Abstract
Background
Prostaglandin D2 (PGD 2) is primarily produced by mast cells and is contributing to the nasal symptoms including nasal obstruction and rhinorrhea.
Objective
This study aimed to evaluate the efficacy and safety of a novel PGD 2 receptor 1 (DP1) antagonist, ONO‐4053, in patients with seasonal allergic rhinitis (SAR).
Methods
This study was a multicenter, randomized, double‐blind, parallel‐group study of patients with SAR. Following a one‐week period of placebo run‐in, patients who met the study criteria were randomized to either the ONO‐4053, leukotriene receptor antagonist pranlukast, or placebo group for a two‐week treatment period. A total of 200 patients were planned to be randomly assigned to receive ONO‐4053, pranlukast, or placebo in a 2:2:1 ratio. Nasal and eye symptoms were evaluated.
Results
Both ONO‐4053 and pranlukast had higher efficacy than placebo on all nasal and eye symptoms. ONO‐4053 outperformed pranlukast in a total of three nasal symptom scores (T3NSS) as well as in individual scores for sneezing, rhinorrhea, and nasal itching. For T3NSS, the Bayesian posterior probabilities that pranlukast was better than placebo and ONO‐4053 was better than pranlukast were 70.0% and 81.6%, respectively, suggesting that ONO‐4053 has a higher efficacy compared with pranlukast. There was no safety‐related issue in this study.
Conclusions
We demonstrated that the efficacy of ONO‐4053 was greater than that of pranlukast with a similar safety profile. This study indicates the potential of ONO‐4053 for use as a treatment for SAR (JapicCTI‐142706).
Keywords: allergic rhinitis, Bayesian posterior probability, leukotriene receptor antagonist, mast cell degranulation, prostaglandin D2 receptor 1 antagonist
Abbreviations
- ALT
alanine aminotransferase
- ancova
analysis of covariance
- AR
allergic rhinitis
- AST
aspartate aminotransferase
- DP1
prostaglandin D2 receptor 1
- FAS
full analysis set
- GGTP
gamma‐glutamyl transpeptidase
- IgE
immunoglobulin E
- JC
Japanese cedar
- JRQLQ
Japanese rhinoconjunctivitis quality of life questionnaire
- LS‐mean
least squares mean
- LTRA
leukotriene receptor antagonist
- PGD2
prostaglandin D2
- RQLQJ
Japanese translation of the rhinoconjunctivitis quality of life questionnaire
- SAR
seasonal allergic rhinitis
- SE
standard error
- T3NSS
total of three nasal symptom scores
- T4NSS
total of four nasal symptom scores
- TP
thromboxane receptor
- TXA2
thromboxane A2.
1. Introduction
Allergic rhinitis (AR) is a type 1 hypersensitivity in the nasal mucosa of affected individuals, characterized by episodic paroxysmal sneezing, rhinorrhea, nasal obstruction, and nasal itching. The disease is triggered by the inhalation of putative antigens and mediated by immunoglobulin E (IgE).1, 2, 3, 4 A conservative estimate of the number of worldwide sufferers is 500 million,1 and it is a disease with significant morbidity, affecting 10‐30% of the adult population in the United States and 39.4% of adults in Japan.2, 3, 4 Causative mechanisms are triggered by antigen inhalation and are believed to include the production of IgE antibodies from activated B lymphocytes and the sensitization of basophilic cells (mast cells and basophils) via antibody binding to IgE receptors on cell surfaces. When antigen binds to surface IgE antibodies on sensitized mast cells, mediators such as histamine, leukotriene, and prostaglandin D2 (PGD2) are produced. These mediators induce the symptoms of sneezing, rhinorrhea, nasal obstruction, and nasal itching. AR is currently treated with antihistamines, leukotriene receptor antagonists (LTRAs), steroids, and vasoconstrictors.1, 2, 3, 4 Moderate‐to‐severe sufferers or patients who do not show sufficient improvement with monotherapy alone require combination therapy.1, 2, 3, 4 In these circumstances, the administration of multiple drugs is associated with reduced compliance and consequent lower quality of life (QOL) and can also be a source of economic loss from the cost of the medications.
PGD2 is primarily produced by mast cells5 and exerts its biological activity by binding to the PGD2 receptor 1 (DP1) or the chemoattractant receptor‐homologous molecule expressed on T helper type 2 cells (CRTH2).6, 7 The observation that asapiprant, a DP1 antagonist, reduced ovalbumin‐induced nasal obstruction and rhinorrhea in a guinea pig model of AR8 suggests that DP1 antagonists have the potential to reduce patients’ nasal symptoms in AR.
ONO‐4053 is a novel synthetic DP1 antagonist which has proven to effectively expand the nasal cavity in a cynomolgus monkey model of AR, and to suppress sneezing and nasal rubbing in a mouse model of AR (internal data of Ono Pharmaceutical). Based on these observations, we have designed an exploratory study of ONO‐4053 in patients with seasonal allergic rhinitis (SAR) to examine its efficacy as a treatment.
2. Method
2.1. Study design
This study was conducted after the review and approval of the clinical trial protocol and informed consent form by the institutional review board. Written informed consent was obtained from all patients before study inclusion.
The study was a placebo and LTRA‐controlled, randomized, double‐blind, parallel‐group study of patients with diagnosed Japanese cedar (JC) pollinosis and was conducted during the JC pollen season. The patients were randomly assigned using a stratified randomization to receive ONO‐4053, LTRA, or placebo in a 2:2:1 ratio. After completing a one‐week screening period of single‐blinded placebo administration, patients who met the study inclusion criteria were enrolled in a two‐week treatment period. The LTRA used in this study was pranlukast whose efficacy profile is superior to placebo9, 10 and comparable with montelukast.11 ONO‐4053 or matching placebo was administered at a dose of 300 mg once daily after food in the evening and pranlukast or matching placebo was administered at a dose of 225 mg twice daily after food in the morning and evening, and each matching placebo is not recognizable whether the actual drug or placebo by both patients and physicians. This study employed the double dummy technique.
