment strategies. There were differences in baseline characteristics between patients treated with warfarin and NOACs, such as the increased use of aspirin in patients on NOACs. Statistical power was limited by the low number of patients in our registry who underwent device implantation on anticoagulation and number of complications across groups, so analyses controlling for possible confounders could not be performed. Anticoagulation strategies were categorized as uninterrupted or interrupted, but the precise timing of interruption prior to device surgery was not reported. Unfortunately, pocket hematoma formation was not a specifically reported adverse event. The centers included in the ORBIT‐AF registry may not be entirely representative of the general population of patients with AF in the United States, and our data may not be applicable to other regions of the world where practice patterns may differ. Finally, these data were obtained before the availability of NOAC reversal agents. It is not yet known how the availability of these agents will alter practice patterns.
5. CONCLUSION
Management of anticoagulation during cardiac device implantation is variable, regardless of whether patients are treated with warfarin or NOACs. Although the majority of patients in our cohort who suffered a stroke/TIA had valvular disease, anticoagulation management in these patients did not differ from the general population. Despite data from prior studies demonstrating excess bleeding complications, patients with AF continue to receive bridging therapy. Adverse event rates 30 days after device implantation are rare regardless of strategy, and prospective randomized data are necessary to guide NOAC management in the device implant setting.
Conflicts of interest
Dr. Ansell: consultant/advisory fees from Bristol‐Myers‐Squibb, Pfizer, Janssen, Daiichi, Boehringer‐Ingelheim, Alere. Dr. Hylek: consultant/advisory boards for Bayer, Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, Daiichi Sankyo, Janssen, Medtronic, Pfizer. Dr. Freeman: consultant of Janssen Scientific. Dr. Kowey: consultant/advisory board for Boehringer‐Ingelheim, Bristol‐Myers‐Squibb, Johnson & Johnson, Portola, Merck, sanofi‐aventis, Daiichi Sankyo. Dr. Gersh: advisory board for Boston Scientific, Medtronic, Johnson & Johnson, St. Jude Medical. Dr. Naccarelli: consulting fees from GlaxoSmithKline, Pfizer, Bristol‐Myers Squibb, Janssen Pharmaceuticals, Otsuka, Daiichi‐Sankyo, Boehringer Ingelheim, Xention. Dr. Go: consulting fees from Janssen Pharmaceuticals. Dr. Fonarow: consultant/advisory board member for Ortho McNeil. Dr. Mahaffey: research support from AstraZeneca, Amgen, Bayer, Boehringer‐Ingleheim, Bristol‐Myers‐Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Portola, POZEN Pharmaceutical, Schering‐Plough, The Medicines Company; consulting agreements with Amgen, AstraZeneca, Glaxo SmithKline, Johnson & Johnson, Merck. Dr. Piccini: research support from Boston Scientific Corporation, Janssen; consultancies to Forest Laboratories, Janssen, Medtronic. Dr. Peterson: research support from Eli Lilly, Janssen Pharmaceutical Products; consulting fees from Boehringer Ingelheim, Janssen Pharmaceutical Products, Merck & Co., sanofi‐aventis.
Black‐Maier E, Kim S, Steinberg BA, Fonarow GC, Freeman J, Kowey PR, Ansell J, Gersh BJ, Mahaffey KW, Naccarelli G, Hylek EM, Go AS, Peterson ED, Piccini JP and for the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) Investigators . Oral anticoagulation management in patients with atrial fibrillation undergoing cardiac implantable electronic device implantation. Clin Cardiol. 2017;40:746–751. 10.1002/clc.22726
Funding information The ORBIT‐AF registry is sponsored by Janssen Scientific Affairs. ClinicalTrials.gov identifier: NCT01165710.
REFERENCES
- 1. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 2. Tang AS, Wells GA, Talajic M, et al. Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med. 2010;363:2385–2395. [DOI] [PubMed] [Google Scholar]
- 3. Cheng A, Nazarian S, Brinker JA, et al. Continuation of warfarin during pacemaker or implantable cardioverter‐defibrillator implantation: a randomized clinical trial. Heart Rhythm. 2011;8:536–540. [DOI] [PubMed] [Google Scholar]
- 4. Marquie C, De Geeter G, Klug D, et al. Post‐operative use of heparin increases morbidity of pacemaker implantation. Europace. 2006;8:283–287. [DOI] [PubMed] [Google Scholar]
- 5. Birnie DH, Healey JS, Wells GA, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368:2084–2093. [DOI] [PubMed] [Google Scholar]
- 6. Piccini JP, Fraulo ES, Ansell JE, et al. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT‐AF. Am Heart J. 2011;162:606–612. [DOI] [PubMed] [Google Scholar]
- 7. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 8. Nascimento T, Birnie DH, Healey JS, et al. Managing novel oral anticoagulants in patients with atrial fibrillation undergoing device surgery: Canadian survey. Can J Cardiol. 2014;30:231–236. [DOI] [PubMed] [Google Scholar]
- 9. Kosiuk J, Koutalas E, Doering M, et al. Treatment with novel oral anticoagulants in a real‐world cohort of patients undergoing cardiac rhythm device implantations. Europace. 2014;16:1028‐1032. [DOI] [PubMed] [Google Scholar]
- 10. Rowley CP, Bernard ML, Brabham WW, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol. 2013;111:1165–1168. [DOI] [PubMed] [Google Scholar]
- 11. Jennings JM, Robichaux R, McElderry HT, et al. Cardiovascular implantable electronic device implantation with uninterrupted dabigatran: comparison to uninterrupted warfarin. J Cardiovasc Electrophysiol. 2013;24:1125–1129. [DOI] [PubMed] [Google Scholar]
- 12. Heidbuchel H, Verhamme P, Alings M, et al. European heart rhythm association practical guide on the use of new oral anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2013;15:625–651. [DOI] [PubMed] [Google Scholar]
- 13. Deharo JC, Sciaraffia E, Leclercq C, et al. Perioperative management of antithrombotic treatment during implantation or revision of cardiac implantable electronic devices: the European Snapshot Survey on Procedural Routines for Electronic Device Implantation (ESS‐PREDI). Europace. 2016;18:778–784. [DOI] [PubMed] [Google Scholar]
- 14. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernard ML, Shotwell M, Nietert PJ, et al. Meta‐analysis of bleeding complications associated with cardiac rhythm device implantation. Circ Arrhythm Electrophysiol. 2012;5:468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghanbari H, Phard WS, Al‐Ameri H, et al. Meta‐analysis of safety and efficacy of uninterrupted warfarin compared to heparin‐based bridging therapy during implantation of cardiac rhythm devices. Am J Cardiol. 2012;110:1482–1488. [DOI] [PubMed] [Google Scholar]


