Abstract
Objective
Rheumatoid arthritis (RA) patients have increased risk of cardiovascular disease (CVD) and total mortality. We measured anti-cyclic citrullinated peptide (anti-CCP) antibodies and use of disease modifying anti-rheumatic drugs (DMARDs) and total mortality over 10 years of follow up among 9,988 white, black, or Hispanic women who self-reported RA in the WHI.
Methods
Using stored baseline serum, we measured anti-CCP, rheumatoid factor (RF), and antinuclear antibodies (ANA) in 9,988 of the women who reported RA. Based on a previous chart review study, “probable” RA was defined as self-report of RA and anti-CCP+ or anti-CCP-with use of DMARDs. Cox proportional hazards regression was used to model the relationships of self-reported RA, DMARDs and anti-CCP+ to total mortality using follow up data through April 2009.
Results
At baseline, mean age was 64±7 years, with 24.5% black and 10% Hispanic. Prevalence of anti-CCP+ was 8.1% (n=812), with n= 217 anti-CCP- but reporting DMARDs, resulting in 1,029/9988 women classified as probable RA, and 8,958 classified as probable non-RA. Age-adjusted mortality rates were about 2-fold higher for anti-CCP+, 20.2/1,000 person-years (PYs) compared to anti-CCP-/no DMARD use women who reported RA, 11.4/1,000 PYs and for women who did not report any arthritis at baseline, 8.3/1,000 PYs. The increased risk with anti-CCP+ RA was not explained by age, RF+, ANA+ or DMARD use.
Conclusions
Anti-CCP+ RA was associated with substantial excess mortality among postmenopausal women in WHI that was not explained by measured risk factors.
Rheumatoid arthritis (RA) patients have a shortened life expectancy, (1) with ∼ 1.5-fold higher mortality rates than controls. (1,2) Excess mortality is largely due to cardiovascular disease (CVD) (3) and is greater in cohorts with existing RA than in inception cohorts because risk increases both with the duration and severity of RA. (4, 5) Mortality is higher among men than women with RA and at older ages. (6) Studies have reported that the excess mortality may be declining over time due possibly to a decrease in disease severity and/or improved drug therapies, especially starting medications earlier in the course of disease and new biological agents. (4, 7-10) No clinical trial has been carried out long enough to look at mortality as an outcome, (11-13) but observational data suggests that methotrexate may reduce both CVD and mortality. (14)
The specific pathophysiology for the excess mortality has not been firmly established in spite of numerous observational studies over the past 60+ years. Possible explanations include accelerated aging, persistent inflammation related to severity of RA as measured by both rheumatoid factor (RF), anti-cyclic citrullinated peptide (anti-CCP) antibody, higher levels of specific cytokines, T cell abnormalities, other immunological changes and secondary fibrosis, thrombosis, amyloid deposition, etc. (15-22) Infectious diseases, B-cell lymphoma and possibly renal and pulmonary diseases are also reported in excess in RA patients. (23,24)
Few large epidemiological cohort studies have included RA as a specific outcome because of the relatively low incidence and prevalence of RA and the difficulties of documenting reported RA diagnoses. (25) The availability of anti-CCP antibody assays that are both sensitive (70-75%) and highly specific (95%) for RA has provided an opportunity to include RA as an outcome in large epidemiological cohort studies that did not select RA patients from the clinic or community registries. (26-28)
In the Women's Health Initiative (WHI), we have previously shown that classification of self-reported RA according to the combination of anti-CCP and use of disease-modifying antirheumatic drugs (DMARDs) at baseline or during the study, resulted in a very high positive predictive and negative predictive value for physician-validated diagnosis of RA based on review of hospital and other medical records in two WHI clinical centers. Very few women who were anti-CCP- and not on DMARDs had clinical evidence of RA. (29) In that study, there were 286 women in two centers who reported a history of RA at either baseline or follow up. Physicians reviewed medical records and information from physicians and validated 42 cases as probable RA, of which 20 (47.6%) were anti-CCP+, whereas of 244 validated as not RA, 5 (2.1%) were anti-CCP+, an 80% positive predictive value. In contrast, the positive predictive value of anti-CCP+ and DMARD use was 100%, of RF+ was 44% and of self-reported DMARD use alone was 62%.
The focus of this report is a detailed evaluation of total mortality over 10 years of follow up among 9,988 women who reported RA in the WHI, stratified by likely clinical RA, reported risk factors, and serum markers measured at baseline-only including anti-CCP by a second generation assay (anti-CCP2), RF, antinuclear antibodies (ANA), and use of DMARDs. Other papers will focus on cytokines, genetics and specifically on risk of coronary heart disease (CHD). Most of the results, except in Table 1, are based on women who reported RA at the baseline visit or at both baseline and follow up visits.
Table 1. All-cause mortality by anti-CCP+ and DMARD use at baseline for women who reported history of RA – unweighted and weighted (total sample) per 1000 PYs.
| Un-weighted | ||||
|---|---|---|---|---|
|
| ||||
| N | Death | Age-adjusted death rate/1000 PYs | Excluding RA at follow up only - Age-adjusted death rate/1000 PYs | |
|
| ||||
| Probable clinical RA | ||||
| Anti-CCP+ | 812 | 162 | 20.2 (15.6-26.1) | 21.3 (16.0-28.5) |
| Anti-CCP-, DMARD use | 217 | 42 | 17.5 (10.6-29.5) | 16.6 (9.8-29.2) |
| Not likely clinical RA | ||||
| Anti-CCP-,DMARDs- | 8951 | 1120 | 11.4 (10.3-12.5) | 12.8 (11.3-14.6) |
| Weighted | ||||
|
|
||||
| N | Death | Age-adjusted death rate | Excluding RA at follow up only - Age-adjusted death rate/1000 PYs | |
|
| ||||
| Probable clinical RA | ||||
| Anti-CCP+ | 1082 | 201 | 18.7 (14.9-23.5) | 21.3 (16.5-27.7) |
| Anti-CCP-, DMARD use | 282 | 55 | 17.8 (11.4-28.0) | 16.3 (10.2-27.0) |
| Not likely clinical RA | ||||
| Anti-CCP-,DMARDs- | 13812 | 1540 | 10.0 (9.2-10.9) | 12.3 (11.0-13.7 |
| No history of reported RA Other arthritis at baseline* | ||||
| Yes | 57572 | 6398 | 9.2 (8.9-9.6) | |
| No | 76160 | 6187 | 8.3 (7.9-8.6) | |
| Total | 133732 | 12585 | 8.7 (8.5-9.0) | |
not included in study sample for biomarker testing
Methods
Participants and Data Collected in WHI
Many previous papers have described the WHI. Briefly, 40 clinical centers enrolled 161,808 women between 1993 and 1997. The mean age of the participants was 62.8 years and 64.5% were white. (30) At baseline, 76,192 (47.1%) of women reported arthritis. At baseline or follow up visits, 16,461 (10.2%) women reported a history of RA. This study is limited to whites, blacks and Hispanics, excluding 713 American Indians, 4,190 Asian/Pacific Islander and 2,362 of other ethnicity or missing blood samples, leaving 15,188 who reported RA at baseline or follow up visits, of whom a sample of 9,988 (62.8%) were included in the study.
