Lesson
This report provides a rare histological example and the appropriate management of spontaneous aortic dissection secondary to giant cell arteritis.
Keywords: giant cell arteritis, dissection, repair, Stanford type A, type B
Introduction
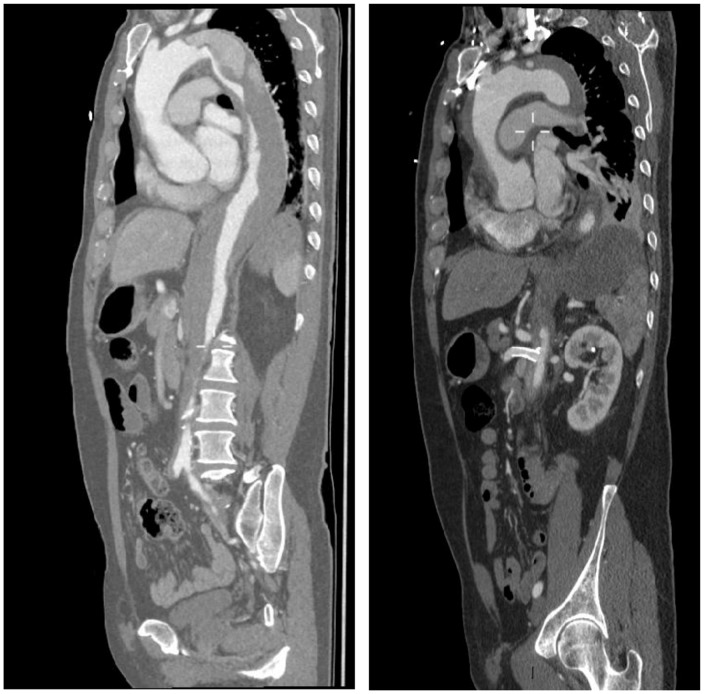
A 69-year-old gentleman presented to the Accident and Emergency Department at a local district general hospital with severe sudden-onset chest, back and abdominal pain. He was assessed and diagnosed, radiologically, to have acute Stanford type B aortic dissection beginning at the distal aortic arch at the level of the left subclavian artery and transferred to a regional teaching hospital. He received a stent to the superior mesenteric artery for malperfusion, with good restoration of mesenteric flow. The following morning, he was noted on a repeat computed tomography scan to have the dissection extending proximally to involve his ascending aorta and aortic root, thus becoming a Stanford type A dissection (Figure 1). He was transferred as an emergency to a tertiary care centre in Edinburgh, in preparation for emergency surgical repair. Postoperatively, he made an uneventful recovery. The histopathology report of the excised dissected aorta specimen had shown features consistent with a histological diagnosis of giant cell arteritis and elastic fragmentation, suggestive of degenerative vascular disease (Figures 2 and 3). Contact with Rheumatology was made urgently and he was commenced on high-dose steroid therapy. He was transferred back to his local district general hospital for further medical optimisation and rehabilitation.
Figure 1.
CT aorta showing (a) Stanford type B dissection (left) and (b) superior mesenteric artery stent in situ and Stanford type A dissection.
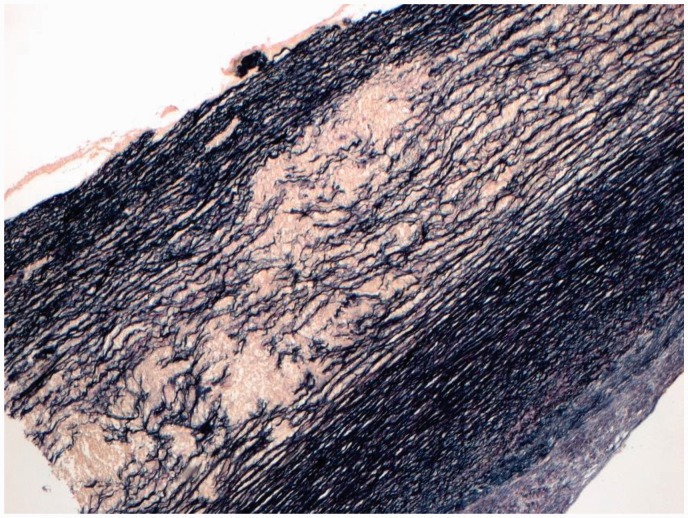
Figure 2.
Aortic wall stained with Verhoeff-Van Gieson stain, showing disruption of the elastic fibres within the elastic media.
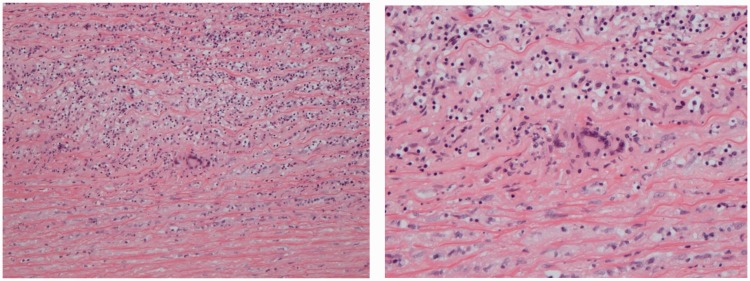
Figure 3.
Microscopy from aorta taken intraoperatively: the aortic wall shows a chronic inflammatory cell infiltrate comprising lymphocytes and scattered admixed multinucleated giant cells.
