Abstract
Prostate cancer (CaP) is often androgen-sensitive malignancy and regresses upon inhibition of androgen signaling. However, CaP, nearly always develops androgen resistance and progresses to aggressive and lethal androgen-independent CaP, which lacks satisfactory therapy. For metastatic CaP, patients are often treated with Taxotere (docetaxel), a cytoskeleton-targeted chemotherapy drug, that provides transient palliative benefit but to which patients rapidly develop drug-resistance. Combination chemotherapy may be used instead, but is more toxic and adds little clinically relevant benefit over docetaxel. Therefore, novel strategies to enhance docetaxel efficacy are needed to effectively treat patients with metastatic CaP. The mercapturic acid pathway, which metabolizes genotoxic and pro-apoptotic toxins, is over-expressed in CaP and plays an important role in carcinogenesis, metastasis and therapy-resistance of CaP. Vicenin-2, a flavonoid derived from Tulsi (holy basil) as an active compound, inhibits the growth of CaP and increases the anti-tumor activity of docetaxel in-vitro and in-vivo. Taken together, the combination of vicenin-2 and docetaxel could be highly effective in the treatment of advanced and metastatic CaP due to their multi-targeting anti-tumor potential.
Keywords: Prostate cancer, vicenin, RLIP76 (RalBP1), drug-resistance, glutathione-conjugate transport, clathrin-dependent endocytosis
Introduction
Prostate cancer (CaP, carcinoma of prostate) constitutes a major health problem in Western countries. CaP is a type of malignancy that arises in the prostate gland, tends to develop in older men, aged 50 and over, and in many cases CaP develops slowly, although in some cases it is aggressive and metastasizes to other parts of the body [1–3]. Dietary factors, lifestyle-related factors, and androgens (AR) contribute to the risk of developing CaP. Hypermethylation of CpG island sequences encompassing the regulatory region of glutathione S-transferase (GSTP1) may link exposure to genome-damaging stress to increased genomic instability during prostatic carcinogenesis. GSTs are detoxifying enzymes that catalyze conjunction of glutathione (GSH) with harmful, electrophilic molecules, thereby protecting cells from carcinogenic agents. The identification of key molecular alterations in CaP cells has implicated carcinogen defenses, such as GSTP1, growth-factor-signaling pathways (i.e., NKX3.1, PTEN, and p27), and AR as critical determinants of the phenotype of CaP cells. In addition, this has defined specific targets that can be used to detect, diagnose, and treat CaP [3–5]. For example, in prostatic intraepithelial neoplasia (PIN), cells that lack GSTP1, undergo genomic damage mediated by carcinogens. NKX3.1, PTEN, and p27 regulate the growth and survival of prostate cells in the normal prostate. NKX3.1 binds DNA and represses expression of the prostate-specific antigen (PSA) gene. The gene for phosphatase and tensin homologue (PTEN), a tumor-suppressor gene, is a common target for somatic alteration during the progression of CaP. PTEN is expressed in normal epithelial cells and in PIN. However, in CaP, levels of PTEN are frequently reduced, particularly in cancers of a high grade or stage [4,5]. Reduced levels of PTEN and NKX3.1 lead to reduced levels of p27, a cyclin-dependent kinase inhibitor encoded by the CDKN1B gene, which contributes to increased proliferation and decreased apoptosis of CaP cells and thus to poor prognosis. In this way, haploinsufficiency for PTEN and NKX3.1 may promote abnormal proliferation of prostate cells [3,4].
Treatment of CaP usually involves therapeutic reduction in the levels of testosterone and dihydrotestosterone. However, this is complicated by the eventual emergence of androgen-independent prostate cancer (AICaP), which is associated with mutations in the androgen-receptor that permit receptor activation by other ligands, increased expression of androgen-receptors accompanying AR amplification, and ligand-independent androgen-receptor activation [5–8]. During androgen withdrawal therapy, the AR signal transduction pathway can also be activated by amplification of the AR gene, by AR gene mutations, or by altered activity of AR co-activators. All of these mechanisms contribute to the emergence of AICaP [6–8].
Furthermore, CaP is molecularly complex, having a multifocal and heterogeneous nature, which presents challenges to interventions that act on a single target [9–12]. The fact that 1 in 3 men develop PIN and that 1 in 6 men develop CaP reflects the associated life-time risk for CaP [9]. In addition, given the dynamic molecular plasticity of incident and progressing CaP tumors, rapid emergence of drug-resistance, and dose-limiting toxicities of currently used drugs like docetaxel (DTL) [13, 14], there is high interest in identifying novel, safe, and non-toxic natural anticancer compounds that can inhibit development of CaP and/or synergistically enhance its sensitivity to anticancer drugs. A variety of natural herbal compounds that have inherent chemical antioxidant properties offer a potential means to overcome these challenges because they display multi-targeted anticancer activities [15–17]. Therefore it may be beneficial to exploit these herbs for human therapy, but challenges remain in identifying appropriate agents or compounds for study.
Phenolic antioxidant compounds that have apoptotic effects on cancer cells appear to work through pleiotropic mechanisms: that simultaneously inhibits multiple pro-cancer pathways [18, 19]. How this happens remains unclear? Assuming directional signaling (up-stream to down-stream), one must consider at least four possible explanations: 1) the drug acts on one target, and all of the observed effects (i.e., inhibition of Akt, MEK, p53, AR, cMyc, etc.) are down-stream of that target; 2) the compound acts on many targets and each effects arises from direct inhibition of each target by the compound; 3) the compound is does not directly act on any of the targets, but changes the cellular milieu such that the up- and down-stream targets are simultaneously affected; or 4) a combination of 1, 2 and 3. The first explanation is unlikely because of the known ability of phenolic antioxidants to directly bind many different proteins. The second is also unlikely because, even if the compound binds all targets, the structural differences in the target proteins make it highly unlikely that a single molecule (or even its metabolites) could inhibit the activity of all targets. Given that antioxidants, by nature, reduce overall concentrations of oxidizing species, a change in milieu appears much more likely. However, given the demonstrated ability of some phenolic compounds to bind to and specifically inhibit one or more signaling proteins, and that inhibition of an upstream signaling protein will necessarily reduce signaling to the next, explanation 4 (a combination of 1–3) is the most likely scenario. In addition, the third scenario should predominate because each of the targets under consideration here is known to be directly affected by overall oxido-reductive balance. Indeed, the in-vitro measures of the catalytic or binding activities of all of the signaling proteins being considered are directly regulated by the ratio of reduced vs. oxidized thiols (i.e., a requirement for β-mercaptoethanol in the buffers used to measure their activities). The ratio of the oxidized:reduced cellular thiols is inextricably linked and in equilibrium with all redox pairs. Thus, the chemical antioxidant effect of any such compound would alter the general milieu such that the activities of all potential targets in a cascade should be affected. Because oxidative stress can activate many of these signaling proteins (i.e., Akt, p53, cMyc, MEK), it seems reasonable that reducing this stress by using an antioxidant should inhibit their activation. However, activation of apoptosis through the intrinsic Bcl-2/Bax mechanism generally happens in concert with an oxidative stress [20–22]. Thus, none of these explanations are entirely satisfactory, and an alternative model must be considered.
The cancer-preventive actions of polyphenolic phytochemicals were originally defined by studying their effects on the mercapturic acid pathway (MAP). Specifically, compounds that induced GST (phase-II detoxification and rate limiting enzyme of MAP) and suppressed cytochrome p450 (phase I detoxification enzymes, upstream of GSTs in the MAP) were identified as being antioxidant cancer-preventives and those that exerted the opposite effect were pro-oxidants. Because all antioxidants are also pro-oxidants at higher concentrations, it stands to reason that those compounds that could prevent cancer by reducing oxidative stress at low concentrations could at high concentrations exert oxidative stress that might trigger apoptosis in cancer cells [23–25]. In this regard, it is interesting that polyphenols exert antioxidant effects in the nanomolar concentration range (which induces GSTs), but apoptosis of cancer cells requires micromolar concentrations (which suppresses or inhibits GSTs). Although this model is conceptually reasonable, it does not explain why increased oxidative stress (due to very high concentrations of polyphenols) could inhibit the activation of p53, cMyc, or Akt.