Priority in the sampling frame was given for women with reported RA and had a CHD event during the 10 year follow up, to Black and Hispanic race/ethnicity and to those women who had available DNA for genetic analysis. The sample included 100% (1,650) of women who reported RA at baseline and follow-up, 72% (4,007 of 5,578) of women who self-reported RA at baseline only, 63% (2,625 of 4,134) of women who reported RA at follow-up but other arthritis at baseline, and 44% (1,706 of 3,826) of women who reported RA at follow-up but no arthritis at baseline. The final sample included 6,487 white, 2,442 Black, and 1,058 Hispanic women. (Supplemental Figure 1) Much of the analysis presented in this paper is restricted to women who reported RA at baseline or baseline and follow-up, 78% of eligible WHI women (5,657 of 7,228), because of lack of follow up blood samples and limited information on subsequent DMARD use after baseline and in order to avoid biasing results by shorter follow-up time. Weighted and un-weighted analyses are included in the paper with the weights based on the specific sampling fractions above. There was little difference in weighted/un-weighted results for participants who had RA at baseline or at baseline and follow up because a very high percentage (78%) who were included in the study. All of the independent variables, except as noted in the text, for DMARD use are based only on baseline data.
At the baseline examination, women were asked about current severity of joint pain and swelling of joints during the past four weeks but not the specific joint or number of joints that were affected. The severity of joint symptomatology varied from none to severe. Women reported their current health status, disability, physical functioning, and employment status. (31) Detailed pharmacological drug histories were also obtained, both at baseline and follow up, every three years in the CT and at third year of follow up in the OS, including drugs used in the treatment of RA (DMARDs). Eligible DMARDs included hydroxychloroquine, sulfasalazine, minocycline, methotrexate, leflunomide, azathioprine, cyclosporine, gold, cyclophosphamide use, anti-rheumatic biologic agents, oral steroids, tumor necrosis factor-α inhibitors and interleukin-1 antagonist. (29) The data is not detailed enough to provide accurate dose of drug history.
Serum Biomarkers
Using baseline serum samples stored at -70° and not previously thawed, anti-CCP2, RF and ANA assays were done in the Rheumatology Clinical Research Laboratory at the University of Colorado. Briefly, anti-CCP (IgG) antibodies were measured using commercially available second-generation (Anti-CCP2) enzyme linked immunosorbent assay kits (Diastat, Axis-Shield Diagnostics Ltd., Dundee, Scotland, UK). Anti-CCP2 antibodies were measured in arbitrary units (U) per ml and considered positive at a cut-off value ≥ 5 U/ml that has been demonstrated to be >98% specific for RA. (29) RF was measured quantitatively by the reactivity of diluted test serum with heterologous IgG in solution by nephelometry which provided continuously variable quantitative results in international units (IU) (Dade Behring, Newark, DE). Per the 1987 American College of Rheumatology (ACR) RA Classification Criteria, the positive cut-off value for these tests is set so that 5% of a population of 490 randomly-selected healthy anonymous blood donors were positive. Quality control was routinely assessed by a procedure where all autoantibody positive sera (anti-CCP2 and/or RF) were re-tested in a blinded fashion, along with 5% of the negative sera, with >97.5% agreement in repeat testing. (29)
ANA testing was performed in a two-step process. First, serum samples were tested using the Bio-Rad Laboratories, Inc. Autoimmune EIA ANA Screening Test (Bio-Rad Laboratories, Inc., Hercules, California USA) using the methods and reagents specified by the manufacturer. Positivity for this assay was determined based on in-house experiments and a level that was >95% sensitive for patients with systemic lupus erythematosus (SLE) who were all ANA positive by indirect immunofluorescence (IIF) and who all met ≥4/11 ACR criteria for classification of SLE, as well as >95% sensitive for patients with a variety of other non-SLE rheumatic diseases known to be positive for ANA by IIF. Second, all WHI samples positive by EIA testing were subsequently tested using IIF and HEp-2 nuclei, and final ANA positivity was determined based on ≥2+ immunofluorescent intensity at a titer of ≥1:320. No follow up blood samples were evaluated in this paper.
Two other comparison groups were included in the analysis: 1) women who reported arthritis but not RA (n=57,572); and 2) never reporting any arthritis (n=76,160) at baseline. No study-specific blood tests were done for those two comparison groups.
Other variables measured in all of the women in WHI and included in this analysis have been previously shown to be related to morbidity and mortality, including history of cigarette smoking (none, past, current), history of hypertension, diabetes, and CHD at baseline, current levels of physical activity, waist circumference, reported general health, age, education and ethnicity. These variables have been described in previous WHI publications. (32,33)
Deaths were identified by semiannual or annual follow up to family, friends, etc., medical care providers, the National Death Index, and obituaries. Only about 1-2% of participants have been lost to follow up. Cardiovascular- and cancer-related morbidities were adjudicated centrally. Cardiovascular mortality included deaths from CHD, stroke, congestive heart failure and other CVDs. The underlying cause of death was used for classification of other cause-specific mortality.
