Abstract
A critical limitation in the cultivation of cartilage for tissue engineering is the dedifferentiation in chondrocytes, mainly during in vitro amplification. Despite many previous studies investigating the influence of various conditions, no data exist concerning the effects of hypothermia. Our aim has been to influence chondrocyte dedifferentiation in vitro by hypothermic conditions. Chondrocytes were isolated from cartilage biopsies and seeded in monolayer and in three-dimensional pellet-cultures. Each cell culture was either performed at 32.2°C or 37°C during amplification. Additionally, the influence of the redifferentiation of chondrocytes in three-dimensional cell culture was examined at 32.2°C and 37°C after amplification at 32.2°C or 37°C. An 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay was used to measure cell proliferation in monolayer, whereas the polymerase chain reaction and immunohistochemical and histological staining were used in three-dimensional pellet-cultures. Real-time polymerase chain reaction was employed to measure the relative expression of the target genes collagen II, collagen I, aggrecan and versican. Ratios were estimated between collagen II/collagen I and aggrecan/versican to evaluate differentiation. A higher value of these ratios indicated an advantageous status of differentiation. In monolayer, hypothermia at 32.2°C slowed down the proliferation rate of chondrocytes significantly, being up to two times lower at 32.2°C compared with culture at 37°C. Simultaneously, hypothermia in monolayer decelerated dedifferentiation. The ratio of aggrecan/versican was significantly higher at 32.2°C compared with that at 37°C. In three-dimensional pellet-culture, the chondrocytes redifferentiated at 32.2°C and at 37°C, and this process is more distinct at 37°C than at 32.2°C. Similar results were obtained for the ratios of collagen II/collagen I and aggrecan/versican and were supported by immunochemical and histological staining. Thus, hypothermic conditions for chondrocytes are mainly advantageous in monolayer culture. In three-dimensional pellet-culture, redifferentiation predominates at 37°C compared with at 32.2°C. In particular, the results from the monolayer cultures show potential in the avoidance of dedifferentiation.
Keywords: Cartilage, hypothermia, differentiation, redifferentiation, pellet-culture
Introduction
Despite current examples of the clinical application of extensive cartilage tissue engineering products, for example, tracheal reconstruction1 or nasal augmentation,2 these methods are experimental and far distant from general clinical use. Before the establishment of new standards in reconstruction, problems have to be solved concerning the long-term stability and survival of such tissues and, in particular, with regard to the quality of the cells that form the newly designed product.3,4 A major problem in using chondrocytes as a cell source in tissue engineering is dedifferentiation. This process appears during in vitro amplification undertaken in order to obtain large amounts of chondrocytes. Many attempts have been made to prevent the dedifferentiation process or at least to induce a redifferentiation process, with most trying to simulate a more physiological environment for cell growth in vitro. To date, the use of bio-reactors for cell growth,5–7 biological scaffolds made of collagen8 or biomechanical optimization3 have been described. Moreover, the physiological–mechanical stimulation of cartilage under culture conditions has been shown to improve cartilage quality significantly.
A study by Lindemann et al.9 has indicated that the temperature within nasal cartilage has a mean of 32.2°C, which is below the mean core body temperature of 37°C. The aim of this study has therefore been to examine the influence of hypothermia during the amplification period of chondrocytes with regard to the grade of dedifferentiation, that is, to determine whether a temperature of 32.2°C during amplification stops or retards the dedifferentiation process, in contrast to a temperature of 37°C during amplification. Additionally, we have examined the influence of the redifferentiation of chondrocytes in three-dimensional (3D) cell culture at 32.2°C and 37°C.
Materials and methods
All reagents, cell culture media, and supplements were purchased from Sigma-Aldrich (Munich, Germany), Invitrogen (Darmstadt, Germany), and Biochrom (Berlin, Germany), unless indicated otherwise. Cell culture material was obtained from Greiner Bio-One GmbH (Frickenhausen, Germany).
Cartilage samples
Cartilage biopsies were collected during septum or septum rhinoplasty operations at the Department of Oto-Rhino-Laryngology, Head and Neck Surgery at the University of Ulm. The biopsies were collected after the patients’ written consent had been obtained and the protocol was approved by the local ethics committee (University of Ulm, no.152/08). All patients were anonymized, although age and gender were recorded. The cells from a donor were only used in this experiment if there could enough cells be isolated to analyse them on each point of time.
The biopsies were transported to the laboratory in 0.9% sodium chloride (B. Braun, Melsungen, Germany). On arrival, each biopsy was cut into smaller pieces (approximately 1 × 1 mm); this was followed by digestion in standard culture medium Dulbecco’s modified Eagle’s medium and Ham’s F-12 nutrient mixture (DMEM/HAMS F-12; 10% standardized foetal bovine serum (FBS); 0.5% gentamicin) with 0.3% collagenase II (Worthington Biochemical Corporation, Lake Wood, USA) for 16 h at 37°C in a waterbath. The cells were then washed in standard culture medium, counted using a Neubauer counting chamber, cryopreserved for later use with freezing media (DMEM/HAMS F-12; 40% foetal calf serum (FCS); 20% dimethyl sulfoxide (DMSO)) and stored in liquid nitrogen.
Cell culture
After isolation, thawing or trypsinization (see below), the cells of each patient were seeded at a density of 5000 cells/cm2 in standard culture medium (as mentioned above) in a tissue-culture flask to start monolayer culture or were centrifuged (1000g) into pellets at a density of 25 × 104 cells and placed for 48 h in differentiation culture media (high-glucose DMEM; 50 µg/mL l-ascorbic acid in phosphate-buffered saline (PBS); 2.5 µg/mL human insulin; 46 µg/mL proline in PBS; 10% FBS and 0.5% Gentamicin) in a 1.5 mL reaction tube to start a 3D pellet-culture. The pellets were allowed to stabilize for 48 h in the tubes and were then transferred into 24-well plates. Depending on the experiment, each cell culture was incubated either at 32.2°C or at 37°C, but always under 5% CO2 and humidified conditions. The culture media were exchanged after 3–4 days. Cell growth in monolayer was analysed at 32.2°C and 37°C by MTS assay (Promega, Madison, USA) and was measured at 490 nm absorption using the microplate reader infinity M200 (Tecan, Maennedorf, Switzerland) in accordance with the manufacturer’s instructions. In 3D pellet-cultures, the diameters of the pellets among the various groups were captured via a light microscope.