We argue that the MAP-Ral hypothesis could explain some of these paradoxical findings and link the chemical/biochemical effects of phenolic antioxidants with well-understood kinase signaling pathways. In this model, high concentrations of polyphenols inhibit the MAP at GST, resulting in further exacerbation of oxidative stress. Compounds that block the glutathione-electrophile-conjugate (GS-E) transporter, RLIP76, could inhibit the Ras/Raf/MEK/Myc or AKT/mTOR/MDM/p53 pathways by simultaneously inhibiting endocytosis [26, 27]. The resultant loss of stress-resistance and cell-cycling checkpoint combined with increased oxidative stress due to the chemical effect of high polyphenol concentrations could shift the balance between Bcl2 and Bax towards apoptosis [20–22]. In recent years, the importance of the Ral/MEK/Myc pathway in cancer has been recognized [28–30]. Blockade of the Ral effector RLIP76 could also exacerbate the tendency towards apoptosis [27–32]. Because Ral is a primary regulator of exosomes, a tendency towards apoptosis would be further compounded if exosomes function to potentiate ligand-receptor signaling.
Flavonoids can modulate drug action through multiple mechanisms such as influencing intestinal absorption, altering the rate and nature of metabolism, bio-distribution of drugs and, most importantly, affecting parallel signaling networks in targeted cancers when co-administered with chemotherapy drugs resulting in additive, antagonistic or synergistic effects [16–19]. We initially revealed a synergistic effect between the flavonoid VCN-2 and docetaxel (DTL), and in in-vivo studies, showed that orally administered VCN-2 in combination with DTL was effective in AICaP [33].
Androgen-independent metastatic prostate cancer (AICaP) is a highly prevalent, morbid, debilitating and lethal disease of men for which satisfactory prevention and treatment are lacking [10]. The use of DTL in advanced CaP provides transient palliative benefit, followed by resistance [13], which necessitates combinatorial tertiary chemopreventive strategies. Novel pharmacological methods for primary prevention of CaP and to overcome tumor burden of metastatic disease are urgently needed. We have sought to decipher the role of the MAP enzymes in carcinogenesis, metastasis, and drug-resistance, and have shown that this pathway plays a key role in apoptosis caused by diverse stressors ranging from radiation and chemotherapy, to hormone-withdrawal [28, 31–36]. Recent studies have directly linked the MAP enzymes to carcinogenesis, survival-promoting, and proliferation-promoting pathways, including cMyc, p53, Rb, and Akt, in a variety of cancers in which MAP enzymes are highly enriched, including CaP [28, 31–33, 37]. Based on this rationale, we performed in-silico docking studies with MAP enzymes to screen a group of plant-derived antioxidants, and identified a lead candidate, VCN-2. We examined VNC-2 effects in cell lines and human xenograft models of CaP and found that VCN-2 exerts strong antineoplastic activity alone and in synergy with DTL in both CaP and AICaP [33]. In this regard, herein we describe the anticancer properties of the Tulsi-derived novel flavonoid VCN-2 as a potential inhibitor of CaP growth because of its strong predicted interactions with multiple enzymes and regulatory nodes in signaling pathways that regulate MAP. The novel features of this compound include its oral bioavailability, lack of toxicity, and mechanistic relevance to central signaling proteins implicated in CaP.
Vicenin-2 (VCN-2)
VCN-2 is a commercially available flavonoid (Quality Phytochemicals LLC., Edison, NJ) that is enriched in Tulsi (also known as holy basil, Ocimum sanctum Linn, OSL; family: Lamiaceae), an extensively cultivated Indian herb that has high religious significance and is believed to prolong the life of men (Fig. 1). OSL is known for its anti-inflammatory, anticancer and anti-diabetic properties in Ayurveda, and has a safe record of human consumption as a raw paste or as an herbal tea for millennia [38–45]. Oxidative stress is a well established cause in the initiation and progression of cancers [46]. Anti-oxidant and anti-inflammatory compounds like cyclooxygenase-2 inhibitors, natural compounds like silibinin and other flavonoids are currently being investigated as potential CaP treatments [47–49]. OSL has been investigated for its chemopreventive properties in dimethyl-benzanthracene (DMBA)-induced oral cancer [50], and also functions as a radio-protectant for bone marrow cells [51–53]. Overall, VCN-2 targets multiple nodes of CaP pathogenesis and progression, which also exhibiting a potent synergistic effect with orally given DTL. This provides a strong rationale for further studying VCN-2 as a single agent for the prevention of CaP and the combination of VCN-2 and DTL for targeting AICaP [33]. Further supporting this, other key features of VCN-2 are: a) hepatoprotective activity, b) antioxidant and anti-inflammatory activities, c) could act as a UV light barrier to protect the plants, d) anticancer activity, and DTL co-administration is more effective than either of the single agents in AlCaP, and e) may be a useful lead for developing multiple target-oriented therapeutic modalities for the treatment of diabetes and diabetes-associated complications [39, 41–45, 53].
Figure 1.
Chemical structure of vicenin-2 (VCN-2)
Docetaxel (DTL)
Currently, DTL is the drug of choice for treating AICaP and is administered intravenously (i.v.) at a dose of 60 – 100 mg/m2 (75 mg/ m2 is the recommended starting dose for AICaP) once every three weeks in clinical practice. However, i.v. administration of DTL is associated with dose-limiting toxicities such as febrile neutropenia and associated myelotoxicity, which has lead to characterization of alternate routes of administration and potential combinatorial regimens. Even anemia and non-febrile neutropenia, which occur in approximately 41% and 67% of patients given DTL, can severely affect the quality of life and consequent survival in elderly patients, especially given that many CaP patients have extensive comorbidities (e.g., diabetes mellitus and age related decline in immune function). Myelotoxicity or bone marrow toxicity results in direct, high level of exposure to bolus doses of DTL, as is typical with i.v. administration. Although, some of the previous studies focused on trying to switch from three week to weekly doses involving moderate dosage reduction, substantial adverse effects still occurred, including hyperlacrimation, and skin- and nail-toxicity [13]. In this regard, VCN-2 in combination with DTL synergistically inhibited the growth of prostate tumors in-vivo with a greater decrease in the levels of AR, pIGF1R, pAkt, PCNA, cyclin D1, Ki67, CD31, and increase in E-cadherin [33]. The salient feature of this study was the potent synergistic effect of VCN-2 and low dose DTL (0.03 mg/m2 DTL and 3 mg/m2 VCN-2 given orally on alternate days as compared to the clinical dose in metastatic AICaP - 75 mg/m2 of DTL i.v. once every 3 weeks plus 5 mg prednisone taken twice daily), which strongly supports further development of VCN-2 and DTL combinatorial regimens for clinical use in aggressive AICaP.