2.2. Key inclusion and exclusion criteria
The patients included in this study were those aged ≥20 and <65 years who had experienced nasal symptoms during the JC pollen season in at least the most recent two years, were positive for the JC pollen specific IgE antibody, and had the total of three nasal symptom scores (T3NSS, total scores for the symptoms of sneezing, rhinorrhea, and nasal obstruction) of ≥6, daytime nasal obstruction scores ≥2 and <4, and nocturnal nasal symptom scores ≥6 in one‐week screening period before randomization.
The following patients were excluded from the study: Patients who had a disease which could interfere with the evaluation of nasal symptoms, such as an upper respiratory tract infection, or a morphological abnormality affecting nasal obstruction; patients who responded to exposure to multiple allergens besides JC pollen with symptoms of AR in order to improve the sensitivity of the evaluation of the study drug; patients with bronchial asthma in order to improve the sensitivity of the evaluation of the study drug; patients who had used anti‐allergic agents such as antihistamines and LTRAs within 14 days before, or during the screening period; patients who had used steroids within 28 days before, or during the screening period; patients who had used Omalizumab within 120 days before, or during the screening period; patients who have started allergy shots within 6 months; patients who had undergone surgical procedures such as turbinectomy for reducing the size of or changing the conditions of the nasal mucosa within five years before, or during the screening period; and patients who had a history of serious drug allergy.
2.3. Evaluation parameters
During the screening and treatment periods, each patient assessed and recorded their AR symptoms daily using an electronic diary (Fujitsu ePRO Solution). Symptoms were scored between 0 and 3 or 0 and 4 following the scoring systems shown in Table 1.3, 12, 13, 14 Efficacy was evaluated based on T3NSS, T4NSS (total of T3NSS plus the symptom of nasal itching), nocturnal nasal symptoms, eye symptoms, troubles with daily life, and the Japanese Rhinoconjunctivitis Quality of Life Questionnaire (JRQLQ), which was confirmed to correlate highly with the Japanese translation of the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQJ).15 Mean scores were calculated for the two‐week treatment period overall, and also for the first week and the second week of the treatment period, individually. Safety was evaluated based on the incidence of adverse events and adverse drug reactions.
Table 1.
Classification of the severity of allergic rhinitis symptoms
| Severity types | ++++(score 4) | +++(score 3) | ++(score 2) | +(score 1) | −(score 0) |
|---|---|---|---|---|---|
| Paroxysmal sneezing (average number of episodes of paroxysmal sneezing in a day) | ≥21 times | 20‐11 times | 10‐6 times | 5‐1 times | Below + |
| Rhinorrhea (average number of episodes of nose blowing a day) | ≥21 times | 20‐11 times | 10‐6 times | 5‐1 times | Below + |
| Nasal obstruction | Completely obstructed all day | Severe nasal obstruction causing prolonged oral breathing in a day | Severe nasal obstruction causing occasional oral breathing in a day | Nasal obstruction without oral breathing | Below + |
| Nasal itching | – | Itchy nose and frequently rubbing or blowing it | Itchy nose and occasionally rubbing or blowing it (between (+++) and (+)) | Itchy nose, which is almost negligible | No itchy nose |
| Difficulty in falling asleep | Unable to sleep at all | Very bad | Bad | Slightly bad | Not at all |
| Nocturnal nasal obstruction | Severe nasal obstruction with persistent oral breathing | Persistent nasal obstruction with oral breathing | Nasal obstruction occasionally bothered | Nasal obstruction, but not bothering | No nasal obstruction |
| Awakening at night | 4 times | 3 times | 2 times | Once | None |
| Itchy eyes | Above +++ | Itchy eyes and frequently rubbing them | Itchy eyes and occasionally rubbing them | Itchy eyes, but feel no need to rub them | Almost no itchy eyes |
| Watery eyes | Above +++ | Watery eyes and frequently wiping them | Watery eyes and occasionally wiping them | Watery eyes, but feel no need to wipe them | Almost no watery eyes |
| Troubles with daily lifea | Impossible | Painful and complicating daily life | Intermediate between (+++) and (+) | Few troubles | Below + |
Scores were assessed every day throughout the study period by each patient using ePro.
Troubles with daily life: Troubles with work, study, household work, sleep, going out, etc.
2.4. Statistical analysis
Sample sizes of 80, 80, and 40 individuals were predetermined for the ONO‐4053 group, the pranlukast group, and the placebo group, respectively, based on practical considerations. Assuming that 5% of the patients were excluded from the efficacy analyses, we calculated that a sample size of 76 patients in the ONO‐4053 and pranlukast groups would give a 70% probability of detecting a 0.16 or greater difference (i.e, more than half of a true difference) between the point estimates of the mean changes from baseline of T3NSS in the ONO‐4053 and pranlukast groups with a true between‐group difference of −0.33 and a common standard deviation of 2.0. As we did not have sufficient power to detect a between‐group difference at a two‐sided significance level of 0.05, all statistical inferences were considered to be exploratory. Our hypothesis was that we would see the half of the difference which had been observed between the montelukast and placebo groups in a point estimate of the difference between the ONO‐4053 and pranlukast groups in this study. We used a Bayesian approach to evaluate the hypothesis.