Monolayer culture
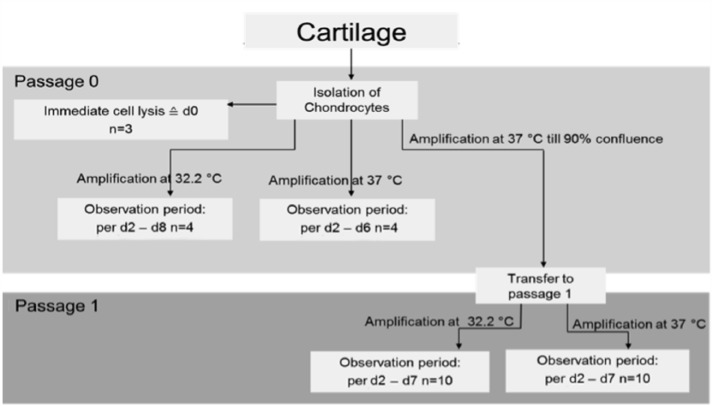
In monolayer, isolated chondrocytes from each patient were cultured separately in standard culture media (as mentioned above) until they reached ~90% confluence (passage 0). At passage 1, cells underwent trypsinization using 0.05% trypsin and 0.02% ethylenediaminetetraaceticacid (EDTA) in PBS for 5 min to create passage 1 monolayers or passage 1 pellets (see below). An overview of the experiment in monolayer is shown in Figure 1.
Figure 1.
The three main parts of the experiment in monolayer. Key: n = number of examined patients, d = harvest day. Initially, the chondrocytes of three patients underwent the lysis process directly after isolation. The cells were then examined at passage 0; cells were grouped equally into two populations, grown at 32.2°C and 37°C and harvested when confluence was expected. The cells were also examined in passage 1; cells were grouped equally into two populations, grown at 32.2°C and 37°C and harvested when confluence was expected.
3D pellet-culture
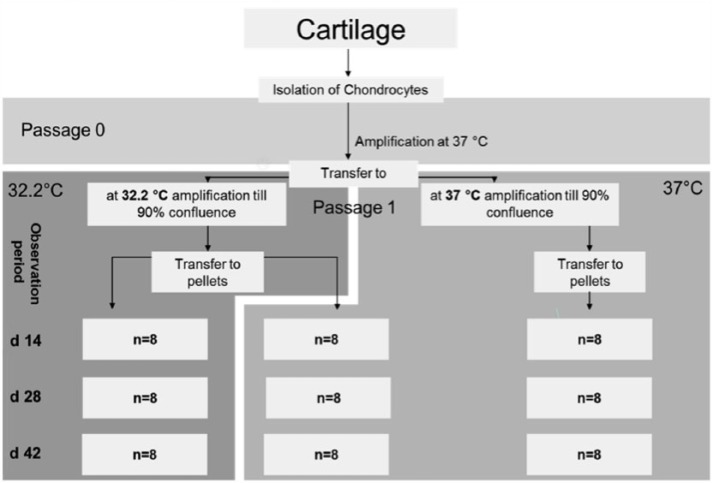
In the 3D pellet-culture models, each pellet was cultivated separately in differentiation culture media (as mentioned above) in the well of a 24-well plate. Examination took place at passage 0 and passage 1. For the passage 0 experiment, which was started immediately after cell isolation, chondrocytes of 7 patients were pooled to obtain enough cells and centrifuged (as mentioned above) into passage 0 pellets. They were divided into two groups equally at 32.2°C and 37°C. The examination points for harvesting were day 14, day 28 and day 42. In the passage 1 pellet experiment, which was started after transfer to passage 1, chondrocytes were divided into two groups equally at 32.2°C and 37°C and grown to confluence. Because of the amplification process, each patient could be treated separately. At confluence, chondrocytes were trypsinized, centrifuged to pellets and regrouped at 32°C and 37°C. The points for harvesting for the cell lysis process or for obtaining a preparation sample were day 14, day 28 and day 42. An overview of the 3D pellet-culture experiment is shown in Figure 2.
Figure 2.
Experiment structure for pellet-culture, passage 1. Key: n = number of examined patients, d = harvest day. Initially, passage 1 cells were divided equally into groups at 32.2°C and 37°C, amplified to confluence and then centrifuged into pellets. The pellets were separated into three groups. In the first group, the cells were amplified at 32.2°C followed by pellet-culture at 32.2°C. In the second group, the cells were amplified at 32.2°C followed by pellet-culture at 37°C. In the third group, the cells were amplified at 37°C followed by pellet-culture at 37°C. The points in time for pellet harvest were days 14, 28 and 42.
Cell lysis and extraction of RNA
After the observation period, culture medium was removed. The chondrocytes in monolayers were lysed in flasks by the addition of 600 µL RLT lysis puffer (Qiagen, Hilden, Germany) in 1% ß-mercaptoethanol (Sigma-Aldrich) and mechanically broken open with a cell scraper (Greiner Bio-One GmbH). The pellets from the 3D pellet-cultures were each transferred into a 2 mL safe-seal tube and snap-frozen. They were then lysed in the same lysis solution as the cells from the monolayers but were mechanically broken open with a Tissue Lyser (Qiagen, Hilden, Germany). Total RNA was isolated and purified using an RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The RNA concentration was measured spectrophotometrically in a 96-well plate and the RNA quality analysis was measured by absorption at 260 and 280 nm. RNA was adjusted to a concentration of 50 ng/μL and kept at −20°C. All measurements were performed using a microplate reader infinity M200 (Tecan, Maennedorf, Switzerland) in accordance with the manufacturer’s instructions.