Advancing the field of CaP research
A major barrier to improving the effectiveness of treatment for AICaP is an incomplete understanding of why AICaP is so aggressive and resistant to therapy. The role of Ral, the MAP, and exosome secretion in resistance to AICaP treatment is unclear. Although, these pathways are known to be linked to therapy resistance in CaP, the underlying mechanism was not known. The recent discovery thatVCN-2 can simultaneously exploit these mechanisms to improve the effectiveness of DTL provides a paradigm shifting approach for understanding how new treatments of AICaP can be devised. This concept is supported by a wide array of literature and studies from our [28, 31–37, 54–58] and others [59–62] laboratories on the role of the MAP in the formation, growth, and metastasis of CaP. The novelty is based on a broadly-supported scientific basis: a) Ral-binding protein (RALBP1/RLIP76) functions at the intersection of the Ral and MAP pathways, b) Blockade of RLIP76 exerts potent anticancer effects on CaP, c) RLIP76, a cancer survival-promoting enzyme, is a major constituent of the exosomes secreted by CaP, d) a novel mechanism for the association of exosomes with the aggressive behavior of AICaP, e) identification of VCN-2 as an anticancer compound, and f) the ability of VCN-2 to potentiate DTL though the MAP, a pathway not affected by DTL. This suggests that the VCN-2 and DLT should have non-overlapping toxicities, the holy grail of combination chemotherapies. Thus, the proposed characterization of the detailed molecular and pharmacokinetic mechanisms of action would lay strong scientific foundation for further clinical development of VCN-2 and DTL regimen for the effective management of human CaP (Fig. 2).
Figure 2.
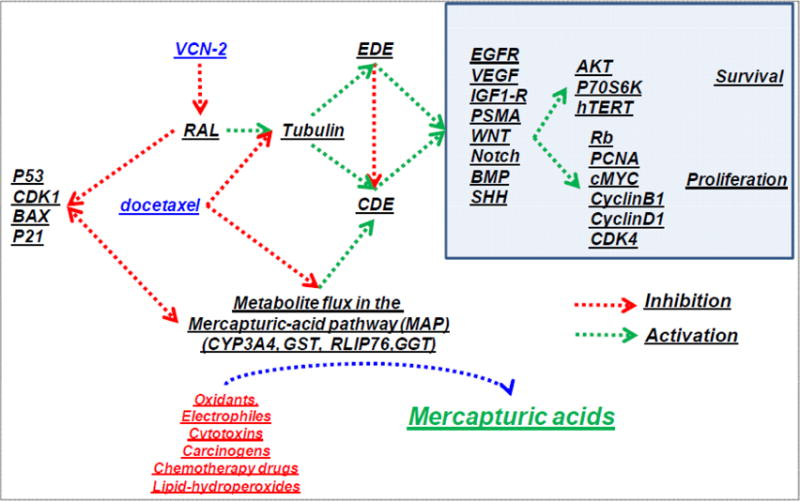
Mechanism of synergistic action of VCN-2 and docetaxel (DTL)
Ultimate applicability
The proposed strategy would benefit patients with metastatic AICaP by improving the effectiveness of DTL chemotherapy. VCN-2 could be given orally during DTL chemotherapy of AICaP. BecauseVCN-2 blocks the androgen receptor; it could be used in the treatment of androgen-sensitive CaP (ASCaP) to avoid castration (surgical or chemical), a therapeutic approach with numerous highly undesirable side effects. If shown in further studies to prevent CaP development, VCN-2 could also be an effective preventative agent. The ability of VCN-2 to affect the composition of exosomes could be an exciting finding. The oral bioavailability of VCN-2 is particularly attractive for its pharmacological development. In addition, VCN-2 qualifies as a ‘generally regarded as safe’ substance, with no known toxicities at present. Our studies [33] will advance scientific knowledge on the role of Ral-signaling in CaP biology, identify an entirely novel mechanism of action of antioxidant antineoplastic agents, and allow development of new strategies to overcome DTL-resistance in AICaP. Overall, VCN-2 caused apoptosis and enhances DTL activity by blocking signaling down-stream of EGF and IGF by inhibiting MAP enzymes that regulate Ral-mediated exosome-secretion and cytoskeletal remodeling.
Interaction of VCN-2 and DTL with the MAP
The MAP is a critical biochemical pathway (phase II biotransformation) that disposes of exogenous and endogenous toxins [23–25, 28]. It defends against exogenous electrophilic (electron-deficient) compounds that cause mutations by forming adduct with DNA-bases. The MAP is also responsible for metabolism of both DTL and VCN-2. DTL is mainly metabolized by the p450 isoenzymes CYP3A4 and CYP3A5 into well-characterized inactive hydroxyl and hydroxyoxazolidinone metabolites. Because polyphenols inhibit the metabolism of taxanes by p450 enzymes [63], it is quite possible that potentiation of DTL could be due to inhibition of its metabolism. This possibility could be tested by measuring concentrations of un-metabolized DTL (active constituent) in cells without of with VCN-2 treatment to determine whether the parent drug concentration is increased upon exposure to VCN-2. These studies are critical to elucidating the mechanism of synergy.
In contrast to DTL, nothing specific is known about VCN-2 metabolism. In general, polyphenols can also be metabolized by p450 into electrophilic derivatives that are then conjugated with GSH. It is possible that VCN-2 also has a similar fate. This is relevant because GS-Es are potent product-inhibitors of GSTs, and their formation could significantly reduce MAP activity [64]. Taken together, analyses of the effects of the MAP on these drugs and the effects of these drugs on MAP can shed substantial light on the possibility that synergy between the drugs is due to mutual inhibition of their biotransformation either directly or through their metabolites, and that signaling-changes are secondary. Competitive inhibition of either GST or RLIP76 due to metabolites would be a potential reason for increased accumulation of pro-apoptotic lipid aldehydes in CaP cells.
Role of Ral signaling in the antineoplastic activity of VCN-2 and DTL
Cancer generally is considered as a neoplastic disease with particular causative and etiologic factors as well as protective elements. Although it has remained difficult to treat, it is preventable. Recently, interest in evaluating dietary phytochemical intake as a potential means for chemoprevention of cancer has increased greatly. Regular intake of phytochemicals has been shown to have a cancer protective role during various stages if cancer, including initiation, promotion and progression and, can modulate oncogenic processes through their antioxidant and anti-inflammatory activities of the phytochemicals and their ability to mimic the chemical structure and activity of hormones. The Ral pathway was recently recognized as playing a significant role in malignant transformation, proliferation, invasion, metastasis, and apoptosis pathways in cancers [28, 65, 66]. A role for the Ral pathway in CaP bone metastases is supported by the loss of bone metastatic activity in PC3 cells that have shRNA-mediated decreases in RalA expression [65, 66]. RLIP76 was the first identified, and one of few known effectors of Ral [67–70]. RLIP76 is over-expressed in CaP, and inhibition of RLIP76 leads to apoptosis as well as xenograft regression [37]. RLIP76 provides a direct link between the Ras and Ral pathways, linking Ras signaling to metastasis and invasion [28, 67]. Thus, the Ral-RLIP76 interaction could regulate cell motility and invasion through Ral/Rac/cdc42, and cell growth and transformation through Myc. Since Myc regulates p53 [71] and Akt can regulate p53 through MDM2, it is possible that the Ral pathway could regulate Myc and p53 through the effects of RLIP76 on Akt, Rac, or cdc42 [28]. Because Ral signaling has not been explored in CaP, and our studies to date indicate that a Ral effector plays an important role in CaP [37], these findings point to a significant role of Ral signaling in CaP biology (Fig. 3). Further investigation in this area will delineate the mechanisms through which VCN-2 exerts its anticancer effects in CaP and perhaps the mechanisms of VCN-2/DTL interactions.
Figure 3. Ral-MAP pathway interactions.
