Abstract
Background:
Significant controversy exists regarding the anterolateral structures of the knee.
Purpose:
To determine the layer-by-layer anatomic structure of the anterolateral complex of the knee.
Study Design:
Descriptive laboratory study.
Methods:
Twenty fresh-frozen cadaveric knees (age range, 38-56 years) underwent a layer-by-layer dissection to systematically expose and identify the various structures of the anterolateral complex. Quantitative measurements were performed, and each layer was documented with high-resolution digital imaging.
Results:
The anterolateral complex of the knee consisted of different distinct layers, with the superficial and deep iliotibial band (ITB) representing layer 1. The superficial ITB had a distinct connection to the distal femoral metaphysis and femoral condyle (Kaplan fibers), and the deep layers of the ITB were identified originating at the level of the Kaplan fibers proximally. This functional unit, consisting of the superficial and deep ITB, was reinforced by the capsulo-osseous layer of the ITB, which was continuous with the fascia of the lateral gastrocnemius and biceps femoris muscles. These 3 components of the ITB became confluent distally, and the insertion spanned from the Gerdy tubercle anteriorly to the lateral tibia posteriorly on a small tubercle (lateral tibial tuberosity). Layer 3 consisted of the anterolateral capsule, in which 35% (7/20) of specimens had a discreet mid-third capsular ligament.
Conclusion:
The anterolateral complex consists of the superficial and deep ITB, the capsulo-osseous layer of the ITB, and the anterolateral capsule. The anterolateral complex is defined by the part of the ITB between the Kaplan fibers proximally and its tibial insertion, which forms a functional unit. A discrete anterolateral ligament was not observed; however, the anterolateral ligament described in recent studies likely refers to the capsulo-osseous layer or the mid-third capsular ligament.
Clinical Relevance:
The anterolateral knee structures form a complex functional unit. Surgeons should use caution when attempting to restore this intricate structure with extra-articular procedures designed to re-create a single discreet ligament.
Keywords: knee, anterolateral, iliotibial band, ITB, capsulo-osseous layer, capsule, anatomy, pivot shift
Injuries to the anterior cruciate ligament (ACL) are accompanied by combined anterior and rotatory instability.3,9,22 Although numerous advances have been made in surgical reconstruction techniques and graft choices, it is well recognized that some patients continue to experience persistent rotatory knee instability after ACL reconstruction.16,17,34,36 It has been suggested that failure to address additional secondary stabilizers may contribute to this postoperative instability. In fact, unaddressed meniscal tears, underappreciated meniscocapsular injuries, increased posterior tibial slope, and anterolateral capsular injuries have all been demonstrated to play a role in rotatory knee stability.3,10,23–28,30,38
Recently, the scientific focus has centered on the structures of the anterolateral side of the knee, with some authors suggesting that extra-articular procedures are indicated in the setting of ACL injury. For example, numerous studies have promoted the existence of a discreet ligament located on the anterolateral aspect of the knee, which was recently termed the anterolateral ligament (ALL).4–7,13,19,31,37,45 This ligament has been suggested to play an important role in the restraint of internal tibial rotation and the pivot-shift phenomenon.26,27,30 However, published descriptions of the femoral insertion, the obliquity of the ligament position, and other morphologic parameters have varied widely.4–7,19,31,37,45 These discrepant anatomic findings are further magnified when we consider biomechanical studies of the ALL. While some study groups found this ligament to be an important restraint of internal tibial rotation,1,26,27,30 others described the biomechanical role of the ALL to be negligible.20,32,43 Considering the inconsistent terms and conflicting findings, it is crucial that the anterolateral knee structure is clearly defined and a consistent terminology is developed. A thorough appreciation of the anatomic characteristics will clarify the role of the lateral extra-articular structures as well as the indications for lateral-side extra-articular procedures. Therefore, the purpose of this study was to describe the anatomic characteristics of the anterolateral complex of the knee as determined through systematic, detailed dissections of young, fresh-frozen human cadaveric knees.
Methods
Twenty fresh-frozen cadaveric knees (mean age, 47.6 years; range, 38-56 years; 12 males, 8 females) with no history of previous knee injuries or surgeries were used for this study. Prior approval was obtained by the Committee for Oversight of Research and Clinical Training Involving Decedents (CORID No. 224) at the University of Pittsburgh for the use of cadaveric knee specimens. Specimens were obtained from Research for Life and Science Care. The cadaveric knees were stored at –20°C and thawed for 24 hours at room temperature prior to dissection. During all stages of dissection, anatomic structures were critically analyzed, and their physical relationships and functional properties were documented.