Real-time polymerase chain reaction
The relative expression of the target genes collagen II (COL2A1), collagen I (COL1A1), aggrecan (ACAN) and versican (VCAN) was measured in relation to the corresponding housekeeping gene GAPDH via a one-step polymerase chain reaction (PCR; Light Cycler 2.0, Roche, Basel, Switzerland) as described elsewhere.10 An overview of the used primer and probes is shown in Table 1. Relative gene expression was calculated using the 2−ΔΔCT formula.
Table 1.
Base sequences of the used primers (TIB Molbiol, Berlin) and the corresponding catalogue numbers of the used probes (Universal Probe Library, Roche, Basel, Switzerland).
| Genes | Probe | Primer left | Primer right |
|---|---|---|---|
| Housekeeping gene | |||
| GAPDH | #60 | gctctctgctcctcctgttc | acgaccaaatccgttgactc |
| Target genes | |||
| Collagen type I (Col1A1) | #15 | atgttcagctttgtggacctc | ctgtacgcaggtgattggtg |
| Collagen type II (Col2A1) | #19 | ccctggtcttggtggaaac | tccttgcattactcccaactg |
| Aggrecan (hAGCN) | #79 | tgcagctgtcactgtagaaactt | atagcaggggatggtgagg |
| Versican | #54 | gcacctgtgtgccaggata | cagggattagagtgacattcatca |
Immunohistochemical and histological staining
Harvested pellets were fixed in 3.5% neutral buffered formalin (Fischer), embedded in paraffin, and sectioned at 3–5 μm. Sections were heat fixed at 56°C for 24 h. Prior to staining, the sections were deparaffinized and rehydrated.10
Immunohistochemical staining for collagen type I (anti-rat: ab34710, dilution 1:1000; Abcam, Cambridge, UK), collagen type II (anti-rat; dilution 1:4000; Developmental Studies Hybridoma Bank) and aggrecan (anti-rat, dilution 1:100; Millipore) was performed using LSAB + System-HRP (Dako, Agilent, Santa Clara, USA) and AEC chromogen (Dako, Agilent, Santa Clara, USA) as described elsewhere.10
Histological staining with Alcian blue (Carl Roth, Karlsruhe, Germany) and with haematoxylin and eosin (Carl Roth, Karlsruhe, Germany) was carried out as previously described.10
Statistical analysis
A regression coefficient in a mixed linear model was formed to evaluate gene ratios independent of day and temperature. Other values were analysed with the two-tailed Mann–Whitney test using GraphPad Prism (version 6.0b for Mac OS X; GraphPad Software, La Jolla, CA, USA). Values were considered significant when p < 0.05 (*), medium significant when p < 0.01 (**), highly significant when p < 0.001 (***) and of the highest significance when p < 0.0001 (****).
Results
Cartilage samples
The number of isolated chondrocytes from each biopsy was on average 1.3 × 106 (standard deviation: 9 × 105). In passage 0, four samples and in passage 1, 10 samples were evaluated on each day in each group.
Monolayer culture
Observation at phase contrast showed, in monolayers, slower cell growth at passage 0 compared with passage 1 chondrocytes. Within the passages, cell growth at 32.2°C was always slower compared with that at 37°C. The proliferation rate of septum chondrocytes at 32.2°C is up to two times lower compared with their culture at 37°C. This is supported by observations during the culture periods among passage 0 and passage 1 and the results of the MTS assay in passage 0 and passage 1. The time point at which 90% confluence in monolayer is reached appears to be comparable among patients and within the same temperature. Cell survival between passages and temperatures was comparable. These observations were confirmed by absorption measurements during MTS assay (Promega, Madison, USA), as shown in Figure 3.
Figure 3.
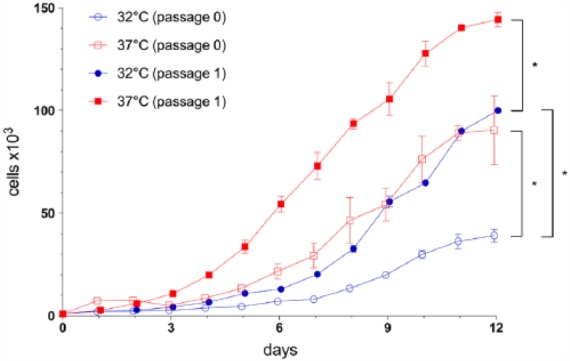
Mean growth curve with standard deviation of chondrocytes in monolayer. Key: d = measure days, cells = number of cells detected. Cell growth of chondrocytes in passage 0 is compared with that at passage 1 and among the different temperature conditions, namely, at 32.2°C and at 37°C; absorption measurements were obtained by MTS (Promega, Madison, USA).