We included the placebo and pranlukast groups to evaluate the assay sensitivity. We also prespecified the criterion for appropriately evaluating the efficacy of the drugs in SAR patients, which stated that the Bayesian posterior probability that the between‐group difference in T3NSS between pranlukast and placebo was <0.00 of the mean changes from the baseline during the two‐week treatment period needed to be > 50%.
Efficacy analyses were performed for the full analysis set (FAS) population, which included all randomized patients who took at least one dose of the test drug and had at least one set of measurements taken following the start of treatment. To evaluate the mean changes from the baseline for continuous endpoints during the entire 2 weeks, and the first week and the second week of the treatment period, an analysis of covariance (ancova) model was used to estimate the least squares mean (LS‐mean) differences and the corresponding 95% confidence intervals between the treatment groups. This model included the treatment group and the baseline value of each endpoint as covariates. The Bayesian posterior probability for the between‐group mean differences was also calculated based on the same ancova model. The Bayesian posterior probabilities for T4NSS, sneezing, rhinorrhea, and nasal itching were calculated post hoc. Missing values were handled as missing without explicit imputations.
The safety analysis population included patients who had taken at least one dose of the test drug. Safety endpoints were analyzed in a descriptive manner.
2.5. Evaluation of human mast cells degranulation
The inhibitory effect of ONO‐4053 on mast cell degranulation was examined because results suggested that ONO‐4053 had an additional inhibitory effect on the symptoms of AR besides those attributable to DP1 antagonism. Human mast cells were derived from CD34+ bone marrow cells and cultured following the method by Saito, et al.16 Briefly, CD34+ bone marrow cells were cultured for six weeks in the presence of stem cell factor (SCF), interleukin‐6 (IL‐6), and interleukin‐3, after which culture was continued in the presence of SCF, IL‐6, insulin‐transferrin‐selenium, and bovine serum albumin. After approximately 10 weeks, more than 90% of the cells were identified as mast cells by means of toluidine blue staining. Mast cells (2 × 105 cells/mL) were sensitized by incubating overnight with human IgE (final concentration: 10 ng/mL); then, the test substances ONO‐4053, laropiprant,17 asapiprant, or BW A868C18 were added before incubating at 37°C for an hour. Degranulation was induced by the addition of anti‐human IgE, and detected by measuring β‐hexosaminidase activity. β‐hexosaminidase activity was assayed as below. The supernatant and substrate solution (p‐nitrophenyl‐nacetyl‐β‐D‐glucosamide) were incubated at 37°C for 2 h, followed by adding stop solution (NaHCO3/Na2CO3, pH 10), and absorbance was determined at 405 nm.
3. Results
3.1. Patient population
Two hundred patients were enrolled in the study, and all were included in the FAS population. Missing data were not explicitly imputed by any other observed values, and as a result, the data for 17 patients were excluded from efficacy analysis, because they had insufficient numbers of ePRO data (<80% of the total) for calculating the mean changes from baseline. A consort diagram is shown in Figure 1, and patient demographics are shown in Table 2. Drug compliance was evaluated by pill counting data and was 100% for both tablets and capsules in all the treatment groups.
Figure 1.
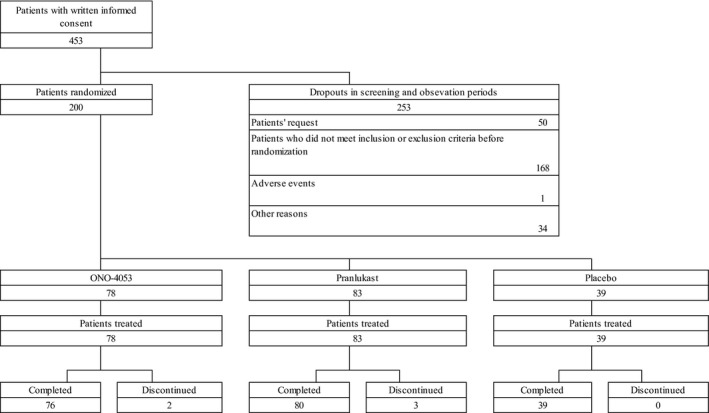
A consort diagram of this study
Table 2.
Demographic and baseline characteristics of patients
| Placebo | Pranlukast | ONO‐4053 | |
|---|---|---|---|
| Number of patients | 39 | 83 | 78 |
| Gender, no. | |||
| Male | 13 | 31 | 25 |
| Female | 26 | 52 | 53 |
| Age (years) | |||
| Mean ± SD | 42.4±9.9 | 37.7±9.7 | 40.4±9.8 |
| Range | 21‐63 | 20‐60 | 21‐61 |
| Rast score (JC pollen) | |||
| Mean | 3.2 | 3.5 | 3.6 |
| Range | 2‐6 | 2‐6 | 2‐6 |
| Rast positive (HD or mite) (%) | 25.6 | 28.9 | 34.6 |
| Baseline Symptomatic scores (Mean ± SD) | |||
| T3NSS | 9.29±1.40 | 9.16±1.22 | 9.23±1.21 |
| T4NSS | 11.90±1.67 | 11.79±1.36 | 11.80±1.46 |
| Sneezing score | 3.01±0.76 | 3.02±0.71 | 3.07±0.64 |
| Rhinorrhea score | 3.43±0.56 | 3.36±0.67 | 3.42±0.56 |
| Nasal obstruction score | 2.84±0.41 | 2.78±0.39 | 2.74±0.38 |
| Nasal itching score | 2.61±0.43 | 2.62±0.35 | 2.56±0.35 |
Baseline symptom scores were recorded by each patient using ePro. HD; house dust.