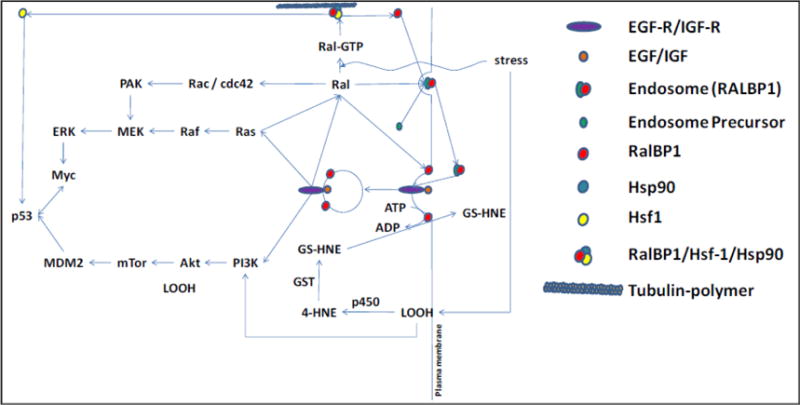
Stress causes activation of Ral to Ral GTP and the formation of LOOH from membrane lipids. This causes dissociation of the RLIP76 (RalBP1)/Hsf1/Hsp90/Tubulin complex, releasing Hsf1 for translocation to the nucleus in a complex with p53. RLIP76 translocates to the cell membrane, where it binds to clathrin adaptors and links its ATPase coupled GS-HNE transport with clathrin-dependent endocytosis. EGF-EGFR or IGF-IGFR complex is endocytosed and, subsequently, downstream kinase regulation is activated leading to p53 and cMyc regulation. LOOH is metabolized to 4HNE by p450, then to GS-HNE by GST. GS-HNE is transported out by RLIP76.
AICaP cells are sensitive to VCN-2, and the VCN-2/DTL combination exerts synergy
Recent studies demonstrated that VCN-2 can potently inhibit the growth of CaP in-vitro as well as in-vivo, as compared to other active constituents of OSL like orientin and luteolin. VCN-2 increased levels of PTEN, decreased levels of hTERT, PCNA, and pRB, and increased p53 levels while inhibiting pEGFR, pAkt, and the mTOR signaling mediator pP70S6K. VCN-2 induced G2/M arrest by decreasing cyclin B1, cyclin D1, and CDK4, and inhibited angiogenesis both in-vitro and in-vivo, as shown by decreases in VEGF, PSMA, and angiogenic marker CD31. Most importantly, VCN-2 as a single agent decreased the levels of AR, C-Myc, and Bcl2 and inhibited the activation of pIGF-1R, which is of specific significance to chemo-dietary therapy of castration resistant CaP (CRCaP). In-vivo mice xenografts studies in which VCN-2 was administered by oral gavage revealed potent regression of CRCaP xenografts with a synergistic effect with DTL, the current drug of choice in CRCaP [33]. VCN-2 alone or in combination with DTL did not cause any overt toxicity in mice. The rationale is based on the entirely novel concept that the Ral-signaling pathway that signals CaP growth and metastasis and the mercapturic acid toxin-biotransformation pathway that protects cells from toxins are together responsible for the aggressive behavior of AICaP. Further studies will facilitate the translation of VCN-2 into a non-toxic drug useful for improving the DTL efficacy of AICaP.
In summary, VCN-2 has been identified as an anticancer compound present in an herb long thought to be beneficial for men’s health and longevity. That VCN-2 can potentiate the anticancer effects of DTL has direct implications in treatment of AICaP. Additional innovation lies in the ability of VCN-2 to recalibrate cellular oncogenic, tumor suppressor and differentiation signaling cascades to induce multi-specific anticancer signaling inputs as revealed by our extensive studies in the context of pathogenesis of CaP [33]. Also, at orally active and non-toxic doses, VCN-2 induced synergistic anticancer effects with DTL.
Discussion
Oxidative stress is a well established as contributing to initiation and progression of CaP [46, 72]. GSH, the predominant soluble physiological thiol in cells, is the primary defense against oxidative stress and its levels are increased as a defense against carcinogenesis. The MAP uses GSH to detoxify exogenous and endogenous carcinogenic toxins through enzymatic formation of conjugates of GSH with oxidant toxins (GS-Ox). GSTs are MAP enzymes that catalyze the formation of GS-Ox. The next step towards the excretion of oxidant toxins is their energy-dependent efflux from cells by energy-dependent transporters. RLIP76 (RALBP1) is the most active of these transporters, the loss of which in knockout mice reduces activity of the MAP by ~80% [28, 31, 34–36]. After efflux from cells, GS-Ox are metabolized to mercapturic acids (MA-Ox) and excreted by the kidneys [23]. Chronic exposure to carcinogens activates the MAP, which is very active in CaP [73]. High levels of carcinogen exposure over-rides the protective effect of this detoxification pathway, resulting in sufficient genotoxicity that cell-growth regulatory signaling proteins are altered and cause unregulated growth of the damaged cells and ultimately resulting in cancer [74, 75]. In addition, expression of this pathway progressively increases during carcinogenesis, such that its activity in cancer cells far exceeds that in normal cells. Indeed, the cancer cells become dependent on this pathway to survive. This is dramatically evident from studies showing that knockout mice lacking RLIP76 are almost entirely resistant to carcinogenesis upon treatment with potent oxidative chemical carcinogens [36, 54]. The importance of RLIP76 for cancer-cell survival is further evident from multiple studies by our [28, 31–37, 54–58] and other [59–62] laboratories showing that blocking the MAP through RLIP76-targeting causes sustained regression of a wide variety of highly treatment-resistant cancers, including CaP [31, 32, 37], in xenograft mouse models of human cancers.
The name RLIP76 derives from the original identification of this protein as the first (and one of only a handful of) effector proteins of the Ral-GTPase signaling pathway [67–69]. Ral is a G-protein that is the master regulator of cell motility, membrane-remodeling and ruffling, endocytosis, and actin-cytoskeleton remodeling that enables cancer cell invasion, metastasis, and stimulation of angiogenesis [28, 76–78]. The importance of RLIP76 in these processes is evident from studies done by us and others showing that angiogenesis as well as invasion or metastasis is severely impaired when RLIP76 is missing or blocked [28, 36, 54–62]. The endocytosis function and GS-Ox efflux functions of RLIP76 are inextricably linked, indicating that this protein serves as a key nexus that links the functions of the Ral pathway with the GSH-linked MAP pathway that mitigates oxidative stress [28, 31, 36]. Endocytosis is a key regulator of ligand-receptor signaling that is critical for cancer-cell growth. In addition, activation of Akt, MAPK, Myc, and other cancer promoting signals is regulated by endocytosis [76–78]. This is evident from the deficient functions of these ligands in RLIP76 knockout mice, which are severely deficient in endocytosis [34–36]. Taken together, these observations suggest that multiple cancer-signaling mechanisms are controlled by the MAP through the enzymatic activity of RLIP76, and that blockade of its function could serve as an important and effective anticancer therapy [28, 31, 32].
We developed a computer algorithm to perform in-silico screening of flavonoid antioxidant compounds with structures similar to known GST-inhibitors. This strategy was based on known interaction with the MAP through direct quenching lipid-peroxidation of ω-6 polyunsaturated fatty acids, suppression of P450s (including CYP3A4, which metabolizes DTL), induction of GSTs and other GSH-linked enzymes that, together, are chemical and biochemical mechanisms for quenching oxidative stress [23–25]. The docking algorithm utilized coordinates of the complete crystal structure of GST-P [79], the partial crystal structure of RLIP76 [80–82], the known GSH-binding residues and the known kinetic constants for these enzymes [36]. These analyses identified VCN-2 [51, 52]. Our studies of the anticancer activity of VCN-2 towards CaP cells, showed significant anticancer activity in-vitro as well as in-vivo [33]. VCN-2 potentiated the effect of DTL on AICaP, both in-vitro and in-vivo. The anticancer activity of VCN-2 towards the CaP lines LNCaP and PC3 was superior to the activity of resveratrol, silibinin, or curcumin [83–85]. EGF and IGF receptors were inhibited, which was associated with pleiotropic inhibitory effects on signaling proteins down-stream of these cancer-promoting peptide hormones important for CaP growth and invasion [1–4]. Exosome-mediated signaling is a recently identified cancer promoting mechanism. Exosomes are membrane-lipid derived nanoparticles that promote cancer growth [86, 87]. Our preliminary proteomic analyses showed that VCN-2 depleted exosomal RLIP76.