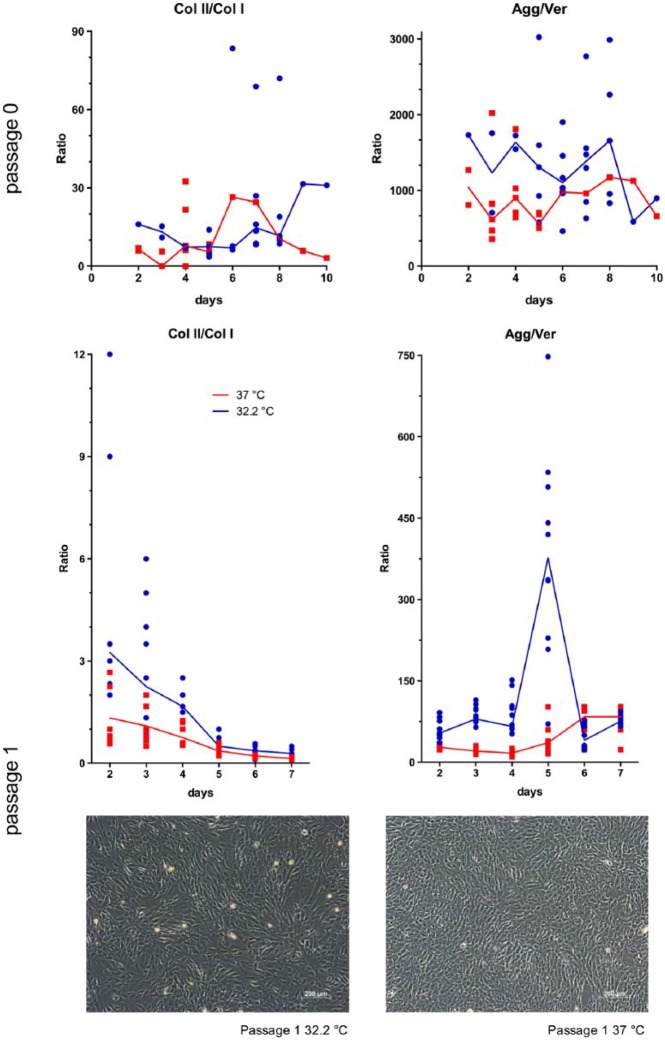
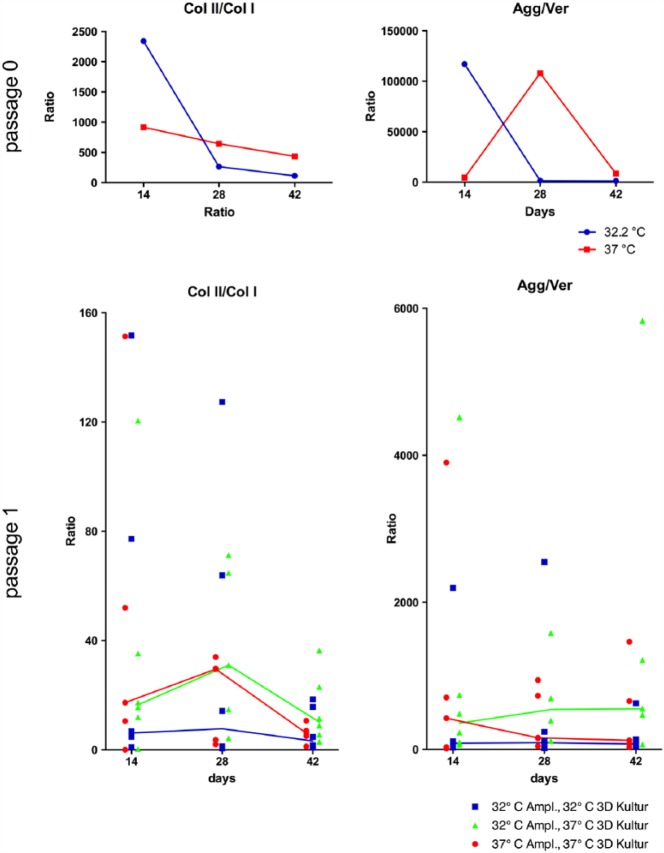
The relative expression of the target genes collagen II (COL2A1), collagen I (COL1A1), aggrecan (ACAN) and versican (VCAN) was evaluated by means of the ratios between collagen II/collagen I (Col II/Col I) and aggrecan/versican (Agg/Ver). A higher value of these ratios indicates an advantageous status of differentiation.11–13 No significant change occurred in the ratio of Agg/Ver during culture, but this ratio was significantly higher at 32.2°C compared with that at 37°C. The ratio of Col II/Col I independent of temperature worsened significantly over time. Independent of time, the difference between the temperatures was not significant. The results were similar in passage 0 and passage 1. Overviews of the median collagen II/collagen I and aggrecan/versican ratios in monolayer are displayed in Figure 4.
Figure 4.
Median ratio curve of collagen II/collagen I (Col II/Col I) and aggrecan/versican (Agg/Ver) in monolayers among the various passages and at temperatures of 32.2°C and 37°C. The median behaviour of these ratios over the measured time (days) is shown at 32.2°C compared with 37°C. Each ratio and median are presented. In the bottom, phase-contrast images of passage 1 cells are presented at 32.2°C and 37°C after 5 days of culture.
3D pellet-culture
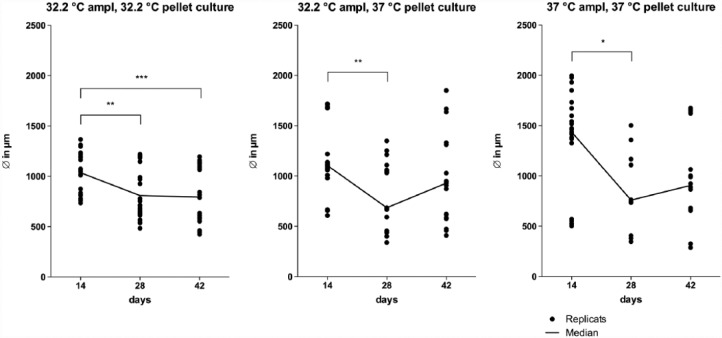
The pellets cultured under various temperatures differed in size. The diameters after 14 days were smallest in 32.2°C amplification following 32.2°C pellet-culture, the 32.2°C amplified, and then, 3D cultured pellets at 37°C showed a bigger diameter which was stable in size from 28 to 42 days. Biggest diameters could be found using 37°C amplification and 37°C pellet-culture. Among all models, a significant median down-swing could be seen. Diameters of the 32.2°C amplified and then 3D cultured pellets at 37°C and the 37°C amplified and cultured pellets were not significantly different after 42 days. An overview of the median pellet diameter behaviour is shown Figure 5.