3.2. Total nasal symptom score
Mean changes in T3NSS and T4NSS from baseline for the two‐week treatment period are shown in Tables 3 and 4, respectively. For T3NSS, changes observed in the pranlukast and placebo groups (LS‐mean ± SE) were −0.83±0.16 and −0.68±0.24, respectively, demonstrating a difference in mean change of −0.15±0.29 for pranlukast compared to placebo. The Bayesian posterior probability that the pranlukast was more effective than placebo in reducing symptoms was 70.0%, which was higher than the criteria of 50% prespecified for this study to be valid.
Table 3.
Changes in T3NSS from the baseline over the two‐week treatment period and their Bayesian posterior probabilities
| Time point | Statistics | Placebo | Pranlukast | ONO‐4053 | |
|---|---|---|---|---|---|
| Mean of the entire 2 weeks | Actual value | N | 35 | 79 | 69 |
| Mean ± SD | 8.62±1.90 | 8.29±1.79 | 8.20±1.97 | ||
| Change from the baseline | LS‐mean (SE) | −0.68 (0.24) | −0.83 (0.16) | −1.04 (0.17) | |
| 95% confidence interval | (−1.15, −0.20) | (−1.15, −0.52) | (−1.38, −0.71) | ||
| Differences compared to placeboa | LS‐mean (SE) | – | −0.15 (0.29) | −0.36 (0.30) | |
| 95% confidence interval | – | (−0.72, 0.42) | (−0.95, 0.22) | ||
| P value | – | .5968 | .2182 | ||
| Bayesian posterior probability <0.00 | – | 0.700 | 0.890 | ||
| Bayesian posterior probability <−0.16 | – | 0.490 | 0.757 | ||
| Bayesian posterior probability <−0.33 | – | 0.269 | 0.547 | ||
| Differences compared to pranlukasta | LS‐mean (SE) | – | – | −0.21 (0.23) | |
| 95% confidence interval | – | – | (−0.67, 0.25) | ||
| Bayesian posterior probability <0.00 | – | – | 0.816 | ||
| Bayesian posterior probability <−0.16 | – | – | 0.587 | ||
| Bayesian posterior probability <−0.33 | – | – | 0.308 | ||
To evaluate the mean changes from the baseline, an analysis of covariance (ancova) model was used which included the treatment group and the baseline value of each endpoint as covariates.
Table 4.
Changes in T4NSS from the baseline over the two‐week treatment period and their Bayesian posterior probabilities
| Time point | Statistics | Placebo | Pranlukast | ONO‐4053 | |
|---|---|---|---|---|---|
| Mean of the entire 2 weeks | Actual value | N | 35 | 79 | 69 |
| Mean ± SD | 10.98±2.36 | 10.60±2.15 | 10.41±2.45 | ||
| Change from the baseline | LS‐mean (SE) | −0.93 (0.31) | −1.14 (0.21) | −1.40 (0.22) | |
| 95% confidence interval | (−1.55, −0.31) | (−1.55, −0.73) | (−1.84, −0.96) | ||
| Differences compared to placeboa | LS‐mean (SE) | – | −0.21 (0.38) | −0.47 (0.38) | |
| 95% confidence interval | – | (−0.95, 0.53) | (−1.23, 0.28) | ||
| P value | – | .5736 | .2192 | ||
| Bayesian posterior probability <0.00 | – | 0.712 | 0.890 | ||
| Bayesian posterior probability <−0.16 | – | 0.554 | 0.792 | ||
| Bayesian posterior probability <−0.33 | – | 0.375 | 0.645 | ||
| Differences compared to pranlukasta | LS‐mean (SE) | – | – | −0.26 (0.30) | |
| 95% confidence interval | – | – | (−0.86, 0.34) | ||
| Bayesian posterior probability <0.00 | – | – | 0.804 | ||
| Bayesian posterior probability <−0.16 | – | – | 0.630 | ||
| Bayesian posterior probability <−0.33 | – | – | 0.412 | ||
To evaluate the mean changes from the baseline, an analysis of covariance (ancova) model was used which included the treatment group and the baseline value of each endpoint as covariates.
The difference in mean change in T3NSS from baseline observed in the ONO‐4053 group compared to the placebo group for the two‐week treatment period was −0.36±0.30. This corresponds to a Bayesian posterior probability of 89.0% for ONO‐4053 that the difference was <0.00. Furthermore, the difference in mean change from baseline in the ONO‐4053 group compared to the pranlukast group for the two‐week treatment period was −0.21±0.23, corresponding to a Bayesian posterior probability of 81.6% for ONO‐4053 that the difference was <0.00. For the mean change, the Bayesian posterior probability of ONO‐4053 being greater than pranlukast was higher than that of pranlukast being greater than the placebo.
The difference in mean changes in T4NSS from baseline observed in the ONO‐4053 and pranlukast groups compared to the placebo group for the two‐week treatment period was −0.47±0.38 and −0.21±0.38, respectively. This corresponds to Bayesian posterior probabilities of 89.0% for ONO‐4053 and 71.2% for pranlukast that these differences were less than 0.00.
For both T3NSS and T4NSS, the reduction in scores was both greater and faster (Figure 2) in the ONO‐4053 group than in the pranlukast or placebo groups.
Figure 2.