Statistical Analysis
The study sample consisted of 9,988 women who were sampled from 15,188 eligible WHI participants who had reported RA at baseline or during follow-up. Sampling fractions varied by whether participants reported RA at baseline or follow up only, CHD status and race/ethnic groups. To correctly represent the WHI RA population, sampling weights, which were defined as 1/sampling fraction, were determined for each woman and incorporated in the analyses. As noted, among women who reported RA, probable RA cases were defined as anti-CCP+, whether on or not on DMARDs, or anti-CCP- but with reported DMARD use, based on our previous chart-review. (34) Women who reported RA but were not anti-CCP+ or on DMARDs were classified as not having RA. The analysis was designed first to compare probable RA vs. no RA among women who reported RA. Other comparison groups were the remaining women who reported other arthritis then RA, or who reported no arthritis during the study but no blood tests were done for these two additional comparison groups. Comparison of HRs within the specific groups in Table 5 should be done carefully because analysis was done specific for each of the 4 classifications of anti-CCP+/- and DMARD use. Results utilize variables measured at baseline only, except when noted for DMARD use. Age-adjusted mortality rates and 95% confidence intervals (CI) were calculated using direct method with the entire WHI population as the standard population. Cox models were used to assess the association between mortality and risk factors. Time to the event was defined as the time from baseline to the date of death or to the end of follow up for deceased and alive subjects, respectively. Analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC). All models were 2-sided at alpha=0.05.
Table 5. Cox regression model of HRs and 95% CI predicting total mortality, among women who reported RA at baseline or baseline and follow up (weighted) for 4 groups based on anti-CCP+/- and DMARDs+/- use at baseline.
| Measurement at baseline | Anti-CCP+/DMARD use HR (95% CI) (n=322/166 deaths) | Anti-CCP+/no DMARD use HR (CI) (n=290/59 deaths) | Anti-CCP-/DMARD use HR (CI) (n=183/33 deaths) | Anti-CCP-/no DMARD use HR (CI) (n=4,566/655 deaths) |
|---|---|---|---|---|
| Log WBC (1 SD) | 2.9 (1.2-7.3) | 2.4 (1.3-4.7) | 4.3 (1.5-12.3) | 1.9 (1.5-2.4) |
| Age (per year) | 1.1 (1.1-1.2) | 1.1 (1.1-1.1) | 1.1 (1.1-1.2) | 1.1 (1.1-1.1) |
| Ethnicity – Black versus white | 0.3 (0.1-0.8) | 0.5 (0.3-1.0) | 1.2 (0.4-3.5) | 1.1 (0.9-1.3) |
| Ethnicity – Hispanic versus white | 0.2 (0.0-1.3) | 0.9 (0.2-3.8) | 1.8 (0.9-3.8) | 0.7 (0.5-1.1) |
| Ever smoked versus nonsmoker | 0.9 (0.6-1.6) | 1.8 (1.0-3.0) | 1.0 (1.0-1.1) | 1.3 (1.1-1.5) |
| Hypertension – yes, no | 1.0 (0.6-1.7) | 1.2 (0.8-2.0) | 1.4 (0.5-3.8) | 1.2 (1.0-1.4) |
| Diabetes – yes, no | 3.4 (1.4-8.4) | 1.2 (0.5-2.8) | 1.2 (0.5-3.2) | 2.2 (1.8-2.7) |
| Education - ≥ College (1) | 0.4 (0.2-0.7) | 0.7 (0.4-1.2) | 2.3 (1.0-5.6) | 0.9 (0.8-1.1) |
| Education - Some College | 0.5 (0.3-0.9) | 0.8 (0.5-1.3) | 1.1 (0.5-2.2) | 1.1 (0.9-1.3) |
| Joint pain – mild (2) | 1.4 (0.3-7.4) | 3.3 (0.7-16.1) | 0.5 (0.2-1.9) | 1.0 (0.7-1.3) |
| Joint pain - moderate | 1.3 (0.2-6.8) | 2.3 (0.5-11.6) | 0.5 (0.2-1.9) | 1.0 (0.8-1.3) |
| Joint pain - severe | 3.0 (0.6-15.8) | 3.6 (0.7-18.7) | 0.4 (0.1-1.6) | 1.3 (1.0-1.7) |
excludes women who only reported RA at follow-up; (1) ≤ high school education; (2) no joint pain
Results
Among women who self-reported RA, 8.1% (812) were anti-CCP+, of whom 467 (57.5%) reported DMARDs at any time during follow-up. Of the 9,179 women who reported RA but were anti-CCP-, 673 (7.3%) were on DMARDs. The prevalence of anti-CCP+ and DMARD use was much higher for women who reported RA at baseline than for women who reported RA at follow up examinations only. When the analysis was restricted to women reporting RA at baseline, 612 were anti-CCP+ and 407 of 612 (67%) reported DMARD use.
Mortality by Reported RA
Over 10 years, 13% of the women in the study sample (1,325 of 9,988) died, including 14% (233 of 1,649) who self-reported RA at both baseline and follow up, 16% (633 of 3,996) who reported RA at baseline only, and 10.5% (456 of 4,331) who self-reported RA at follow up only. Median time to death was approximately 8 years for those who had RA at baseline, 6.6 years for those who reported RA at both baseline and follow up and 6.4 years for those who reported RA at follow up only.
Anti-CCP+ and DMARD Use
In the total study sample, age-adjusted death rates over 10 years of follow up were higher for women with probable RA, i.e., anti-CCP+ or anti-CCP- on DMARDs, than for women reporting RA but not likely to have RA, i.e., anti-CCP- and no DMARD use. (Table 1) Results were similar in weighted analyses, particularly for the increased death rates for anti-CCP+. Death rates were also lower among women who did not report RA, particularly for women who reported no arthritis at baseline. (Table 1)
Age-specific death rates increased across age groups for both anti-CCP+ and anti-CCP-women, but remained substantially higher for anti-CCP+ within each age group (data not shown.) Among anti-CCP+ women, mortality rates were lower for Black compared to White or Hispanic women. CIs were wide due to small numbers of non-white anti-CCP+ women There was no significant difference in percent dead by anti-CCP levels at baseline: <31 units, 20.7%; 31-75, 16.3%; 75-102, 19.7% and >102, 16.7%.