Figure 5.
Median diameter curve of pellets among different culture models in passage 1. Key: d = days when measured, ∅ = diameter in micrometer, ampl = amplification. Each diameter and the median are presented.
The median behaviour of Col II/Col I in passage 0 pellet shows, an increase from day 14 to day 28, following a decrease until day 42. This increase and decrease were most distinct in the pellet-culture models that were incubated during pellet-cultivation at 37°C. This indicates a tendentially stronger redifferentiation of chondrocytes at 37°C during pellet-culture up to day 28 and following dedifferentiation to day 42, but changes were not significant.
In passage 1 pellets, the median behaviour of Agg/Ver showed, among harvest days from cultures with 32.2°C amplification, 32.2°C pellet-culture, a nearly steady state. Under culture conditions of 32.2°C amplification, 37°C pellet-culture, Agg/Ver increased till day 28 and stayed constant at day 42. Under culture condition of 37°C amplification, 37°C pellet-culture, a decrease occurred at day 28 and the level then remained at almost same level at day 42. Under culture conditions of 32.2°C amplification, 37°C pellet-culture, an increase in the RNA level concerning differentiation grade was noted. Overviews of the median collagen II/collagen I and aggrecan/versican ratios in 3D passage 0 and passage 1 pellet-culture are displayed in Figure 6.
Figure 6.
Median ratio curve of collagen II/collagen I (Col II/Col I) and aggrecan/versican (Agg/Ver) in three-dimensional (3D) pellet-culture, among the various passages and at temperatures of 32.2°C and 37°C in passage 0 and of the various culture models in passage 1. Passage 1 pellet-culture models: amplification at 32.2°C, pellet-culture at 32.2°C; amplification at 32.2°C, pellet-culture at 37°C; and amplification at 37°C, pellet-culture at 37°C. The median behaviour of these ratios is shown over the measured time (days) at 32.2°C compared with 37°C in passage 0; in passage 1, the various pellet-culture models (as mentioned above) are compared. Each ratio and median are presented.
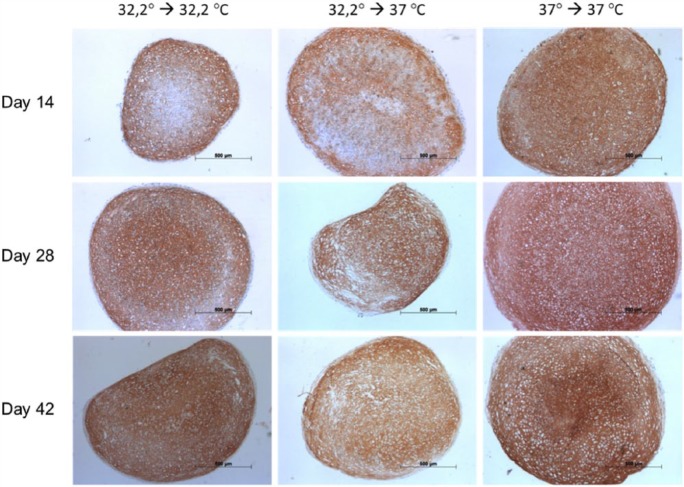
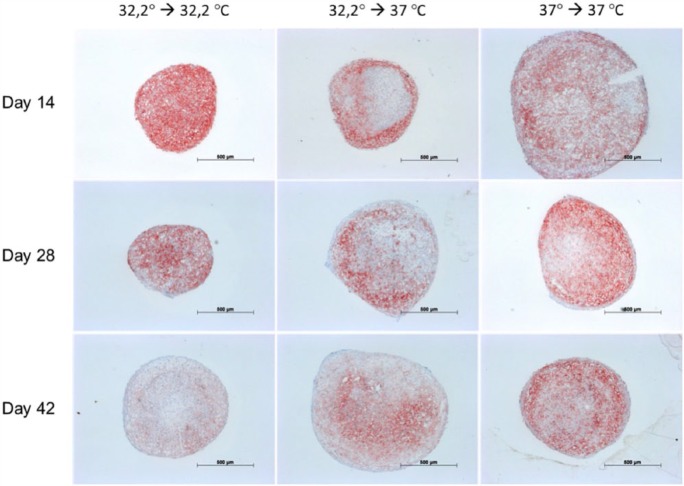
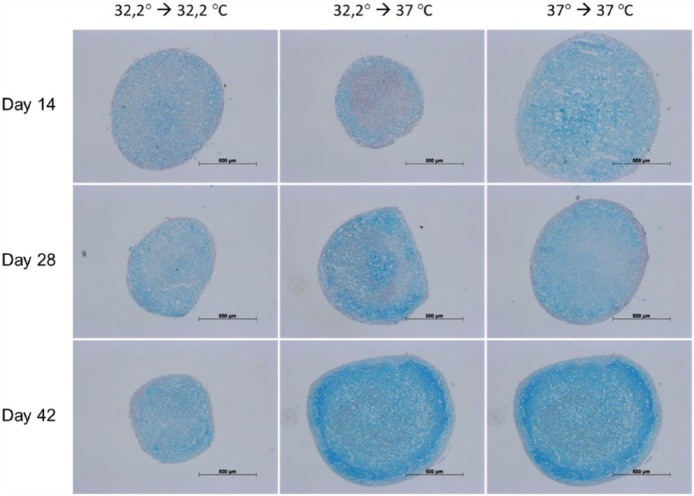
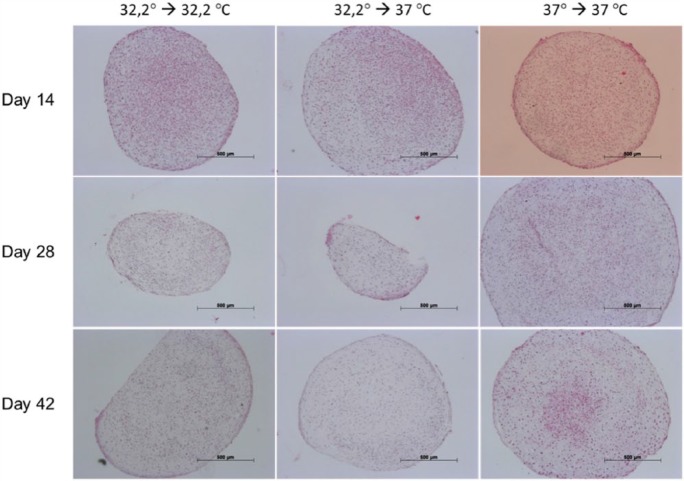
Immunohistochemical staining for collagen II (Figure 7) showed a steady increase during the investigation period and was most distinct in culture involving 32.2°C amplification, 32.2°C pellet-culture and 37°C amplification, 37°C pellet-culture. Additional culture at 37°C during amplification and 37°C during pellet-culture showed augmented lacuna development comparable with that of native cartilage. The concomitant immunohistochemical staining for collagen I (Figure 8) showed a steady decrease during the investigation period in culture, namely, 32.2°C amplification, 32.2°C pellet-culture and 32.2°C amplification, 37°C pellet-culture. This observation was most distinct under culture conditions of 32.2°C amplification, 32.2°C pellet-culture. Under culture conditions of 37°C amplification, 37°C pellet-culture, the staining collagen I was almost steady. The increase or decrease in staining was more discrete for aggrecan (Figure 9) than that for collagen II and collagen I. All culture models showed intense staining among harvesting points. Alcian blue (Figure 10) and haematoxylin and eosin staining (Figure 11) confirmed our findings.
Figure 7.
Collagen II.
Figure 8.
Collagen I.
Figure 9.
Aggrecan.
Figure 10.
Alcian blue.
Figure 11.
Haematoxylin and eosin.
Figures 7–11 show immunohistochemical pellet staining of collagen II (Figure 7), collagen I (Figure 8) and aggrecan (Figure 9) and histological staining with Alcian blue (Figure 10) and haematoxylin and eosin (Figure 11) of the various pellet-culture models on days 14, 28 and 42. (Key: 32.2° → 32.2°C: amplification at 32.2°C, pellet-culture at 32.2°C; 32.2° → 37°C: amplification at 32.2°C, pellet-culture at 37°C; 37° → 37°C: amplification at 37°C, pellet-culture at 37°C.)
Discussion