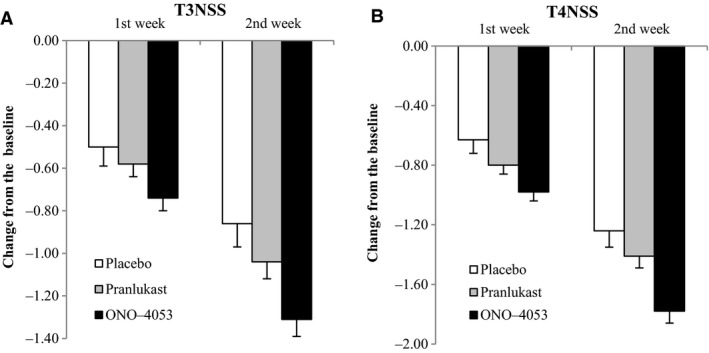
Changes in T3NSS and T4NSS at the first week and the second week in the treatment period. In terms of absolute values, the pranlukast group exhibited a larger change than the placebo group, and the ONO‐4053 group exhibited a larger change than the pranlukast group from the 1st week. Data are presented as mean ± SE
3.3. Individual symptoms
The mean changes in individual symptoms from baseline for the two‐week treatment period are shown in Figure 3, and those observed during the first week and the second week individually are shown in Figure 4. The reduction in all nasal symptom scores was both greater and faster in the ONO‐4053 group than in the pranlukast group. The Bayesian posterior probabilities that mean changes from baseline for the two‐week treatment observed in the ONO‐4053 group were greater than in the pranlukast group for symptoms of sneezing, rhinorrhea, nasal obstruction, and nasal itching were 88.8%, 82.5%, 55.7%, and 77.4%, respectively. For nocturnal nasal and eye symptoms, the reductions observed in the ONO‐4053 and pranlukast groups were of similar magnitude, and greater than those in the placebo group. The reduction in the score for negative impact on daily life was both greater and faster in the ONO‐4053 group than in the pranlukast group. The reduction in JRQLQ scores reported by the ONO‐4053 and pranlukast groups was similar and greater than those reported by the placebo group.
Figure 3.

Changes in individual symptoms over the two‐week treatment period. In terms of absolute values, the ONO‐4053 group exhibited a larger change than both the placebo group and the pranlukast group in various scores. Data are presented as mean ± SE
Figure 4.

Changes in each score at the first week and the second week in the treatment period. In terms of absolute values, the ONO‐4053 group exhibited a larger change than both the placebo and pranlukast groups in various scores at both the first week and the second week of the two‐week in the treatment period. Data are presented as mean ± SE
3.4. Safety
The number of patients in the safety analysis set was 200. The incidences of adverse events and adverse drug reactions are shown in Table 5, and details of adverse drug reactions are provided in Table 6. The incidences of adverse events were similar across the study groups: 15.4% in the placebo group, 18.1% in the pranlukast group, and 17.9% in the ONO‐4053 group. The adverse drug reactions observed in the ONO‐4053 group were rash, alanine aminotransferase increased, gamma‐glutamyl transpeptidase increased, glucose urine present, liver function test abnormal, and protein urine present. All of these adverse drug reactions were mild and reversible. One patient receiving ONO‐4053 developed a rash, which resulted in the termination of drug administration.
Table 5.
Summary of safety: the number of cases and incidence rates of adverse events and adverse drug reactions
| Placebo | Pranlukast | ONO‐4053 | |
|---|---|---|---|
| Adverse events | 6/39 (15.4%) | 15/83 (18.1%) | 14/78 (17.9%) |
| Adverse drug reactions | 2/39 (5.1%) | 8/83 (9.6%) | 7/78 (9.0%) |
There was no significant difference between the three groups. All of the adverse events and adverse drug reactions were mild.
Table 6.
Details of adverse drug reactions: type, numbers of cases, and incidence rates
| Placebo | Pranlukast | ONO‐4053 | |
|---|---|---|---|
| Upper abdominal pain | 1 (1.2%) | ||
| Thirst | 1 (1.2%) | ||
| Rashes | 1 (1.3%) | ||
| ALT increased | 1 (2.6%) | 2 (2.6%) | |
| AST increased | 1 (2.6%) | ||
| Blood bilirubin increased | 1 (2.6%) | 2 (2.4%) | |
| Blood triglycerides increased | 1 (1.2%) | ||
| GGTP increased | 1 (1.3%) | ||
| Glucose urine present | 2 (2.6%) | ||
| Blood urine present | 1 (1.2%) | ||
| Liver function test abnormal | 1 (1.3%) | ||
| White blood cell count increased | 1 (1.2%) | ||
| Protein urine present | 1 (1.2%) | 1 (1.3%) |
All adverse drug reactions were mild and reversible.
3.5. Inhibitory effects on mast cell degranulation
The results of examination of the inhibitory effects of ONO‐4053, laropiprant, asapiprant, and BW A868C on mast cell degranulation are shown in Figure 5. ONO‐4053 had extremely inhibitory effects at a concentration of 10−5 m that approximates to the expected serum concentration from ONO‐4053 in this clinical study, whereas laropiprant, asapiprant, or BW A868C did not.
Figure 5.

Effect of ONO‐4053, laropiprant, asapiprant, and BW A868C on human mast cell degranulation. ONO‐4053 inhibited human mast cell degranulation, while the other DP1 antagonists did not. Data are presented as mean ± SE. Mast cell degranulation was detected by β‐hexosaminidase release from bone‐marrow‐derived mast cells
4. Discussion
The involvement of PGD2 in AR has been suggested. In humans, the concentration of PGD2 in nasal mucus increases after antigen challenge,18, 19, 20, 21, 22, 23, 24, 25 and nasal obstruction and rhinorrhea are induced by nasal administration of PGD2.26, 27, 28 The potential for DP1 antagonists to reduce the nasal symptoms of AR was suggested by the evidence obtained from an animal model of AR.8 We have conducted a study to evaluate the efficacy and safety of a new synthetic DP1 antagonist, ONO‐4053, in patients with SAR. Prior to this phase II study, we have conducted phase I study in healthy volunteers at the maximum dose of 900 mg of ONO‐4053 given for 8 days. Although there were no safety concerns even at 900 mg of ONO‐4053, we selected 300 mg of ONO‐4053 because the blood concentration at 24 h in human after administration was higher than that in cynomolgus monkey when ONO‐4053 suppressed the shrinkage of nasal cavity maximally after antigen provocation. As this was the first study to evaluate the efficacy of ONO‐4053 in real‐world setting, we did not prespecify the primary endpoint. As T3NSS or T4NSS is generally an important endpoint in allergic rhinitis, we focused on these most. We measured PK of ONO‐4053, but there was no particular relationship between PK and these endpoints.