The basic scientific and mechanistic significance of these findings is broader. Many antioxidant compounds that have anticancer activity towards CaP also suppress the MAP pathway and inhibit the enzymatic activity as well as expression of GSTs [88, 89]. Because the MAP is directly linked to Ral, it is possible that the anticancer effects of several plant-derived antioxidants towards CaP could operate in a similar manner. The structural similarity of the GSH-binding site of GST and RLIP76 supports the idea that they could block MAP by simultaneously inhibiting both enzymes. The observed synergy between DTL and VCN-2 could also be explained by this model because the mechanism of action of DTL is through targeting the functions of the cellular cytoskeleton (Fig. 2). Because DTL does not directly affect the MAP, the combination of DTL and VCN-2 could have significant advantages, namely that the functional target of both agents, membrane and cytoskeletal function, would be inhibited through distinct routes such that overlapping toxicities could be avoided. This is supported by the lack of apparent systemic toxicity of the combination of VCN-2 and DTL in mice [33].
VCN-2 exerts highly effective and multi modal regulatory effects on critical nodes of CaP signaling while also exerting a potent synergistic effect with DTL. In this review, we have laid specific emphasis on the critical factors contributing to both primary chemopreventive potential of VCN-2 as well as signaling and metabolic changes that contribute to VCN-2 and DTL synergy, which is relevant for tertiary chemoprevention of even advanced metastatic prostate tumors. We recently showed the efficacy of VCN-2 as a single agent and in combination with DTL in CaP. VCN-2 effectively induced anti-proliferative, anti-angiogenic and pro-apoptotic effects in CaP cells (PC-3, DU-145 and LNCaP) regardless of their androgen responsiveness or p53 status. VCN-2 inhibited the EGFR/Akt/mTOR/p70S6K pathway while decreasing c-Myc, cyclin D1, cyclin B1, CDK4, PCNA and hTERT in-vitro. The potent anticancer effects of VCN-2 seen in in-vitro studies were confirmed in in-vivo nude mice xenograft studies. VCN-2 reached a level of 2.6 ± 0.3 μmol/L in serum after oral administration in mice, which reflected that VCN-2 is absorbed after oral administration. The i.v. administration of DTL is associated with dose-limiting toxicities such as febrile neutropenia, which has led to characterization of alternate routes of administration and potential combinatorial regimens. In this regard, in a preclinical model, VCN-2 in combination with DTL synergistically inhibited the growth of prostate tumors in-vivo with a greater decrease in the levels of AR, pIGF1R, pAkt, PCNA, cyclin D1, Ki67, CD31, and increase in E-cadherin [33]. VCN-2 has also been investigated for radioprotection and anti-inflammatory properties [51, 52]. These findings collectively provide strong evidence that VCN-2 is effective against CaP progression along with indicating that VCN-2 and DTL co-administration is more effective than either of the single agents in AICaP. Therefore, we believe that chronic administration of VCN-2 and/or its chemical derivatives have strong potential for use as preventive agents for CaP, and that VCN-2 could be used to enhance the anticancer efficacy of chemotherapy drugs. High intensity acute exposure to electrophilic compounds (i.e., chemotherapy drugs) causes apoptosis and necrosis; chronic long-term exposure accelerates carcinogenesis. The endogenous toxin metabolism is primarily responsible for biotransformation of lipid-hydroperoxides (LOOH) and their degradation products into mercapturic acids (Fig. 3). LOOH decompose into toxic electrophilic aldehydes that are pro-apoptotic and mutagenic alkylating agents [23]. Currently, the clinical use of DTL is associated with need for higher doses in elderly patients, who form the largest group of individuals affected with CRCaP, leading to substantial toxicity [13]. The pharmacokinetic studies will provide requisite rationale and dosage calibrations that are essential for further studies. Spontaneous tumor models represent the best means by which to test the primary chemo-preventative effect of VCN-2 and in which to establish a reliable set of VCN-2 regulated secretory biomarkers.
Conclusion
Regression of AICaP cell line PC3 upon RLIP76 blockade, inhibition of growth of PC3 by a novel plant polyphenol, VCN-2, and the synergy between VCN-2 and DTL in xenografts are novel findings [33, 37]. The interactions between Ral and the MAP are novel and the potential role of these in regulation of exosome signaling could represent a paradigm shift in CaP biology. VCN-2 causes apoptosis in ASCaP as well as in AICaP. It simultaneously inhibits multiple signals downstream of EGF and IGF that are important for the aggressive behavior of AICaP. These include signals that regulate cell cycle check points (p53, Rb, PCNA), proliferation (PCNA), differentiation (cMyc, E-cadherin, fibronectin), survival (Akt, mTOR), senescence (hTERT), and apoptosis (bcl-2, Bax, Bak, caspases) [33]. In ASCaP, these signaling changes are accompanied by down-regulation of AR. The underlying mechanisms of the wide spectrum of anticancer signaling effects are incompletely understood. Studies by Nagaprashantha et al. indicate that VCN-2 depletes RLIP76 in exosomes secreted by CaP. These CaP-secreted exosomes function in an autocrine manner to activate the Ral-A pathway, which controls the movement and remodeling of membranes and cytoskeleton, and serves as a generalized modulator of ligand-receptor signaling by regulating endocytosis [86, 87]. Because DTL also targets the cytoskeleton, we postulate that the mechanism of action of VCN-2, the synergy between DTL and VCN-2, and pleiotropic signaling effects occur through the effects of VCN-2 on Ral, a pathway that is regulated by MAP. Recent studies revealed the effect of VCN-2 on signaling networks of Ral and their relationship to proteins that serve to promote AICaP growth. These studies addressed generalizability and mechanisms in-vitro and confirmed in animal xenograft studies that should simultaneously examine potential pharmacokinetic and toxicological interaction of VCN-2 and DTL combination.
The overall objective of our proposed combinatorial (VCN2 + DTL) approach is to improve the length and quality of life of men with metastatic AICaP by increasing the effectiveness of DTL chemotherapy by using the non-toxic and active natural compound VCN-2. Our immediate future objectives are to understand whether these observations are broadly applicable to AICaP in cell lines, to define the mechanism of action of VCN-2, and to determine the mechanism underlying its anticancer synergy with DTL. These studies will provide a much needed understanding of how AICaP cells survive and grow, and could lead to additional treatment approaches. This review has summarized our current understanding of VCN-2 is a potent, natural anticancer compound that broadly targets MAP and causes cancer-selective apoptosis that can be used to prevent and treat CaP, and that the effects of VCN-2 can be monitored through the CaP-specific exosomal fraction in peripheral blood. Therefore, the present preclinical findings of detailed cellular, genetic and pharmacokinetic mechanisms of action would lay strong scientific foundation for further clinical development of VCN-2 chemo-dietary regimens for effective management of human CaP. Future studies are needed to advance scientific knowledge in the role of Ral-signaling in CaP biology, define this novel mechanism of action of antioxidant antineoplastic agents, and allow development of new strategies to defeat DTL-resistance in AICaP.
Relevance to Human Health.
VCN-2 is a safe flavonoid that is enriched in the time-tested Indian herb Tulsi, represents a potent and effective chemopreventive compound whose multi-specific anticancer signaling properties are highly relevant to the extensive genetic and molecular complexity associated with the primary pathogenesis of CaP, as well as the emergence of AICaP. VCN-2 has potent anticancer activity in both ASCaP and AICaP, and remarkable synergy when used with DTL in cell and human xenograft models. VCN-2 effectively and simultaneously targets many essential molecular networks that influence CaP incidence, acquisition and maintenance of pluripotency, tumor angiogenesis, apoptosis resistance, and development of AICaP and metastasis. Our future studies aim to delineate the mechanistic basis underlying the usefulness of VCN-2 alone for chemoprevention of CaP and AICaP, as well as to evaluate the combination of VCN-2 and DTL as a tertiary chemo-dietary approach to prevent aggressive and metastatic CaP. In this regard, determining the efficacy of VCN-2 in pre-clinical models of CaP could have significant impact by informing the rational use of VCN-2 as well as VCN-2 and DTL combinatorial regimens in human CaP prevention and control.