Dedifferentiation in chondrocytes is a crucial limitation factor in the cultivation of cartilage under the principles of tissue engineering. This process mainly takes place during amplification in monolayer and increases over cultivation time.14 However, dedifferentiation is not irreversible and chondrocytes maintain redifferentiation. Redifferentiation is mainly induced by a stimulus in 3D culture under physiological conditions.15–17 Although redifferentiation takes place, it is an incomplete process, and to date, no cartilage can be cultured with comparable quality to that of native cartilage. The aim of this study has been to attempt to influence chondrocyte dedifferentiation in vitro by hypothermic conditions. A positive effect can be expected, because in vivo elastic cartilage is found in regions that are physiologically cooler than 37°C. We have further examined various passages both in monolayer and in 3D pellet-cultures.
Independent of the various culture systems that try to induce a physiological stimulus,18–21 controllable and adjustable conditions in established culture systems are of interest. Wilkins and Hall22 showed a 50% higher matrix synthesis of chondrocytes cultured at pH values between 7.1 and 7.4. Obradovic et al.23 could culture more cartilage-like tissue in aerobic metabolism by changing only 50% of the culture medium once per week. Yodmuang et al.11 found a higher level of collagen II und aggrecan under transient hypoxia. Nevertheless, despite the many publications with regard to the influence of such adjustable conditions, no data are currently available concerning the influence of hypothermia on the grade of dedifferentiation of chondrocytes.
In addition to its possible use in tissue engineering, mild hypothermia is of special interest, for example, in the commonly accepted observation that mild-to-moderate hypothermia (30°C–36°C) has a better outcome in various brain insults.24,25 These circumstances and the knowledge of the mean mucosal nose temperature in vivo at 32.2°C9 has led to the hypothesis that mild hypothermia might have a significant influence on septum cartilage and thus on the grade of differentiation.
This study has shown sufficient proliferation of chondrocytes at 32.2°C in monolayer. The results from the MTS assay indicate that the different proliferation rates are attributable to a slower metabolism rate in the cells at 32.2°C.
The ratios between Col II/Col I and Agg/Ver in our monolayer showed that chondrocytes cultured at 32.2°C also underwent dedifferentiation. However, hypothermia in monolayer decelerates dedifferentiation. This is indicated by the median behavior that resembles but that is time-delayed with respect to that of monolayer-cultured chondrocytes at 37C and their ratios of Col I/II and Agg/Ver (Figure 4).
A regression coefficient in a mixed linear model was formed to evaluate ratios independent of day and temperature. This reassessment was necessary as data from different cell numbers would have been compared because of the different proliferation rates. In monolayer, this adjustment showed that no significant change occurred in the ratio of Agg/Ver during culture, but that this ratio was significantly higher at 32.2°C compared with that at 37°C. The ratio of Col II/Col I independent of temperature changed significantly over time. Independent of time, the difference between the temperatures was not significant. The results were similar in passage 0 and passage 1 and indicate the similar behaviour of differentiation in both passages. This is of special interest because we were unable to carry out the same number of experimental replications in passage 0 as in passage 1. This circumstance was unavoidable both in monolayer and in 3D pellet-culture, because only a limited amount of donor cartilage per day and, therefore, only a limited number of cells could be collected. Additionally, the quantity of cells isolated from each donor cartilage varied. Because of the different differentiation capacity in different donors, they were only included into the experiment when the number of cells was sufficient to evaluate them on every time point and in every method from one donor. Thus, a special model for passage 0 was formed. We estimated the time point when ~90% confluence was reached and performed a higher number of repeats around this time. Early culture times were not investigated because of the lack of clinical relevance. Cell harvesting before ~90% confluence implies fewer cells, whereas harvesting after ~90% of confluence implies an intolerable grade of dedifferentiation.