Dissection
After the skin and subcutaneous tissue were removed, a longitudinal incision was made in the anterior-most part of the iliotibial band (ITB) to separate it from the iliopatellar band. Distally, the incision traveled along the lateral border of the patellar tendon and ended at the level of the tibial tubercle. Next, the superficial ITB was reflected posteriorly via blunt, proximal-to-distal dissection, leaving its posterior fibers and distal insertion at the Gerdy tubercle intact. With anterior retraction of the vastus lateralis muscle, the lateral intermuscular septum and the distinct distal femoral insertion of the ITB (Kaplan fibers) were visualized.18 Thereafter, the ITB was released in stepwise fashion from its insertion on the Gerdy tubercle to further reflect it posteriorly and to assess its deeper layers.
Next, an incision was made between the ITB (posterior to the lateral intermuscular septum) and the fascia of the short head of the biceps femoris muscle.40,41 The long and short heads of the biceps femoris muscle were carefully removed from proximal to distal, and its connections to the posterolateral capsule and deep layers of the ITB as well as its distal insertions on the tibia and fibula were documented.40
After removal of the biceps femoris muscle and fascia, the components of the ITB were assessed and documented from the posterior direction. Then, the capsulo-osseous layer39 of the ITB was sharply separated from the superficial ITB to evaluate its course, proximal and distal insertions, and attachments with the surrounding structures.
After removal of the deep layers of the ITB, the anterolateral capsule was assessed. First, the superficial layer of the capsule according to Seebacher et al33 was dissected to visualize the lateral collateral ligament (LCL) followed by an anterior capsular incision. The meniscofemoral and meniscotibial ligaments (coronary ligament) were identified, and the presence or absence of a mid-third capsular thickening (ie, mid-third capsular ligament14,15,41) was recorded.
Quantitative Measurements
Quantitative measurements of the capsulo-osseous layer of the ITB and the Kaplan fibers were obtained with a digital caliper (Fisher Scientific; ISO 17025 calibrated; accuracy 0.03 mm) at 90° of knee flexion. Every step of the dissection was documented with a digital camera (Canon EOS Rebel T5i). Measurements were expressed as mean ± 1 SD.
Results
Superficial Iliotibial Band
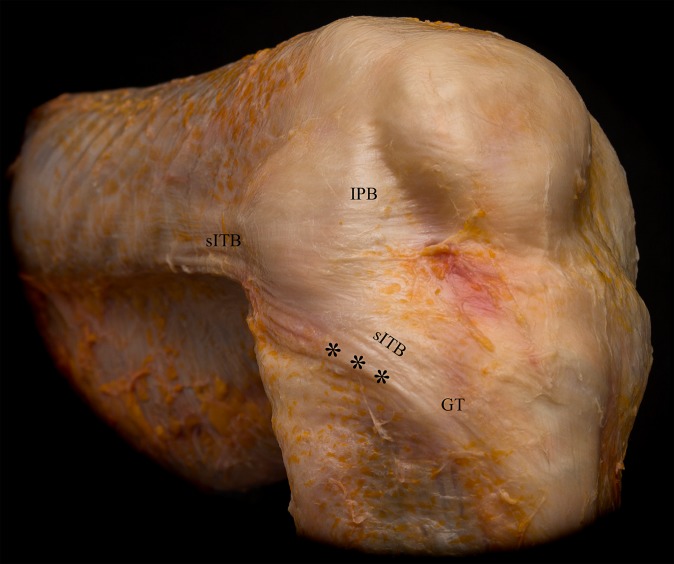
The superficial ITB was located in layer 1 (as described by Seebacher et al33). It inserted in a wide area spanning from the Gerdy tubercle anteriorly to the anterolateral and lateral tibia posteriorly. Anteriorly, curved fibers ran from the ITB to the lateral aspect of the patella and patellar tendon (iliopatellar band). Posteriorly, the superficial ITB reinforced the fascia of the biceps femoris muscle (Figure 1). Posterior reflection of the superficial ITB demonstrated its femoral insertion along the linea aspera of the femur via the lateral intermuscular septum. Posteriorly, the fascia of the biceps femoris muscle showed fascial and aponeurotic extensions, which inserted on the proximal tibia, posterior to the Gerdy tubercle.
Figure 1.
Layer 1 including the superficial iliotibial band (sITB) and iliopatellar band (IPB). Asterisks indicate the folding of the posterior part of the sITB at higher degrees of knee flexion. GT, Gerdy tubercle.