Future Perspectives.
The use of VCN-2 as an anti-prostate cancer natural agent is novel. The potent interaction of VCN-2 with DTL is novel and of immediate significance in the current therapy of AICaP. The prediction algorithm for anticancer activity of antioxidants based on in-silico models of interaction between detoxification enzymes and signaling proteins is novel, and appears to be highly significant based on correct predictions of the activity of VCN-2 in CaP. The results described herein have provided strong preclinical rationale for the anticancer effects of VCN-2 in CaP by revealing the mechanisms of regulation of critical nodes of CaP such as cMyc, AR, and p53. The systematic investigation of secretory and exosomes proteins in-vitro along with analysis of differentially detected proteins in in-vivo spontaneous models of control and VCN-2 treated groups will help to establish a reliable set of VCN-2 response predictive, CaP-specific biomarkers for further studies. The spontaneous models will also help to strengthen the requisite preclinical rationale to pursue clinical trials. Collectively, these studies will bring about a paradigm shift in the field of CaP research by laying greater emphasis on developing the non-toxic flavonoid VCN-2 for effective interventional strategies for CaP.
Highlights.
The salient features of this review article with potential clinical relevance include:
VCN-2 is a safe and non-toxic flavonoid from the herb “Ocimum Sanctum Linn (OSL)”, also known as “holy basil” or “Tulsi” which has been used for thousands of years in Ayurvedic medicine.
VCN-2 induces potent anticancer effects in CaP irrespective of AR responsiveness or p53 status by inhibiting critical nodes of tumor signaling such as c-Myc, AR, EGFR, pIGFR, PCNA, hTERT and increasing the pro-differentiation marker E-cadherin.
VCN-2 inhibits angiogenesis and decreases VEGF expression in CaP.
In preclinical models, VCN-2 was effectively absorbed after oral administration, which correlated with tumor regression.
The i.v. administration of DTL, the current drug of choice in AICaP, is associated with dose-limiting complications like febrile neutropenia which has lead to testing potential combinatorial regimens and alternate routes of administration. In this regard, the described studies provide strong evidence supporting a synergistic inhibitory effect of oral administration of VCN-2 and DTL in mouse xenografts of CaP without any toxicity and weight loss.
The VCN-2 and DTL combination caused greater inhibition of the CaP signaling networks compared to either of the single agents.
The clinical significance is based on the high probability of clinical translation of VCN-2 into a non-toxic strategy for enhancing the efficacy of DTL in AICaP.
Acknowledgments
This work was supported in part by the Department of Defense grant (W81XWH-16-1-0641) and funds from the Perricone Family Foundation, Los Angeles, CA. Funding from the Beckman Research Institute of City of Hope is also acknowledged. We apologize to all colleagues whose work we could not cite due to space constraints.
The abbreviations used are
- AICaP
androgen-independent prostate cancer
- AR
androgen receptor
- ASCaP
androgen-sensitive prostate cancer
- CaP
carcinoma of prostate
- CRCaP
castration resistant carcinoma of prostate
- CD31
cluster of differentiation 31 protein
- cdc2
cyclin-dependent kinase 1 also known as CDK1 or cell division cycle protein 2 homolog
- CDE
clathrin-dependent endocytosis
- CDK4
cyclin dependent kinase 4
- DTL
docetaxel
- EGFR
epidermal growth factor receptor
- GSH
glutathione
- GS-E
glutathione electrophile conjugates
- GST
glutathione S-transferase
- 4HNE
4-hydroxy nonenal
- Hsf1
heat shock transcription factor 1
- hTERT
human telomerase reverse transcriptase
- IGF-1R
insulin growth factor 1 receptor
- LOOH
lipid-hydroperoxides
- MAP
mercapturic acid pathway
- mTOR
mammalian target of rapamycin
- OSL
Ocimum Sanctum Linn
- PCNA
proliferative cell nuclear antigen
- PIN
prostatic intraepithelial neoplasia
- PSA
prostate specific antigen
- PSMA
prostate specific membrane antigen
- PTEN
phosphatase and tensin homologue
- RB
retinoblastoma protein
- RLIP76 (RALBP1)
a 76 kDa Ral-interacting protein
- VCN-2
vicenin-2
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: No conflict of interest exists for any of the authors.
References
- 1.Attard G, Parker C, Eeles RA, Schröder F, Tomlins SA, Tannock I, Drake CG, de Bono JS. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 2.Chang AJ, Autio KA, Roach M, Scher HI. High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol. 2014;11:308–323. doi: 10.1038/nrclinonc.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. The New Eng J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 4.Aschelter AM, Giacinti S, Caporello P, Marchetti P. Genomic and epigenomic alterations in prostate cancer. Front Endocrinol. 2012;3:128. doi: 10.3389/fendo.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24(18):1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault. Endocr Relat Cancer. 2002;9(1):61–73. doi: 10.1677/erc.0.0090061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nature Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 8.Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12(6):1665–1671. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]
- 9.Tomlins SA, Mehra R, Rhodes DR, Cao X, Wang L, Dhanasekaran SM, Kalyana-Sundaram S, Wei JT, Rubin MA, Pienta KJ, Shah RB, Chinnaiyan AM. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 10.Andreoiu M, Cheng L. Multifocal prostate cancer: biologic, prognostic, and therapeutic I mplications. Hum Pathol. 2010;41:781–793. doi: 10.1016/j.humpath.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Delacroix SE, Ward JF. Prostate cancer multifocality: impact on cancer biology and treatment recommendations. Panminerva Med. 2010;52:209–216. [PubMed] [Google Scholar]
- 12.Lee MC, Moussa AS, Yu C, Kattan MW, Magi-Galluzzi C, Jones JS. Multifocal high grade prostatic intraepithelial neoplasia is a risk factor for subsequent prostate cancer. J Urol. 2010;184:1958–1962. doi: 10.1016/j.juro.2010.06.137. [DOI] [PubMed] [Google Scholar]
- 13.Engels FK, Verweij J. Docetaxel administration schedule: from fever to tears? A review of randomised studies. Eur J Cancer. 2005;41:1117–1126. doi: 10.1016/j.ejca.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Kenmotsu H, Tanigawara Y. Pharmacokinetics, dynamics and toxicity of docetaxel: Why the Japanese dose differs from the Western dose. Cancer Sci. 2015;106(5):497–504. doi: 10.1111/cas.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji HF, Li XJ, Zhang HY. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009;10(3):194–200. doi: 10.1038/embor.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqui AA, Iram F, Siddiqui S, Sahu K. Role of natural products in drug discovery process. Int J Drug Dev Res. 2014;6(2):172–204. [Google Scholar]