We have calculated Bayesian posterior probabilities for the between‐group mean differences. In a Bayesian framework, uncertainty about any differences observed is expressed via probability distributions, enabling quantitative comparisons between experimental treatment and control. In addition, it has been reported that Bayesian statistics are useful for “go/no‐go” decision‐making in proof‐of‐concept studies.29 The Bayesian posterior probability was therefore appropriate for making comparisons between drug groups in this study. The Bayesian posterior probability that the positive control, pranlukast, was more effective than placebo in reducing T3NSS was not <50%, which proves the validity of this study. In addition, the Bayesian posterior probability that ONO‐4053 was more effective than pranlukast was not <50%, suggesting that ONO‐4053 demonstrated greater efficacy. For T3NSS, the Bayesian posterior probability that pranlukast was better than placebo was 70.0%, and the Bayesian posterior probability that ONO‐4053 was better than pranlukast was 81.6%. These values suggest that ONO‐4053 could demonstrate superior efficacy than pranlukast in a confirmatory study by direct comparison. It is known the effect of LTRA is superior to placebo for AR nasal symptoms, and LTRA is widely used for relief of AR symptoms. In this study, we found ONO‐4053 would be more effective than LTRA, pranlukast, for T3NSS or T4NSS. These facts indicate the efficacy of ONO‐4053 would be clinically meaningful in T3NSS or T4NSS. A sample size of about 600 to 1500 patients per group in the ONO‐4053 and pranlukast groups will give a 90% power of detecting a significant difference at a two‐sided significance level of 0.05 between the mean changes from baseline of T4NSS in the ONO‐4053 and pranlukast groups with a true between‐group difference of −0.26 and a common standard deviation of 1.4 to 2.2. Although it is generally difficult to detect a significant difference against an active treatment, we think it might be still feasible in this patient population. As the sample size of this study is small (40‐80 patients per arm), considering the montelukast study (approximately 300 patients per arm),13 the estimate accuracy of the between‐group difference might be low. Therefore, further examination is needed to precisely quantify the difference between the ONO‐4053 and pranlukast groups in the subsequent studies.
We made a hypothesis that the difference observed between ONO‐4053 and the LTRA, pranlukast, would be similar to the difference observed between the LTRA, montelukast, and placebo (−0.33 for the mean change in T3NSS from the baseline).13 We conducted this study with the assumption that it would be possible to detect a change that was approximately half of this magnitude (−0.16). For the between‐group differences in mean changes in T3NSS from baseline between the ONO‐4053 and pranlukast groups, the Bayesian posterior probability for this being lower than −0.33 was 30.8% (<50%), but the Bayesian posterior probability for this being lower than −0.16 was 58.7%. The actual value calculated for the Bayesian posterior probability confirmed the expected efficacy, indicating the validity of using of Bayesian statistics for this exploratory study.
Laropiprant has been found to have insufficient efficacy as a treatment for AR.30 Laropiprant acts as an antagonist to the thromboxane receptor (TP) as well as to DP1.17 Reduction in mucosal size at the inferior turbinate as a result of exposure to thromboxane A2 (TXA2)31 and induction of nasal obstruction by inhibition of TXA2 synthetase have been reported.32 Laropiprant presumably demonstrated poor efficacy in reducing AR nasal obstruction because its DP1 antagonistic action was canceled by its TP antagonistic action.
ONO‐4053 demonstrated greater efficacy than the LTRA, pranlukast, in relieving AR symptoms, particularly for symptoms of sneezing, rhinorrhea, and nasal itching, for which involvement of histamine is known. However, the fact that ONO‐4053 is not a histamine antagonist and that laropiprant lacks efficacy for sneezing, rhinorrhea, and nasal itching indicates that the effectiveness of ONO‐4053 in relieving these symptoms, which are primarily histamine‐induced, cannot be due to its DP1 antagonistic action. From these observations, we made the hypothesis that ONO‐4053 has an additional mechanism besides its DP1 antagonistic action. As a result of examination based on this hypothesis, we found that ONO‐4053 had an inhibitory effect on mast cell degranulation. Neither laropiprant, asapiprant, nor BW A868C inhibited mast cell degranulation. These results suggest that the high efficacy of ONO‐4053 in treating the symptoms of AR derives from the absence of any TP antagonistic action and the presence of an inhibitory effect on mast cell degranulation. The mechanism of ONO‐4053's inhibitory effect on mast cell degranulation is still unknown. As ONO‐4053 has demonstrated robust efficacy for symptoms of sneezing, rhinorrhea, and nasal itching, the elucidation of these inhibitory mechanisms could provide a clue for the discovery of a novel target drug to replace antihistamines and LTRAs.
Levels of PGD2 expression are high in patients with chronic rhinosinusitis, chronic cough, and bronchial asthma, as well as in patients with AR.25, 33, 34, 35, 36, 37, 38 It has also been reported that the asthma of DP1‐deficient mice tended to be mild39 and that DP1 mediated PGD2‐induced VEGF release from nasal polyp fibroblasts.40 In addition to its DP1 antagonistic action, ONO‐4053 inhibits the production of chemical mediators such as histamine and leukotriene due to its inhibitory effect on mast cell degranulation. ONO‐4053 has also been found to reduce antigen‐induced urticaria in monkeys (internal data of Ono Pharmaceutical). These observations highlight a potential for ONO‐4053 to be effective in treating chronic rhinosinusitis with nasal polyps, and diseases associated with mast cell chemical mediators, such as bronchial asthma, and urticaria, in addition to AR.