Despite the results in monolayer being promising, this culture model is only an interim stage for clinical use, being essential for the sufficient and extensive proliferation of chondrocytes. A real useable structure emerges in a 3D model, which is required for redifferentiation. To date, many scaffolds, their materials, shapes and properties have been described,26 but the exact mechanism of the way that a scaffold influences redifferentiation is not clear.3,27 A redifferentiation process also takes place in pellets and cell aggregates that form a stable network.17 Pellet models allow investigations into cellular processes without the influence of a scaffold or of seeding processes.28 Commonly, these pellets are formed from 1–5 × 105 chondrocytes that are incubated at the presence of transforming growth factor beta (TGF-ß)29 or other growth factors and that reach a size of 1–2 mm. Despite this size being critical for a sufficient supply of nutrients throughout the pellet and matrix synthesis and the redifferentiation varying within pellets, macropellets represent a gold standard in investigations because of the redifferentiation in chondrocytes.28 In our study, the pellets had a mean density of 2.5 × 105 cells. To investigate the influence of amplification and 3D culture at various temperatures, three culture models were generated in total: amplification at 32.2°C, with following pellet-culture at 32.2°C; amplification at 32.2°C, with following pellet-culture at 37°C and amplification at 37°C, with following pellet-culture at 37°C.
PCR and the immunohistochemical and histological staining of the pellets provide no evidence of proliferation in the 3D pellet-culture. The diameter behaviour within the various culture models can only give hints of proliferation. Although, among all models, a median down-swing can be seen, no evidence for apoptosis processes has been found following histological staining. Therefore, this indicates, instead, the occurrence of contraction processes within the pellet.
Chondrocytes were found to redifferentiate at 32.2°C and 37°C. This process was more distinct at 37°C than at 32.2°C. These conclusions are based, on one hand, on the results of the ratios of Col II/Col I and Agg/Ver in passage 0 and passage 1. On the other hand, the results from immunochemical staining in passage 1 are also supported by those from the histological staining in passage 1.
None of the changes in the ratios in 3D pellet-cultures in passage 0 were significant because of the small number of replicates. A comparison between the passages was more challenging compared with that for the monolayer experiment because fewer repetitions were possible as described above. The alternative would have been frozen cells that influence differentiation as well or animal cells that has less clinical relevance. Although PCR can give hints about the cellular differentiation grade of chondrocytes, this does not necessarily correlate with the existing protein within the pellet. These proteins, however, define the quality of newly designed products and are therefore the norm for clinical use. Thus, the differentiation grade is best interpreted with a combination of PCR and immunohistochemical or histological staining.
We have seen a wide variety of values, especially in passage 0. The reason for this could be the different differentiation capacity of septum chondrocytes, dependent on donor site within the septum and the age of the donor patient.30
Hypothermic conditions for chondrocytes seem mainly to be advantageous in monolayer culture. In a 3D pellet-culture, as performed in this study, redifferentiation predominates at temperatures at 37°C compared with culture at 32.2°C.
Although hypothermic conditions involve disadvantages with regard to proliferation speed, this can be advantageous for clinical use in particular cases. For instance, after cell biopsy, the health of a patient does not allow the early implantation of designed tissues. Hypothermia at 32.2°C slows down the proliferation rate of cells significantly; simultaneously, it has a benefit concerning differentiation, as shown by the ratio for Agg/Ver. Thus, chondrocytes can be kept for a longer period in monolayer at 32.2°C, with no risk of proceeding dedifferentiation, which occurs when they are cultured at 37°C. This permits a more flexible passage to a 3D model and hence a longer reaction time when unexpected delays occur.