- 18.Wang H, Khor TO, Shu L, Su Z, Fuentes F, Lee JH, Tony Kong AN. Plants against cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12(10):1281–1305. doi: 10.2174/187152012803833026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Anticancer efficacy of polyphenols and their combinations. Nutrients. 2016;8:552. doi: 10.3390/nu8090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao D, Lew KL, Kim YA, Zeng Y, Hahm ER, Dhir R, Singh SV. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;12:6836–6843. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- 23.Jakoby WB. The glutathione S-transferases: a group of multi-functional detoxification protein. Adv Enzymol Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- 24.Awasthi YC, Sharma R, Singhal SS. Human Glutathione S-transferases. Int J Biochem. 1994;26:295–308. doi: 10.1016/0020-711x(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 25.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki T, Hanai S, Kishi H, Liu Z, Bao Y, Kikuchi A, Tsuchida K, Sugino H. Regulation of endocytosis of activin type II receptors by a novel PDZ protein through Ral/Ral-binding protein 1-dependent pathway. J Biol Chem. 2002;277:19008–19018. doi: 10.1074/jbc.M112472200. [DOI] [PubMed] [Google Scholar]
- 27.Gildea JJ, Harding MA, Seraj MJ, Gulding KM, Theodorescu D. The role of Ral A in epidermal growth factor receptor-regulated cell motility. Cancer Res. 2002;62:982–985. [PubMed] [Google Scholar]
- 28.Awasthi S, Singhal SS, Sharma R, Zimniak P, Awasthi YC. Transport of glutathione-conjugates and chemotherapeutic drugs by RLIP76: a novel link between G-protein and tyrosine-kinase signaling and drug-resistance. Int J Cancer. 2003;106:635–646. doi: 10.1002/ijc.11260. [DOI] [PubMed] [Google Scholar]
- 29.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 30.Dang CV. MYC on the path to cancer. Cell. 2012;149(1):22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awasthi S, Singhal SS, Awasthi YC, Martin B, Woo JH, Cunningham CC, Frankel AE. RLIP76 and cancer. Clin Cancer Res. 2008;14:4372–4377. doi: 10.1158/1078-0432.CCR-08-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singhal SS, Yadav S, Roth C, Singhal J. RLIP76: A novel glutathione-conjugate and multi-drug transporter. Biochem Pharmacol. 2009;77:761–769. doi: 10.1016/j.bcp.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagaprashantha LD, Vatsyayan R, Singhal J, Fast S, Roby R, Awasthi S, Singhal SS. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem Pharmacol. 2011;82:1100–1109. doi: 10.1016/j.bcp.2011.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Awasthi S, Singhal SS, Yadav S, Singhal J, Drake K, Nadkar A, Zajac E, Wickramarachchi D, Rowe N, Yacoub A, Boor P, Dwivedi S, Dent P, Awasthi YC. RALBP1 is a major determinant of radiation sensitivity. Cancer Res. 2005;65:6022–6028. doi: 10.1158/0008-5472.CAN-05-0968. [DOI] [PubMed] [Google Scholar]
- 35.Singhal J, Singhal SS, Yadav S, Warnke M, Yacoub A, Dent P, Sharma R, Awasthi YC, Armstrong D, Awasthi S. RLIP76 in defense of radiation poisoning. Int J Rad Oncol Biol Phys. 2008;72:553–561. doi: 10.1016/j.ijrobp.2008.06.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singhal SS, Wickramarachchi D, Yadav S, Singhal J, Leake K, Vatsyayan R, Lelsani P, Chaudhary P, Suzuki S, Yang S, Awasthi YC, Awasthi S. Glutathione-conjugate transport by RLIP76 is required for clathrin-dependent endocytosis and chemical carcinogenesis. Mol Cancer Therap. 2011;10:16–28. doi: 10.1158/1535-7163.MCT-10-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singhal SS, Roth C, Leake K, Singhal J, Yadav S, Awasthi S. Regression of prostate cancer xenografts by RLIP76 depletion. Biochem Pharmacol. 2009;77:1074–1083. doi: 10.1016/j.bcp.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh S, Majumdar DK, Rehan HM. Evaluation of anti-inflammatory potential of fixed oil of ocimum sanctum (holybasil) and its possible mechanism of action. J Ethnopharmacol. 1996;54:19–26. doi: 10.1016/0378-8741(96)83992-4. [DOI] [PubMed] [Google Scholar]
- 39.Rai V, Iyer U, Mani UV. Effect of tulasi (ocimum sanctum) leaf powder supplementation on blood sugar levels, serum lipids and tissue lipids in diabetic rats. Plant Foods Hum Nutr. 1997;50:9–16. doi: 10.1007/BF02436038. [DOI] [PubMed] [Google Scholar]
- 40.Marrassini C, Davicino R, Acevedo C, Anesini C, Gorzalczany S, Ferraro G. Vicenin-2, a potential anti-inflammatory constituent of urtica circularis. J Nat Prod. 2011;74(6):1503–1507. doi: 10.1021/np100937e. [DOI] [PubMed] [Google Scholar]
- 41.Satyamitra M, Mantena S, Nair CKK, Chandna S, Dwarakanath BS, Uma Devi P. The antioxidant flavonoids, orientin and vicenin enhance repair of radiation-induced damage. SAJ Pharma Pharmacol. 2014;1:105. [Google Scholar]
- 42.Islam MN, Ishita IJ, Jung HA, Choi JS. Vicenin-2 isolated from Artemisia capillaris exhibited potent anti-glycation properties. Food Chem Toxicol. 2014;69:55–62. doi: 10.1016/j.fct.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 43.Lee IC, Bae JS. Anti-inflammatory effects of vicenin-2 and scolymoside on polyphosphate-mediated vascular inflammatory responses. Inflamm Res. 2016;65:203–212. doi: 10.1007/s00011-015-0906-x. [DOI] [PubMed] [Google Scholar]
- 44.Dharmani P, Kuchibhotla VK, Maurya R, Srivastava S, Sharma S, Palit G. Evaluation of anti-ulcerogenic and ulcer-healing properties of Ocimum sanctum Linn. J Ethnopharmacol. 2004;93:197–206. doi: 10.1016/j.jep.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Vats V, Yadav SP, Grover JK. Ethanolic extract of Ocimum sanctum leaves partially attenuates streptozotocin-induced alterations in glycogen content and carbohydrate metabolism in rats. J Ethnopharmacol. 2004;90:155–160. doi: 10.1016/j.jep.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 46.Trush MA, Kensler TW. An overview of the relationship between oxidative stress and chemical carcinogenesis. Free Radic Biol Med. 1991;10:201–209. doi: 10.1016/0891-5849(91)90077-g. [DOI] [PubMed] [Google Scholar]
- 47.Sabichi AL, Lippman SM. COX-2 inhibitors and other nonsteroidal anti-inflammatory drugs in genitourinary cancer. Semin Oncol. 2004;31:36–44. doi: 10.1053/j.seminoncol.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 48.Singh RP, Sharma G, Dhanalakshmi S, Agarwal C, Agarwal R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Biomarkers Prev. 2003;12:933–939. [PubMed] [Google Scholar]
- 49.Amjad AI, Parikh RA, Appleman LJ, Hahm ER, Singh K, Singh SV. Broccoli-derived sulforaphane and chemoprevention of prostate cancer: from bench to bedside. Curr Pharmacol Rep. 2015;1(6):382–390. doi: 10.1007/s40495-015-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karthikeyan K, Ravichandran P, Govindasamy S. Chemopreventive effect of ocimum sanctum on DMBA-induced hamster buccal pouch carcinogenesis. Oral Oncol. 1999;35:112–119. doi: 10.1016/s1368-8375(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 51.Nayak V, Devi PU. Protection of mouse bone marrow against radiation-induced chromosome damage and stem cell death by the ocimum flavonoids orientin and vicenin. Radiat Res. 2005;163:165–171. doi: 10.1667/rr3263. [DOI] [PubMed] [Google Scholar]
- 52.Vrinda B, Devi PU. Radiation protection of human lymphocyte chromosomes in vitro by orientin and vicenin. Mutat Res. 2001;498:39–46. doi: 10.1016/s1383-5718(01)00263-7. [DOI] [PubMed] [Google Scholar]
- 53.Mondal S, Mirdha BR, Mahapatra SC. The science behind sacredness of Tulsi (Ocimum sanctum Linn.) Ind J Physiol Pharmacol. 2009;53(4):291–306. [PubMed] [Google Scholar]