The present study demonstrated the efficacy and safety of ONO‐4053 as a treatment for SAR. Its efficacy presumably derived both from its DP1 antagonistic action and its inhibitory effect on mast cell degranulation. Current treatment for AR includes multiple therapeutic agents depending on symptoms. For example, in clinical practice guidelines for allergic rhinitis in the United States as well as Europe and Japan, antihistamines are recommended for sneezing, rhinorrhea, and nasal itching while nasal steroids, vasoconstrictors, and LTRA (only in Japan) are recommended for nasal obstruction.1, 2, 3, 4 The efficacy of ONO‐4053 was demonstrated for all symptoms of AR, including sneezing, rhinorrhea, nasal itching, and nasal obstruction. ONO‐4053 therefore has the potential to become a new class of therapeutic agent that can be used to treat any symptom of AR.
Conflict of Interest
K. Okubo is a medical adviser and a consultant for Ono Pharmaceutical. K. Hashiguchi is a consultant for Ono Pharmaceutical and has investigator's fee for this clinical trial from Ono Pharmaceutical. S. Yamaguchi, M. Odani and H. Yamamotoya are full‐time employees of Ono Pharmaceutical. The rest of the authors have investigator's fee for this clinical trial from Ono Pharmaceutical.
Author Contribution
KO, KH, MO, and HY participated in design of this study, KO, KH, MO, and HY participated in data analysis and interpretation, and SY participated in experiment using mast cells.
Acknowledgments
We thank Masataka Kuroda of Ono Pharmaceutical Co., Ltd. for his assistance of this study.
Okubo K, Hashiguchi K, Takeda T, et al. A randomized controlled phase II clinical trial comparing ONO‐4053, a novel DP1 antagonist, with a leukotriene receptor antagonist pranlukast in patients with seasonal allergic rhinitis. Allergy. 2017;72:1565–1572. https://doi.org/10.1111/all.13174
Funding information
This study was funded by Ono Pharmaceutical Co., Ltd.
Edited by: De Yun Wang
References
- 1. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy. 2008;63(Suppl 86):8‐160. [DOI] [PubMed] [Google Scholar]
- 2. Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1‐S84. [DOI] [PubMed] [Google Scholar]
- 3. Okubo K, Kurono Y, Fujieda S, et al. Japanese guideline for allergic rhinitis 2014. Allergol Int. 2014;63:357‐375. [DOI] [PubMed] [Google Scholar]
- 4. Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152:S1‐S43. [DOI] [PubMed] [Google Scholar]
- 5. Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts LJ 2nd. Prostaglandin D2 generation after activation of rat and human mast cells with anti‐IgE. J Immunol. 1982;129:1627‐1631. [PubMed] [Google Scholar]
- 6. Pettipher R, Hansel TT, Armer R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat Rev Drug Discov. 2007;6:313‐325. [DOI] [PubMed] [Google Scholar]
- 7. Kostenis E, Ulven T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol Med. 2006;12:148‐158. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi G, Asanuma F, Suzuki N, et al. Effect of the potent and selective DP1 receptor antagonist, asapiprant (S‐555739), in animal models of allergic rhinitis and allergic asthma. Eur J Pharmacol. 2015;765:15‐23. [DOI] [PubMed] [Google Scholar]
- 9. Yonekura S, Okamoto Y, Okubo K, et al. Beneficial effects of leukotriene receptor antagonists in the prevention of cedar pollinosis in a community setting. J Investig Allergol Clin Immunol. 2009;19:195‐203. [PubMed] [Google Scholar]
- 10. Endo S, Gotoh M, Okubo K, Hashiguchi K, Suzuki H, Masuyama K. Trial of pranlukast inhibitory effect for cedar exposure using an OHIO chamber. J Drug Assess. 2012;1:48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okubo K, Baba K. A double‐blind non‐inferiority clinical study of montelukast, a cysteinyl leukotriene receptor 1 antagonist, compared with pranlukast in patients with seasonal allergic rhinitis. Allergol Int. 2008;57:383‐390. [DOI] [PubMed] [Google Scholar]
- 12. Okuda M. Nasal Allergies: Basics and Clinical ‐ Revised Edition [in Japanese]. Tokyo, Japan: Iyaku Journal Co., Ltd; 2005. [Google Scholar]
- 13. Okubo K, Baba K. Therapeutic effect of montelukast, a cysteinyl leukotriene receptor 1 antagonist, on Japanese Patients with Seasonal Allergic Rhinitis. Allergol Int. 2008;57:247‐255. [DOI] [PubMed] [Google Scholar]
- 14. Soh N, Kumai M, Nakashima M, Ishikawa T. A comparative study of mometasone furoate nasal spray and fluticasone propionate nasal spray in patients with perennial allergic rhinitis. Allergol Immunol. 2009;16:98‐117. [Google Scholar]
- 15. Okuda M, Ohkubo K, Goto M, et al. Comparative study of two Japanese rhinoconjunctivitis quality‐of‐life questionnaires. Acta Otolaryngol. 2005;125:736‐744. [DOI] [PubMed] [Google Scholar]
- 16. Saito H, Kato A, Matsumoto K, Okayama Y. Culture of human mast cells from peripheral blood progenitors. Nat Protoc. 2006;1:2178‐2183. [DOI] [PubMed] [Google Scholar]