Among participants who reported RA at baseline (or baseline and follow-up), the prevalence of RF positivity measured in baseline serum samples was approximately 20% (1,152 of 5,657), higher for anti-CCP+ vs. anti-CCP- women (69% vs. 12%). (Table 2) Approximately half of the RF+ women were anti-CCP-/no DMARD use, i.e., unlikely to have clinical RA based on our validation study. (29) The overall prevalence of ANA was 15.6% or 881 participants, similar to a recent report of a United States population sample. (34) Of ANA+ women, 16% were anti-CCP+ and 31% RF+, but two-thirds of the ANA+ women were anti-CCP- and RF- and therefore probably did not have clinical RA. (Table 2) Finally, age-adjusted death rates were higher for anti-CCP+ and RF+ but not for ANA+ women. (Table 2) Age-adjusted death rates were not significantly higher for those who were anti-CCP+/RF+ than for anti-CCP+/RF- and not elevated for those who were anti-CCP-/RF+, suggesting that anti-CCP+ rather than RF+ was the correlate of increased death rates. There were very few participants who had a combination of anti-CCP+/ANA+. Death rates were also elevated for RF+ and ANA+, but the sample size was small. Surprisingly, the death rate was only slightly higher for women who were anti-CCP+/RF+/ANA+, compared with those negative for all three, i.e., 14.4/1,000 vs. 12.5/1,000 person-years (PYs).
Table 2. Prevalence of anti-CCP+, RF+ and ANA+ and age-adjusted death rates among women who reported RA at baseline or baseline and follow-up (N=5,657).
| Anti-CCP | RF | ANA | N | Age-adjusted death rate/1000 PYs |
|---|---|---|---|---|
| - | 5042 | 13.0 (11.5-14.7) | ||
| + | 612 | 21.4 (16.0-28.5) | ||
| - | 4492 | 12.8 (11.2-14.6) | ||
| + | 1152 | 17.9 (14.4-22.4) | ||
| - | 4768 | 13.7 (12.1-15.5) | ||
| + | 881 | 14.7 (11.3-19.4) | ||
| - | - | - | 3802 | 12.5 (10.9-14.5) |
| + | - | - | 75 | 20.9 (9.9-48.3) |
| - | + | - | 487 | 14.5 (10.0-21.1) |
| - | - | + | 597 | 13.3 (9.4-18.9) |
| + | + | - | 395 | 23.3 (16.5-33.0) |
| + | - | + | 13 | 30.5 (7.9-136.8) |
| - | + | + | 142 | 19.5 (11.2-36.0) |
| + | + | + | 128 | 14.4 (6.7-31.7) |
Mortality by DMARD and/or Methotrexate Use
Among women who reported RA at baseline in weighted analysis, 68% (337/493) of those who reported methotrexate use at baseline were anti-CCP+, whereas only 40% (125/313) of those who reported use of other DMARDs at baseline were anti-CCP+ and only 4.5% (291/6,422) of women who reported RA at baseline and were not on DMARDs were anti-CCP+. Mortality was similar for use of DMARDs vs. no DMARDs or methotrexate versus any DMARDs but higher for anti-CCP+ versus anti-CCP- for users or nonusers of methotrexate or users and nonusers of all DMARDs. (Table 3)
Table 3. Age adjusted mortality at 2009 by methotrexate and DMARD use* among women who reported RA at baseline or at baseline and follow-up (weighted).
| Anti-CCP | Medication | N | N (%) Deaths | Age-adjusted death rate/1,000 PYs | |
|---|---|---|---|---|---|
| + | Methotrexate use | 337 | 68 (20.1) | 21.3 (14.5-31.6) | |
| + | No Methotrexate, Any DMARD use | 125 | 30 (23.8) | 22.0 (12.1-40.7) | |
| + | No Methotrexate, No DMARD use | 291 | 64 (22.0) | 21.6 (14.3-33.0) | |
| - | Methotrexate use | 156 | 22 (14.3) | 13.1 (6.9-26.7) | |
| - | No Methotrexate, Any DMARD use | 188 | 34 (18.2) | 15.5 (9.1- 27.7) | |
| - | No Methotrexate, No DMARD use | 6131 | 860 (14.0) | 12.4 (11.1-13.9) | |
|
| |||||
| No history of arthritis | 76160 | 6187 (8.1) | 8.3 (7.9-8.6) | ||
DMARD use at baseline, not including prednisone
Causes of Death
The distribution of the causes of death was similar for probable RA (i.e., anti-CCP+, anti-CCP-/DMARD use) and probable not RA (i.e., anti-CCP-/no DMARD use) among women reporting RA at baseline (not shown.) Total CVD, including CHD and stroke, and all cancers were the leading causes of death. There was also little difference in the distribution of cause of death as compared to the total WHI study. However, the number of deaths among anti-CCP+ women is small and limits interpretation of distribution of causes of death.
Risk Factors
Death rates over 10 years were substantially higher among anti-CCP+ women who reported cigarette smoking, diabetes, less physical activity, poor health, history of CHD or poor physical function on the SF-36. (31) (Table 4) Women who were anti-CCP+ had higher total mortality within each of these attributes as compared to women who had never reported RA or who reported history of RA but were anti-CCP-, not on DMARDs and thus presumably did not have clinical RA. The reported health status, i.e. excellent to poor, was a very powerful predictor of mortality across all groups. Approximately 25% of anti-CCP+ women reported poor health as compared to 7% of women who did not report RA, with death rates increased more than 4-fold for anti-CCP+ women who reported poor health compared with women who reported excellent health and who never reported RA.
Table 4. Age-adjusted death rates by baseline variable by anti-CCP and DMARD use in self-reported RA, excluding history of RA at follow up only (weighted).