- 54.Lee S, Wurtzel J, Singhal SS, Awasthi S, Goldfinger LE. RALBP1/RLIP76 depletion in mice suppresses tumor growth by inhibiting tumor neo-vascularization. Cancer Res. 2012;72:5165–5173. doi: 10.1158/0008-5472.CAN-12-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singhal SS, Singhal J, Yadav S, Sahu M, Awasthi YC, Awasthi S. RLIP76: a target for kidney cancer therapy. Cancer Res. 2009;69:4244–4251. doi: 10.1158/0008-5472.CAN-08-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singhal SS, Singhal J, Yadav S, Dwivedi S, Boor P, Awasthi S. Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76 (RALBP1) Cancer Res. 2007;67:4382–4389. doi: 10.1158/0008-5472.CAN-06-4124. [DOI] [PubMed] [Google Scholar]
- 57.Leake K, Singhal J, Nagaprashantha L, Awasthi S, Singhal SS. RLIP76 regulates PI3K/Akt signaling and chemo-radio-therapy resistance in pancreatic cancer. PLoS ONE. 2012;7:e34582. doi: 10.1371/journal.pone.0034582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singhal SS, Awasthi YC, Awasthi S. Regression of melanoma in a murine model by RLIP76 depletion. Cancer Res. 2006;66:2354–2360. doi: 10.1158/0008-5472.CAN-05-3534. [DOI] [PubMed] [Google Scholar]
- 59.Wang Q, Wang JY, Zhang XP, Lv ZW, Fu D, Lu YC, Hu GH, Luo C, Chen JX. RLIP76 is overexpressed in human glioblastomas and is required for proliferation, tumorigenesis and suppression of apoptosis. Carcinogenesis. 2013;34:916–926. doi: 10.1093/carcin/bgs401. [DOI] [PubMed] [Google Scholar]
- 60.Wu Z, Owens C, Chandra N, Popovic K, Conaway M, Theodorescu D. RalBP1 is necessary for metastasis of human cancer cell lines. Neoplasia. 2010;12:1003–1012. doi: 10.1593/neo.101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin TD, Samuel JC, Routh ED, Der CY, Yeh JJ. Activation and involvement of Ral GTPases in colorectal cancer. Cancer Res. 2011;71:206–215. doi: 10.1158/0008-5472.CAN-10-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q, Qian J, Wang JY, Luo C, Chen J, Hu G, Lu Y. Knockdown of RLIP76 expression by RNA interference inhibits invasion, induces cell cycle arrest, and increases chemosensitivity to the anticancer drug temozolomide in glioma cells. J Neurooncol. 2013;112:73–82. doi: 10.1007/s11060-013-1045-2. [DOI] [PubMed] [Google Scholar]
- 63.Václavíková R, Horský S, Simek P, Gut I. Paclitaxel metabolism in rat and human liver microsomes is inhibited by phenolic antioxidants. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:200–209. doi: 10.1007/s00210-003-0781-9. [DOI] [PubMed] [Google Scholar]
- 64.Awasthi S, Srivastava SK, Ahmad F, Ahmad H, Ansari GAS. Interactions of glutathione S-transferase π with ethacrynic acid and its glutathione conjugate. Biochim Biophys Acta. 1993;1164:173–178. doi: 10.1016/0167-4838(93)90245-m. [DOI] [PubMed] [Google Scholar]
- 65.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst. 2001;93:1062–1074. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 66.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 67.Jullien-Flores V, Dorseuil F, Romero O, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis JH. Bridging Ral GTPase to Rho pathways. RLIP76, a Ral effector with CDC42/Rac GTPase-activating protein activity. J Biol Chem. 1995;270:22473–22477. doi: 10.1074/jbc.270.38.22473. [DOI] [PubMed] [Google Scholar]
- 68.Park SH, Weinberg RA. A putative effector of Ral has homology to Rho/Rac GTPase activating proteins. Oncogene. 1995;11:2349–2355. [PubMed] [Google Scholar]
- 69.Cantor SB, Urano T, Feig LA. Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases. Mol Cell Biol. 1995;15:4578–4584. doi: 10.1128/mcb.15.8.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. The exocyst is a Ral effector complex. Nat Cell Biol. 2002;4:66–72. doi: 10.1038/ncb728. [DOI] [PubMed] [Google Scholar]
- 71.Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo YY. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nelson CP, Kidd LC, Sauvageot J, Isaacs WB, De Marzo AM, Groopman JD, Nelson WG, Kensler TW. Protection against 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine cytotoxicity and DNA adduct formation in human prostate by glutathione S-transferase P1. Cancer Res. 2001;61:103–109. [PubMed] [Google Scholar]
- 73.Marzo AM De, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 75.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 76.Rosse C, L’Hoste S, Offner N, Picard A, Camonis JH. RLIP, an effector of the Ral-GTPases, is a platform for Cdk1 to phosphorylate epsin during the switch off of endocytosis in mitosis. J Biol Chem. 2003;278:30597–30604. doi: 10.1074/jbc.M302191200. [DOI] [PubMed] [Google Scholar]
- 77.Morinaka K, Koyama S, Nakashima S, Hinoi T, Okawa K, Iwamatsu A, Kikuchi A. Epsin binds to the EH domain of POB1 and regulates receptor-mediated endocytosis. Oncogene. 1999;18:5915–5922. doi: 10.1038/sj.onc.1202974. [DOI] [PubMed] [Google Scholar]
- 78.Jullien-Flores V, Mahe Y, Mirey G, Leprince C, Meunier-Bisceuil B, Sorkin A, Camonis JH. RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral pathway in receptor-endocytosis. J Cell Sci. 2000;113:2837–2844. doi: 10.1242/jcs.113.16.2837. [DOI] [PubMed] [Google Scholar]
- 79.Kano T, Sakai M, Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987;47:5626–5630. [PubMed] [Google Scholar]
- 80.Mott HR, Owen D. RLIP76 (RalBP1): The first piece of the structural puzzle. Small GTPases. 2010;1:157–160. doi: 10.4161/sgtp.1.3.14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajasekar KV, Campbell LJ, Nietlispach D, Owen D, Mott HR. The structure of the RLIP76 RhoGAP-Ral binding domain dyad: fixed position of the domains leads to dual engagement of small G proteins at the membrane. Structure. 2013;21:2131–2142. doi: 10.1016/j.str.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mott HR, Owen D. Structure and function of RLIP76 (RalBP1): an intersection point between Ras and Rho signalling. Biochem Soc Trans. 2014;42:52–58. doi: 10.1042/BST20130231. [DOI] [PubMed] [Google Scholar]
- 83.Wu KJ, Zeng J, Zhu GD, Zhang LL, Zhang D, Li L, Fan JH, Wang XY, He DL. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta Pharmacol Sin. 2009;30:1162–1168. doi: 10.1038/aps.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh RP, Deep G, Blouin MJ, Pollak MN, Agarwal R. Silibinin suppresses in vivo growth of human prostate carcinoma PC-3 tumor xenograft. Carcinogenesis. 2007;28:2567–2574. doi: 10.1093/carcin/bgm218. [DOI] [PubMed] [Google Scholar]
- 85.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemo-sensitization, and radio-sensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 86.Villarroya-Beltria C, Baixaulia F, Gutiérrez-Vázqueza C, Sánchez-Madrida F, Mittelbrunna M. Sorting it out: Regulation of exosome loading. Seminars Cancer Bio. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun Y, Liu J. Potential of cancer cell–derived exosomes in clinical application: a review of recent research advances. Clin Therap. 2014;36:863–872. doi: 10.1016/j.clinthera.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 88.Cheng S, Gao N, Zhang Z, Chen G, Budhraja A, Ke Z, Son YO, Wang X, Luo J, Shi X. Quercetin induces tumor-selective apoptosis through downregulation of Mcl-1 and activation of Bax. Clin Cancer Res. 2010;16:5679–5691. doi: 10.1158/1078-0432.CCR-10-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meybodi NM, Mortazavian AM, Monfared AB, Sohrabvandi S, Meybodi FA. Phytochemicals in cancer prevention: a review of the evidence. Iran J Cancer Prev. 2017:e7219. [Google Scholar]