The results from the monolayer cells stimulate new considerations with regard to stopping dedifferentiation, for example, further investigation with temperature ranges other than 32.2°C and 37°C. Because many studies have shown that a dynamic load on cartilage constructs has an impact leading to a better differentiation grade,31,32 a dynamic temperature change rather than a constant temperature might improve the grade of differentiation. Moreover, a combination of hypoxia and hypothermia might be of interest in investigations of an additive effect.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1. Walles T. Tracheobronchial bio-engineering: biotechnology fulfilling unmet medical needs. Adv Drug Deliv Rev 2011; 63: 367–374. [DOI] [PubMed] [Google Scholar]
- 2. Yanaga H, Koga M, Imai K, et al. Clinical application of biotechnically cultured autologous chondrocytes as novel graft material for nasal augmentation. Aesthetic Plast Surg 2004; 28: 212–221. [DOI] [PubMed] [Google Scholar]
- 3. Schuh E, Hofmann S, Stok KS, et al. The influence of matrix elasticity on chondrocyte behavior in 3D. J Tissue Eng Regen Med 2012; 6: e31–e42. [DOI] [PubMed] [Google Scholar]
- 4. Von Bomhard A, Veit J, Bermueller C, et al. Prefabrication of 3D cartilage constructs: towards a tissue engineered auricle – a model tested in rabbits. PLoS ONE 2013; 8: e71667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arnold HJ, Müller M, Waldhaus J, et al. A novel buoyancy technique optimizes simulated microgravity conditions for whole sensory organ culture in rotating bioreactors. Tissue Eng Part C Methods 2010; 16: 51–61. [DOI] [PubMed] [Google Scholar]
- 6. Mantero S, Sadr N, Riboldi SA, et al. A new electro-mechanical bioreactor for soft tissue engineering. J Appl Biomater Biomech 2007; 5: 107–116. [PubMed] [Google Scholar]
- 7. Santoro R, Olivares AL, Brans G, et al. Bioreactor based engineering of large-scale human cartilage grafts for joint resurfacing. Biomaterials 2010; 31: 8946–8952. [DOI] [PubMed] [Google Scholar]
- 8. Lee H, Yeo M, Ahn S, et al. Designed hybrid scaffolds consisting of polycaprolactone microstrands and electrospun collagen-nanofibers for bone tissue regeneration. J Biomed Mater Res B Appl Biomater 2011; 97: 263–270. [DOI] [PubMed] [Google Scholar]
- 9. Lindemann J, Leiacker R, Rettinger G, et al. Nasal mucosal temperature during respiration. Clin Otolaryngol Allied Sci 2002; 27: 135–139. [DOI] [PubMed] [Google Scholar]
- 10. Bermueller C, Schwarz S, Elsaesser AF, et al. Marine collagen scaffolds for nasal cartilage repair: prevention of nasal septal perforations in a new orthotopic rat model using tissue engineering techniques. Tissue Eng Part A 2013; 19: 2201–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yodmuang S, Gadjanski I, Chao PH, et al. Transient hypoxia improves matrix properties in tissue engineered cartilage. J Orthop Res 2013; 31: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malda J, Van Blitterswijk CA, Van Geffen M, et al. Low oxygen tension stimulates the redifferentiation of dedifferentiated adult human nasal chondrocytes. Osteoarthritis Cartilage 2004; 12: 306–313. [DOI] [PubMed] [Google Scholar]
- 13. Martin I, Jakob M, Schafer D, et al. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage 2001; 9: 112–118. [DOI] [PubMed] [Google Scholar]
- 14. Von der Mark K, Gauss V, Von der Mark H, et al. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 1977; 267: 531–532. [DOI] [PubMed] [Google Scholar]
- 15. Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982; 30: 215–224. [DOI] [PubMed] [Google Scholar]
- 16. Bonaventure J, Kadhom N, Cohen-Solal L, et al. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res 1994; 212: 97–104. [DOI] [PubMed] [Google Scholar]
- 17. Jakob M, Demarteau O, Schafer D, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem 2001; 81: 368–377. [DOI] [PubMed] [Google Scholar]
- 18. Haugh MG, Meyer EG, Thorpe SD, et al. Temporal and spatial changes in cartilage-matrix-specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Eng Part A 2011; 17: 3085–3093. [DOI] [PubMed] [Google Scholar]
- 19. Heyland J, Wiegandt K, Goepfert C, et al. Redifferentiation of chondrocytes and cartilage formation under intermittent hydrostatic pressure. Biotechnol Lett 2006; 28: 1641–1648. [DOI] [PubMed] [Google Scholar]
- 20. Hoenig E, Winkler T, Mielke G, et al. High amplitude direct compressive strain enhances mechanical properties of scaffold-free tissue-engineered cartilage. Tissue Eng Part A 2011; 17: 1401–1411. [DOI] [PubMed] [Google Scholar]
- 21. Ogawa R, Mizuno S, Murphy GF, et al. The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue Eng Part A 2009; 15: 2937–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilkins RJ, Hall AC. Control of matrix synthesis in isolated bovine chondrocytes by extracellular and intracellular pH. J Cell Physiol 1995; 164: 474–481. [DOI] [PubMed] [Google Scholar]
- 23. Obradovic B, Carrier RL, Vunjak-Novakovic G, et al. Gas exchange is essential for bioreactor cultivation of tissue engineered cartilage. Biotechnol Bioeng 1999; 63: 197–205. [DOI] [PubMed] [Google Scholar]
- 24. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346: 549–556. [DOI] [PubMed] [Google Scholar]
- 25. Yokobori S, Gajavelli S, Mondello S, et al. Neuroprotective effect of preoperatively induced mild hypothermia as determined by biomarkers and histopathological estimation in a rat subdural hematoma decompression model. J Neurosurg 2013; 118: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rotter N, Bucheler M, Haisch A, et al. Cartilage tissue engineering using resorbable scaffolds. J Tissue Eng Regen Med 2007; 1: 411–416. [DOI] [PubMed] [Google Scholar]
- 27. Miot S, Woodfield T, Daniels AU, et al. Effects of scaffold composition and architecture on human nasal chondrocyte redifferentiation and cartilaginous matrix deposition. Biomaterials 2005; 26: 2479–2489. [DOI] [PubMed] [Google Scholar]
- 28. Babur BK, Ghanavi P, Levett P, et al. The interplay between chondrocyte redifferentiation pellet size and oxygen concentration. PLoS ONE 2013; 8: e58865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldberg AJ, Lee DA, Bader DL, et al. Autologous chondrocyte implantation. Culture in a TGF-beta-containing medium enhances the re-expression of a chondrocytic phenotype in passaged human chondrocytes in pellet culture. J Bone Joint Surg Br 2005; 87: 128–134. [PubMed] [Google Scholar]
- 30. Vetter U, Pirsig W, Heinze E. Growth activity in human septal cartilage: age-dependent incorporation of labeled sulfate in different anatomic locations. Plast Reconstr Surg 1983; 71: 167–171. [DOI] [PubMed] [Google Scholar]
- 31. Correia C, Pereira AL, Duarte AR, et al. Dynamic culturing of cartilage tissue: the significance of hydrostatic pressure. Tissue Eng Part A 2012; 18: 1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Waldman SD, Spiteri CG, Grynpas MD, et al. Effect of biomechanical conditioning on cartilaginous tissue formation in vitro. J Bone Joint Surg Am 2003; 85(suppl. 2): 101–105. [DOI] [PubMed] [Google Scholar]