- 17. Sturino CF, O'Neill G, Lachance N, et al. Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)‐4‐(4‐Chlorobenzyl)‐7‐fluoro‐5‐ (methylsulfonyl)‐1,2,3,4‐tetrahydrocyclopenta[b]indol‐3‐yl]‐acetic Acid (MK‐0524). J Med Chem. 2007;50:794‐806. [DOI] [PubMed] [Google Scholar]
- 18. Giles H, Leff P, Bolofo ML, Kelly MG, Robertson AD. The classification of prostaglandin DP‐receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br J Pharmacol. 1989;96:291‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Creticos PS, Adkinson NF Jr, Kagey‐Sobotka A, et al. Nasal challenge with ragweed pollen in hay fever patients. J Clin Invest. 1985;76:2247‐2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagenmann M, Baroody FM, Desrosiers M, et al. Unilateral nasal allergen challenge leads to bilateral release of prostaglandin D2. Clin Exp Allergy. 1996;26:371‐378. [PubMed] [Google Scholar]
- 21. Small P, Biskin N, Barrett D. Effects of intensity of early response to allergen on the late phase of both the nose and skin. Ann Allergy. 1994;73:252‐258. [PubMed] [Google Scholar]
- 22. Naclerio RM, Proud D, Togias AG, et al. Inflammatory mediators in late antigen‐induced rhinitis. N Engl J Med. 1985;313:65‐70. [DOI] [PubMed] [Google Scholar]
- 23. Naclerio RM, Hubbard W, Lichtenstein LM, Kagey‐Sobotka AK, Proud D. Origin of late phase histamine release. J Allergy Clin Immunol. 1996;98:721‐723. [DOI] [PubMed] [Google Scholar]
- 24. Horak F, Toth J, Hirschwehr R, et al. Effect of continuous allergen challenge on clinical symptoms and mediator release in dust‐mite‐allergic patients. Allergy. 1998;53:68‐72. [DOI] [PubMed] [Google Scholar]
- 25. Naclerio RM, Meier HL, Kagey‐Sobotka AK, et al. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1983;128:597‐602. [DOI] [PubMed] [Google Scholar]
- 26. Doyle WJ, Boehm S, Skoner DP. Physiologic responses to intranasal dose‐response challenges with histamine, methacholine, bradykinin, and prostaglandin in adult volunteers with and without nasal allergy. J Allergy Clin Immunol. 1990;86:924‐935. [DOI] [PubMed] [Google Scholar]
- 27. Howarth PH. Mediators of nasal blockage in allergic rhinitis. Allergy. 1997;52(Suppl 40):12‐18. [DOI] [PubMed] [Google Scholar]
- 28. Hecken AV, Depre M, Lepeleire ID, et al. The effect of MK‐0524, a prostaglandin D(2) receptor antagonist, on prostaglandin D (2)‐induced nasal airway obstruction in healthy volunteers. Eur J Clin Pharmacol. 2007;63:135‐141. [DOI] [PubMed] [Google Scholar]
- 29. Fisch R, Jones I, Jones J, Kerman J, Rosenkranz GK, Schmidli H. Bayesian design of proof‐of‐concept trials. Ther Innov Regul Sci. 2015;49:155‐162. [DOI] [PubMed] [Google Scholar]
- 30. Philip G, Adelsberg JV, Loeys T, et al. Clinical studies of the DP1 antagonist laropiprant in asthma and allergic rhinitis. J Allergy Clin Immunol. 2009;124:942‐948. [DOI] [PubMed] [Google Scholar]
- 31. Komori M, Miwa M, Hirano M, et al. Effects of arachidonic acid metabolites [in Japanese]. Japanese J Rhinol. 1999;38:69‐73. [Google Scholar]
- 32. Szczeklik A, Nizankowska E, Splawinski J, Dworski R, Gajewski P, Splawinska B. Effects of inhibition of thromboxane A2 synthesis in aspirin‐induced asthma. J Allergy Clin Immunol. 1987;80:839‐843. [DOI] [PubMed] [Google Scholar]
- 33. Murray JJ, Tonnel AB, Brash AR, et al. Prostaglandin D2 is released during acute allergic bronchospasm in man. Trans Assoc Am Physicians. 1985;98:275‐280. [PubMed] [Google Scholar]
- 34. Murray JJ, Tonnel AB, Brash AR, et al. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med. 1986;315:800‐804. [DOI] [PubMed] [Google Scholar]
- 35. Sampson SE, Sampson AP, Costello JF. Effect of inhaled prostaglandin D2 in normal and atopic subjects, and of pretreatment with leukotriene D4. Thorax. 1997;52:513‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Birring SS, Parker D, Brightling CE, Bradding P, Wardlaw AJ, Pavord ID. Induced sputum inflammatory mediator concentrations in chronic cough. Am J Respir Crit Care Med. 2004;169:15‐19. [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto M, Okano M, Fujiwara T, et al. Expression and characterization of PGD2 receptors in chronic rhinosinusitis: modulation of DP and CRTH2 by PGD2. Int Arch Allergy Immunol. 2009;148:127‐136. [DOI] [PubMed] [Google Scholar]
- 38. Maher SA, Birrell MA, Adcock JJ, et al. Prostaglandin D2 and the role of the DP1, DP2 and TP receptors in the control of airway reflex events. Eur Respir J. 2015;45:1108‐1118. [DOI] [PubMed] [Google Scholar]
- 39. Matsuoka T, Hirata M, Tanaka H, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013‐2019. [DOI] [PubMed] [Google Scholar]
- 40. Kanai K, Okano M, Fujiwara T, et al. Effect of prostaglandin D2 on VEGF release by nasal polyp fibroblasts. Allergol Int. 2016;65:414‐419. [DOI] [PubMed] [Google Scholar]