| Age-adjusted Death Rates/1000 PYs | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Baseline Measurement | Weighted Total | Anti-CCP+ (N=812) | Weighted total | Anti-CCP- /DMARD use at baseline (N=217) | Weighted total | Anti-CCP-/No DMARD use at baseline (N=8951) | Never self-reported RA (N=122,275) |
| Age | |||||||
| 50-59 | 262 | 9.2 (6.3-13.5) | 74 | 4.9 (1.9-12.8) | 1894 | 5.3 (4.4-6.3) | 3.2 (3.1-3.3) |
| 60-69 | 313 | 17.8 (13.8-22.9) | 102 | 16.0 (10.2-25.3) | 2617 | 10.6 (9.5-11.8) | 7.4 (7.2-7.6) |
| 70-79 | 178 | 43.8 (34.9-55.1) | 69 | 32.7 (21.7-49.3) | 1713 | 24.7 (22.5-27.1) | 17.6 (17.1-18.1) |
|
| |||||||
| Ethnicity | |||||||
| Black | 149 | 12.1 (6.4-23.3) | 31 | 17.6 (6.1-52.5) | 979 | 11.5 (9.3-14.3) | 10.8 (9.8-12.0) |
| Hispanic | 41 | 22.6 (8.5-62.8) | 4 | 0 | 322 | 7.3 (4.6-11.4) | 7.1 (5.8-8.8) |
| White | 563 | 20.3 (15.8-26.1) | 210 | 18.3 (11.4-29.7) | 4928 | 9.9 (9.1-10.9) | 8.6 (8.3-8.9) |
|
| |||||||
| Education | |||||||
| ≤High school | 194 | 24.2 (16.0-37.1) | 58 | 13.3 (4.8-39.7) | 1752 | 10.2 (8.7-11.9) | 9.7 (9.1-10.3) |
| Some college | 306 | 16.3 (11.1-23.9) | 94 | 25.3 (13.9-47.1) | 2486 | 10.5 (9.2-11.9) | 9.0 (8.6-9.5) |
| ≥College | 243 | 17.0 (11.4-25.7) | 92 | 14.0 (6.1-32.5) | 1929 | 9.52 (8.2-11.1) | 7.9 (7.6-8.3) |
|
| |||||||
| Smoking | |||||||
| Never | 303 | 14.9 (10.1-22.4) | 105 | 15.7 (7.7-32.6) | 3120 | 8.7 (7.7-9.9) | 6.9 (6.6-7.3) |
| Past | 360 | 18.7 (13.5-26.0) | 121 | 20.9 (11.6-38.3) | 2506 | 10.2 (8.9-11.6) | 9.5 (9.1-9.9) |
| Current | 81 | 27.8 (15.4-50.5) | 18 | 0 | 491 | 20.0 (15.6-25.6) | 19.3 (17.7-21.1) |
|
| |||||||
| General health | |||||||
| Excellent/very good | 191 | 12.1 (7.7-19.6) | 89 | 7.3 (6.3-8.5) | 2521 | 7.3 (6.3-8.5) | 6.7 (6.4-7.0) |
| Good | 350 | 18.3 (13.0-26.1) | 124 | 10.1 (8.9-11.6) | 2477 | 10.1 (8.9-11.6) | 10.2 (9.7-10.7) |
| Fair/poor | 205 | 29.9 (20.1-44.6) | 31 | 17.5 (14.9-20.5) | 1190 | 17.5 (14.9-20.5) | 19.0 (17.6-20.5) |
|
| |||||||
| Diabetes | |||||||
| No | 712 | 17.8 (14.0) | 219 | 9.1 (8.3-10.0) | 5623 | 9.1 (8.3-10.0) | 8.1 (7.9-8.4) |
| Yes | 41 | 32.3 (16.1-66.7) | 25 | 21.4 (17.4-26.4) | 600 | 21.4 (17.4-26.4) | 18.9 (17.3-20.6) |
|
| |||||||
| CHD at baseline | |||||||
| No | 687 | 17.5 (13.7-22.5) | 224 | 8.9 (8.1-9.8) | 5329 | 8.9 (8.1-9.8) | 8.2 (7.9-8.4) |
| Yes | 66 | 33.3 (18.0-63.2) | 21 | 19.2 (16.0-23.3) | 900 | 19.2 (16.0-23.3) | 15.9 (14.6-17.4) |
|
| |||||||
| Physical function construct (SF36) | |||||||
| ≤ 85 | 589 | 20.2 (15.6-26.2) | 208 | 12.1 (11.0-13.3) | 4143 | 12.1 (11.0-13.3) | 11.0 (10.6-11.4) |
| >85 | 134 | 13.6 (7.7-24.1) | 35 | 6.4 (5.3-7.6) | 1939 | 6.4 (5.3-7.6) | 6.2 (5.9-6.6) |
|
| |||||||
| Waist circumference (cm) | |||||||
| ≤88 | 460 | 16.3 (12.0-22.4) | 134 | 8.7 (7.7-9.8) | 3113 | 8.7 (7.7-9.8) | 7.6 (7.3-7.9) |
| >88 | 291 | 22.6 (16.2-31.6) | 108 | 11.5 (10.2-12.8) | 3092 | 11.5 (10.2-12.8) | 10.5 (10.0-10.9) |
|
| |||||||
| Total METs per week | |||||||
| <2.5 | 264 | 22.9 (15.7-33.4) | 89 | 13.6 (11.9-15.7) | 1847 | 13.6 (11.9-15.7) | 11.0 (10.4-11.6) |
| 2.5-18.4 | 359 | 18.6 (13.3-26.3) | 124 | 9.4 (8.3-10.7) | 3040 | 9.4 (8.3-10.7) | 8.5 (8.1-8.8) |
| ≥18.25 | 129 | 13.4 (7.7-24.6) | 31 | 7.4 (6.0-9.1) | 1326 | 7.4 (6.0-9.1) | 7.0 (6.6-7.5) |
|
| |||||||
| Hypertension | |||||||
| No | 479 | 18.3 (13.7-24.5) | 146 | 7.7 (6.8-8.8) | 3490 | 7.7 (6.8-8.8) | 7.3 (7.0-7.6) |
| Yes | 270 | 20.1 (13.9-29.1) | 96 | 13.3 (11.8-14.9) | 2678 | 13.3 (11.9-14.9) | 11.3 (10.8-11.8) |
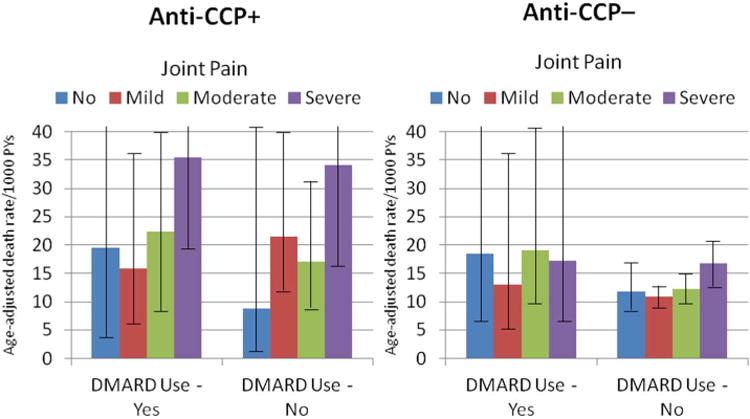
Joint Pain
Age-adjusted death rates were positively related to joint pain reported in the four weeks prior to baseline interview, especially for anti-CCP+ women. (Figure) Severe joint pain was associated with a significant 2-fold higher age-adjusted death rates vs. none or mild joint pain among anti-CCP+ women. Among women who reported severe joint pain, death rates were much higher for anti-CCP+ (35.5/1,000 PYs) than for women who reported RA and were anti-CCP-/no DMARD use (17.2/1,000 PYs), reported arthritis but not RA (12.1/1,000 PYs), or did not report arthritis (11.4/1,000 PYs), i.e., an approximate 3-fold difference across severe joint pain symptomatology by anti-CCP+ status. Furthermore, age-adjusted death rates varied significantly, over 4-fold, between women who were anti-CCP+ and severe joint pain compared with women with no history of arthritis and no joint pain at baseline.