Previous to this, he had been fit and well and a keen golf player. Further questioning revealed that he had been suffering a spell of intractable headaches from November 2015 to January 2016, before settling. He also had a spell of severe gum inflammation with loss of teeth preceding that. He did not have a history of ischaemic heart disease. Operation: emergency repair of Stanford type A aortic dissection with replacement of aortic valve, aortic root and ascending aorta: biological Bentall using a 25-mm Carpentier-Edwards perimount into a 28-mm Gelweave Valsalva graft.
Discussion
Clinically occult giant cell arteritis is evident in approximately 50% of patients preceding aortic dissection.1,2 In a retrospective cohort study by Nuenninghoff et al.,3 nine patients (5%) of those diagnosed with giant cell arteritis, over 50 years of age, developed aortic dissection without aneurysm, with a case mortality of 77%. Liu et al.4 demonstrated that 46% of patients with histopathology-confirmed giant cell arteritis presented with aortic dissection as their first presentation, with 85% involving the proximal aorta, resulting in a two-week mortality rate of 80% mainly due to fatal pericardial tamponade.4,5
The American College of Rheumatology classification criteria for giant cell arteritis include (1) age over 50 years, (2) recent-onset localised headache, (3) tenderness or pulse attenuation on temporal artery palpation, (4) erythrocyte sedimentation rate above 50 mm/h and (5) arterial biopsy showing necrotising vasculitis.6 The diagnostic gold standard is histopathological via arterial wall biopsy, with a positive predictive value of 50–80% based on the clinical disease pattern.7 The estimated median time from diagnosis of giant cell arteritis to identification of thoracic aortic dissection is 1.1 years.2 Given the potential morbidity of delayed treatment, investigation and immediate management should be guided by high clinical suspicion. Typical laboratory findings include normocytic anaemia and reactive thrombocytosis. Hypoalbuminaemia may be evident, and 25–35% of patients will have increased liver transaminases and alkaline phosphatase, with normalisation on commencing steroid treatment. Erythrocyte sedimentation rate is often greater than 100 mm/h, although C-reactive protein levels may correlate better with disease activity.7,8 Early radiological imaging may expedite prompt treatment to prevent long-term complications.
First-line management includes corticosteroid therapy. Prognosis is closely related to the time elapsed before initiation. Guidelines recommend high-dose prednisolone, 40–60 mg, or its equivalent as first-line treatment, although recent evidence supports similar efficacy with 30–40 mg daily.8 Approximately 2–3 months after therapy initiation, once the disease is adequately controlled, tapering of steroid therapy is appropriate and maintenance may continue for years.4 Currently, there are no evidence-based recommendations to guide steroid therapy after aortic dissection.
Conclusion
This case provides a rare histological example of spontaneous aortic dissection secondary to occult and undiagnosed GCA with a good outcome after surgical intervention. It reinforces the importance of histological examination of excised aortic tissue from thoracic aneurysms. Diagnosis and treatment should be based on high clinical suspicion, and follow-up imaging is required to detect large-vessel involvement. Further evidence is needed regarding the surveillance and treatment of dissections in patients with giant cell arteritis, as well as steroid therapy after giant cell arteritis-associated aortic dissection.
Declarations
Competing Interests
None declared.
Funding
None declared.
Ethics approval
Not applicable
Guarantor
KL
Contributorship
AEM and KL conceived and designed the case report. WW, EM, SyT and KL contributed clinical information/radiological imaging/histological imaging. AEM and KL wrote the paper. AEM, WW, EM, SyT and KL contributed to the critical revision of the article. AEM, WW, EM, SyT and KL contributed to the final approval version to be published.
Provenance
Not commissioned; peer-reviewed by Akhtar Husain
References
- 1.Bengtsson BA, Malmvall BE. The epidemiology of giant cell arteritis including temporal arteritis and polymyalgia rheumatica. Incidences of different clinical presentations and eye complications. Arthritis Rheum 1981; 24: 899–904. [DOI] [PubMed] [Google Scholar]
- 2.Giujusa T, Dario C, Risica G, et al. Aortic dissection: an incidence study based on hospital cases. Cardiologia 1994; 39: 107–112. [PubMed] [Google Scholar]
- 3.Nuenninghoff DM, Hunder GG, Christianson TJH, McClelland RL, Matteson EL. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum 2003; 48: 3522–3531. [DOI] [PubMed] [Google Scholar]
- 4.Liu G, Shupak R, Chiu BKY. Aortic dissection in giant-cell arteritis. Semin Arthritis Rheum 1995; 25: 160–171. [DOI] [PubMed] [Google Scholar]
- 5.Berry MF, Woo YJ. Repair of acute type A aortic dissection associated with temporal arteritis. Ann Thorac Surg 2003; 76: 1717–1718. [DOI] [PubMed] [Google Scholar]
- 6.Hunder G, Bloch D, Michel B, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990; 33: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 7.Villa-Forte A, Mandell B. Vasculitis. In: Bonow R, Mann D, Zipes D, Libby P, Braunwald E. (eds). Braunwald’s heart diseases: a textbook of cardiovascular medicine, Philadelphia, PA: Elsevier Saunders, 2012, pp. 1878–1879. [Google Scholar]
- 8.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary – a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, “American Association for Thoracic Surgery, American College of Radiology”. Cathet Cardiovasc Interv 2010; 76: E43–E86. [DOI] [PubMed] [Google Scholar]