Figure 1. Age adjusted Death Rates by Anti-CCP + and -, DMARD Use and Joint Pain Among Women Who Reported RA at Baseline.
Kidney Function
The original WHI did not include kidney function measures. Therefore we evaluated creatinine in a subset (n= 789) including 599 (76%) of participants in the RA study who were anti-CCP+. Mean (SD) creatinine was 0.59 (0.20) mg/dl, with 99th percentile = 1.3 mg/dl and was similar for anti-CCP+ and anti-CCP-. Age-adjusted death rates increased with blood creatinine levels. The percentage deaths was higher with higher creatinine quartile for both anti-CCP+ women, from 15.7% in the lowest quartile of creatinine to 31.1% in the highest quartile, and for anti-CCP-, from 20.8% in the lowest quartile of creatinine to 30.6% in the highest quartile. However, confidence limits are wide because the number of deaths in this subsample was small.
White Blood Cell (WBC) Count
Higher WBC counts were an independent predictor of mortality. For anti-CCP+ women, age-adjusted death rates increased linearly and significantly from approximately 11.9 (4.2-41.5) /1,000 PYs for WBC <4.08, to 35.6 (22.9-57.4)/1,000 PYs for WBC >8.3 (p=<.0001), and for anti-CCP-/no DMARD use, from 8.7 (6.0-12.8)/1,000 PYs to 19.2 (14.9-24.8)/1,000 PYs (p=.0001). Results were similar for weighted or un-weighted analysis. (not shown)
Multivariable Models
In multivariable-adjusted Cox regression models for the total sample, probable RA was a significant predictor of mortality compared with women who did not report a history of RA, with HR (95%CIs) of 2.8 (2.2-3.5) for anti-CCP+/DMARD use at baseline, 2.2 (1.7-2.7) for anti-CCP+ and no DMARD use at baseline, 1.8 (1.4-2.5) for anti-CCP- and DMARD use at baseline.(not shown) Women who reported RA but were not likely not to have RA (i.e., anti-CCP- and not on DMARDs) also had a significant increased risk of total mortality, HR 1.3 (CI: 1.2-1.4), compared with women who did not report a history of RA. Risk factors for mortality were then compared within categories defined by anti-CCP and DMARD use (Table 5). Among anti-CCP+ DMARD users at baseline, significant predictors of mortality were (log) WBC count, age, smoking, hypertension and diabetes. For anti-CCP+ women, HRs for severe joint pain and high WBC count were similar for DMARDs s vs. no DMARDs. In contrast, among anti-CCP-/DMARD use participants (a small sample) joint pain severity was unrelated to mortality, although elevated WBC count remained a powerful predictor of mortality. Among anti-CCP-/no DMARD use participants (unlikely to have RA, the largest sample), both severe joint pain and WBC count were independent predictors of the risk of mortality. (Table 5) These women likely have osteoarthritis. These results were not modified by inclusion of specific cytokine, chemokine levels or distribution of shared epitome which are included in separate papers and not shown here.
Discussion
The large sample size of postmenopausal women in the WHI has provided a unique opportunity to determine the prevalence and risk factors for mortality among women with probable RA (defined as anti-CCP+ or use of DMARDs), those who reported RA at baseline but likely did not have RA and women who reported other arthritis (osteoarthritis) and women who did not report any arthritis at baseline and follow up. This is the first large longitudinal study to evaluate anti-CCP, RF, risk factors and mortality.
The important results of this analysis was that anti-CCP+ is associated with a substantial 2-2.5-fold increased risk of total mortality statistically independent of the baseline use of DMARDs, including methotrexate, and in multivariate models that included many risk factors and lifestyles associated with mortality. Elevated levels of key risk factors such as WBC count, smoking, diabetes and health status increased mortality among women who were anti-CCP+. Epidemiology and genetic studies of RA need to separate anti-CCP+ and anti-CCP- RA. (35)
It is possible that other unmeasured risk factors or residual confounding could explain the excess mortality associated with anti-CCP+. A clinical trial that specifically modified anti-CCP+ independent of other risk factors would be necessary to prove independent effects of anti-CCP on mortality. DMARDs, such as methotrexate, or more recently biological agents substantially reduced symptomatology and seem to offer total (14) and cardiovascular (36,37) risk protection in observations trials, and low dose methotrexate is currently being tested in a secondary prevention trial of CHD with the presumption that the reduction in inflammation and especially C-reactive protein will reduce cardiovascular morbidity and mortality. (38) The new biological agents were not generally available at the beginning of the WHI through 1997 and therefore we have no information of the use of these new biologics and mortality in this study. No clinical trial, possibly due to short follow up and smaller sample size, to date has shown that these new agents decreased total mortality or CHD among RA patients in spite of their great success in reducing symptomatology.
Excess mortality has also been noted in other inflammatory diseases even when treatment has been successful in reducing important biological markers of disease, such as in well-treated HIV patients. Persistence of inflammation and secondary changes in thrombotic markers, such as D-dimer, were reported to be very strong predictors of total mortality in the well-treated HIV population. (39) Persistent inflammation could contribute to in an increase in fibrosis in many organs, such as the heart, lung and kidneys, as well as an increased risk of thrombogenesis and mortality. (40-48) The possibility that inflammatory-type amyloid (serum amyloid A [SAA]) contributes to excess mortality should also be evaluated. (49) The relationship of lipoproteins and markers of thrombosis and fibrinolysis and CHD incidence is a topic of a separate paper. New technologies using magnetic resonance imaging and positron emission tomography imaging provide opportunities to evaluate both extent of fibrosis in different organs and amyloid deposition secondary to levels of SAA as potential contributors to excess mortality in RA. It will also be important to determine whether newer therapies not only reduce morbidity but also excess mortality. (50) The substantial excess mortality of anti-CCP in combination with other risk factors supports the need to prevent and control risk factors among RA patients.
This study has several important limitations. The WHI is not a random population-based study. The failure to identify any effect of DMARDs or methotrexate on mortality may be due, in part, to longer duration of disease prior to entry to the WHI and use of selected DMARDs for the more severe and persistent disease. Despite the large sample of minority participants in the overall study sample, the number of anti-CCP+ (n=301) limited detailed race-specific analysis. The lower age-adjusted mortality for Black anti-CCP+ women needs further analysis in larger sample sizes of Black women. Finally, the WHI was not an inception cohort of RA women. We do not know the age of onset of RA, duration of disease or prior treatment before entering the WHI. The mean age of 63 years suggests that most of the women likely had RA for 10 or more years prior to entry to the study. We have no information about men.
In summary, among a large sample of postmenopausal women in the WHI, self-reported RA was associated with higher mortality, but only a small fraction had probable RA defined as anti-CCP+ or anti-CCP-/ DMARD use. The increased risk of death for anti-CCP+ women was not explained by age, RF+, ANA+ or DMARD use. Traditional risk factors (e.g., smoking, diabetes), kidney disease, joint pain, health status, or WBC count also did not statistically explain the increased risk for anti-CCP+, despite their independent associations with higher mortality. Further longitudinal RA studies and clinical trials should focus on identifying the specific determinants of excess mortality, especially among the anti-CCP+ RA population and whether further modification of systemic inflammation and/or reduction in fibrosis and thrombogenesis will decrease mortality.
Supplementary Material
Supplemental Table 1. RA status and baseline variables
Supplemental Table 2. Characteristics of study sample (N=9.988) according to timing of RA report (baseline or follow-up)
Supplemental Table 3. Cause of death by anti-CCP+ or – and DMARD use for women who self-reported RA at baseline or baseline and follow up
Supplemental Table 4. Creatinine levels and RA baseline and percent dead by anti-CCP+/-
Supplemental Figure 1. Sampling Frame For the Women's Health Initiative Study
Supplemental Figure 2. Mortality at 2009 by Age and Ethnicity Groups, Excluding RA at Follow Up Only
Supplemental Figure 3. Age-adjusted Death Rate by Anti-CCP+, DMARD Use and Joint Pain, Among Women Who Have Not Reported RA
Acknowledgments
Grant Support: This work was funded by BAA NHLBI-WH-09-01 Contract No. HHSN268200960006C. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
References
- 1.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35–61. [PubMed] [Google Scholar]
- 2.Watson DJ, Rhodes T, Guess HA. All-cause mortality and vascular events among patients with rheumatoid arthritis, osteoarthritis, or no arthritis in the UK General Practice Research Database. J Rheumatol. 2003;30:1196–202. [PubMed] [Google Scholar]
- 3.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–7. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 4.Gabriel SE, Crowson CS, O'Fallon WM. Mortality in rheumatoid arthritis: have we made an impact in 4 decades? J Rheumatol. 1999;26:2529–33. [PubMed] [Google Scholar]
- 5.Radovits BJ, Fransen J, Shamma AL, Eijsbouts AM, van Reil PLCM, Laan RFJM. Excess mortality emerges after 10 years in an inception cohort of early rheumatoid arthritis. Arthritis Care Res. 2010;62:362–70. doi: 10.1002/acr.20105. [DOI] [PubMed] [Google Scholar]
- 6.Mikuls TR, Saag KG, Criswell LA, Merlino LA, Kaslow RA, Shelton BJ, et al. Mortality risk associated with rheumatoid arthritis in a prospective cohort of older women: results from the Iowa Women's Health Study. Ann Rheum Dis. 2002;61:994–9. doi: 10.1136/ard.61.11.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjornadal L, Baecklund E, Yin L, Granath F, Klareskog L, Ekbom A. Decreasing mortality in patients with rheumatoid arthritis: results from a large population based cohort study in Sweden, 1964-95. J Rheumatol. 2002;29:906–12. [PubMed] [Google Scholar]
- 8.Meune C, Touze E, Trinquart L, Allanore Y. High risk of clinical cardiovascular events in rheumatoid arthritis: Levels of associations of myocardial infarction and stroke through a systematic review and meta-analysis. Arch Cardiovasc Dis. 2010;103:253–61. doi: 10.1016/j.acvd.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Bergstrom U, Jacobsson LTH, Turesson C. Cardiovascular morbidity and mortality remain similar in two cohorts of patients with long-standing rheumatoid arthritis seen in 1978 and 1995 in Malmo, Sweden. Rheumatology. 2009;48:1600–5. doi: 10.1093/rheumatology/kep301. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez A, Kremers HM, Crowson CS, Nicola PJ, Davis JM, III, Therneau TM, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsson LTH, Turesson C, Nilsson JA, Petersson IF, Lindqvist E, Saxne T, et al. Treatment with TNF blockers and mortality risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:670–5. doi: 10.1136/ard.2006.062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikuls TR, Fay BT, Michaud K, Sayles H, Thiele GM, Caplan L, et al. Associations of disease activity and treatments with mortality in men with rheumatoid arthritis: results from the VARA registry. Rheumatology. 2011;50:101–9. doi: 10.1093/rheumatology/keq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology. 2010;49:295–307. doi: 10.1093/rheumatology/kep366. [DOI] [PubMed] [Google Scholar]
- 14.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–7. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 15.Pincus T, Sokka T, Wolfe F. Premature mortality in patients with rheumatoid arthritis: evolving concepts [editorial] Arthritis Rheum. 2001;44:1234–6. doi: 10.1002/1529-0131(200106)44:6<1234::AID-ART213>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 16.Kroot EJ, De Jong BA, van Leeuwen MA, Swinkels H, van den Hoogen FH, van't Hof M, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000;43:1831–5. doi: 10.1002/1529-0131(200008)43:8<1831::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Van der Helm-van Mil AHM, Verpoort KN, Breedveld FC, Toes REM, Huizinga TWJ. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R949–58. doi: 10.1186/ar1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sihvonen S, Korpela M, Mustila A, Mustonen J. The predictive value of rheumatoid factor isotypes, anti-cyclic citrullinated peptide antibodies, and antineutrophil cytoplasmic antibodies for mortality in patients with rheumatoid arthritis. J Rheumatol. 2005;32:2089–94. [PubMed] [Google Scholar]
- 19.Crowson CS, Liang KP, Therneau TM, Kremers HM, Gabriel SE. Could accelerated aging explain the excess mortality in patients with seropositive rheumatoid arthritis? Arthritis Rheum. 2010;62:378–82. doi: 10.1002/art.27194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson A, Diamond B, Mackay IR, Rosen FS. Autoimmune diseases. N Engl J Med. 2001;345:340–50. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 21.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–5. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hueber W, Tomooka BH, Zhao X, Kidd BA, Drijfhout JW, Fries JF, et al. Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-CCP-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann Rheum Dis. 2007;66:712–9. doi: 10.1136/ard.2006.054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–72. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 24.Sihvonen S, Korpela M, Laippala P, Mustonen J, Pasternack A. Death rates and causes of death in patients with rheumatoid arthritis: a population-based study. Scand J Rheumatol. 2004;33:221–7. doi: 10.1080/03009740410005845. [DOI] [PubMed] [Google Scholar]
- 25.Liang KP, Gabriel SE. Autoantibodies: innocent bystander or key player in immunosenescence and atherosclerosis? J Rheumatol. 2007;34:1203–7. [PubMed] [Google Scholar]
- 26.Rantapaa-Dahlqvist S, de Jang BA, Berglin E, Hallman G, Wadell G, Stenlund H. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal R, Liao K, Nair R, Ringold S, Costenbader KH. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2009;61:1472–83. doi: 10.1002/art.24827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiik AS, van Venrooij WJ, Pruijn GJM. All you wanted to know about anti-CCP but were afraid to ask. Autoimmun Rev. 2010;10:90–3. doi: 10.1016/j.autrev.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Walitt B, Mackey RH, Kuller LH, Deane KD, Robinson W, Holers VM, et al. Predictive value of autoantibody testing for validating self-reported diagnoses of rheumatoid arthritis in the Women's Health Initiative. Am J Epidemiol. 2013;177:887–893.30. doi: 10.1093/aje/kws310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Women's Health Initiative Study Group. Design of the Women's Health Initiative Clinical Trial and Observational Study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 31.Lynch CP, McTigue KM, Bost JE, Tinker LF, Vitolins M, Adams-Campbell L, et al. Excess weight and physical health-related quality of life in postmenopausal women of diverse racial/ethnic backgrounds. J Womens Health. 2010;19:1449–58. doi: 10.1089/jwh.2009.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McTigue KM, Chang Y, Eaton C, Garcia L, Johnson KC, Lewis CE, Liu S, Mackey RH, Robinson J, Rosal MC, Snetselaar L, Valoski A, Kuller L. Severe obesity, heart disease and death among white, African-American and Hispanic postmenopausal women. Obesity. 2012 doi: 10.1002/oby.20224. In Press. [DOI] [PubMed] [Google Scholar]
- 33.Eaton CB, Young A, Allison MA, Robinson J, Martin LW, Kuller LH, et al. Prospective association of vitamin D concentrations with mortality in postmenopausal women: results from the Women's Health Initiative (WHI) Am J Clin Nutr. 2011;94:1471–8. doi: 10.3945/ajcn.111.017715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoh M, Chan EKL, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64:2319–27. doi: 10.1002/art.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padyukov L, Seieslastad M, Ong RTH, Ding B, Ronnelid J, Seddighzadeh M, et al. the Epidemiological Investigation of Rheumatoid Arthritis (EIRA) study group. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann Rheum Dis. 2011;70:259–65. doi: 10.1136/ard.2009.126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenberg JD, Kremer JM, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Corrona Investigators. Tumour necrosis factor antagonist use associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:576–82. doi: 10.1136/ard.2010.129916. [DOI] [PubMed] [Google Scholar]
- 37.Wasko MCM, Dasgupta A, Hubert H, Fries JF, Ward MM. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013:334–42. doi: 10.1002/art.37723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7:332–9. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 39.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sattar N, McCarey DW, Capell H, Mclnnes IB. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–63. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 42.Connolly M, Mullan RH, McCormick J, Matthews C, Sullivan O, Kennedy A, et al. Acute-phase serum amyloid A regulates tumor necrosis factor α and matrix turnover and predicts disease progression in patients with inflammatory arthritis before and after biologic therapy. Arthritis Rheum. 2012;64:1035–45. doi: 10.1002/art.33455. [DOI] [PubMed] [Google Scholar]
- 43.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weyand CM, Fulbright JW, Goronzy JJ. Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp Gerentol. 2003;38:833–41. doi: 10.1016/s0531-5565(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 45.Michel JJ, Turesson C, Lemster B, Atkins SR, Iclozanb C, Bongartz T, et al. CD56-expressing T cells that have features of senescence are expanded in rheumatoid arthritis. Arthritis Rheum. 2007;56:43–57. doi: 10.1002/art.22310. [DOI] [PubMed] [Google Scholar]
- 46.Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380:689–98. doi: 10.1016/S0140-6736(12)61079-4. [DOI] [PubMed] [Google Scholar]
- 47.Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52:412–20. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 48.Young A, Koduri G, Batley M, Kulinskaya E, Gough A, Norton S, et al. Mortality in rheumatoid arthritis Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology. 2007;46:350–7. doi: 10.1093/rheumatology/kel253. [DOI] [PubMed] [Google Scholar]
- 49.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 2007;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 50.Wong TC, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 166:1206–16. doi: 10.1161/CIRCULATIONAHA.111.089409. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. RA status and baseline variables
Supplemental Table 2. Characteristics of study sample (N=9.988) according to timing of RA report (baseline or follow-up)
Supplemental Table 3. Cause of death by anti-CCP+ or – and DMARD use for women who self-reported RA at baseline or baseline and follow up
Supplemental Table 4. Creatinine levels and RA baseline and percent dead by anti-CCP+/-
Supplemental Figure 1. Sampling Frame For the Women's Health Initiative Study
Supplemental Figure 2. Mortality at 2009 by Age and Ethnicity Groups, Excluding RA at Follow Up Only
Supplemental Figure 3. Age-adjusted Death Rate by Anti-CCP+, DMARD Use and Joint Pain, Among Women Who Have Not Reported RA