Middle and Deep Iliotibial Band
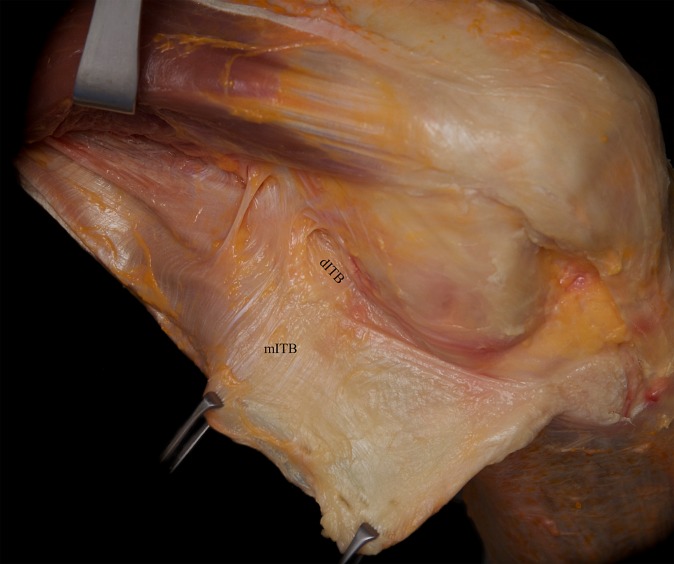
The middle layer could be separated only via sharp dissection from the superficial ITB. This layer was characterized by obliquely aligned fibers running in a proximal-lateral to distal-medial direction (Figure 2). The fibers crossed the superficial ITB and could best be visualized in the supracondylar area of the ITB.
Figure 2.
Posterior reflection and distal release of the superficial iliotibial band (ITB) revealed the obliquely aligned fibers of the middle layer of the ITB (mITB). As seen here, these fibers could best be seen in the supracondylar region. dITB, deep layer of the ITB.
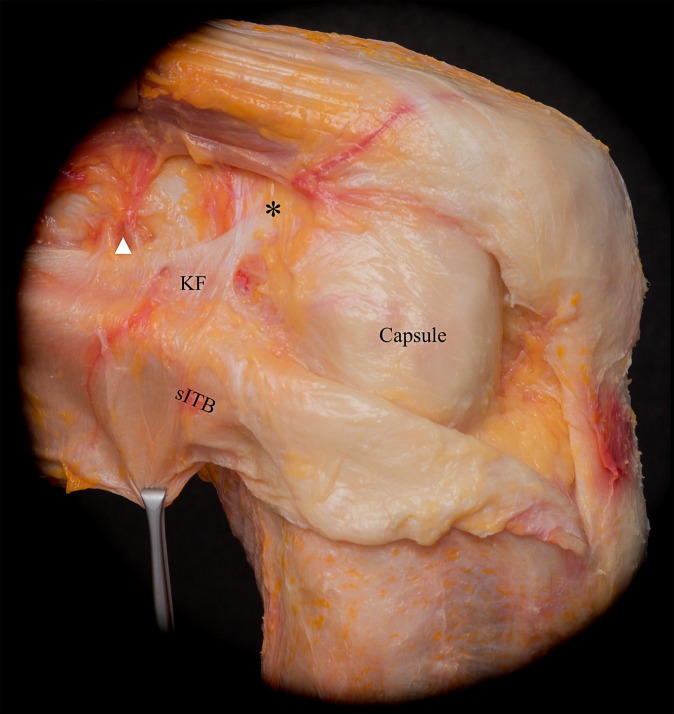
In continuity with the middle layer, the deep layer resided in the posterior-most aspect of the ITB and blended with the superficial ITB distal to the lateral femoral epicondyle. The deep layer inserted slightly posterior to the Gerdy tubercle together with the posterior-most fibers of the superficial ITB. Proximally, the so-called Kaplan fibers18 were part of this deep layer. These firm and distinct fiber bundles connected the superficial ITB to the distal femoral metaphysis and condyle and were in close proximity to the branches of the superior genicular artery (Figure 3). Compared with the obliquely aligned fibers of the intermuscular septum, the distinct and thicker Kaplan fibers were characterized by their transverse course from lateral to medial. The Kaplan fiber insertion onto the lateral distal femoral metaphysis (Table 1) was found in 100% of the dissections, whereas 2 additional thin fiber bundles were seen more distally in 80% (16/20) of the specimens. These accessory condylar insertions were located proximal and anterior to the lateral femoral epicondyle (Figures 3 and 4).
Figure 3.
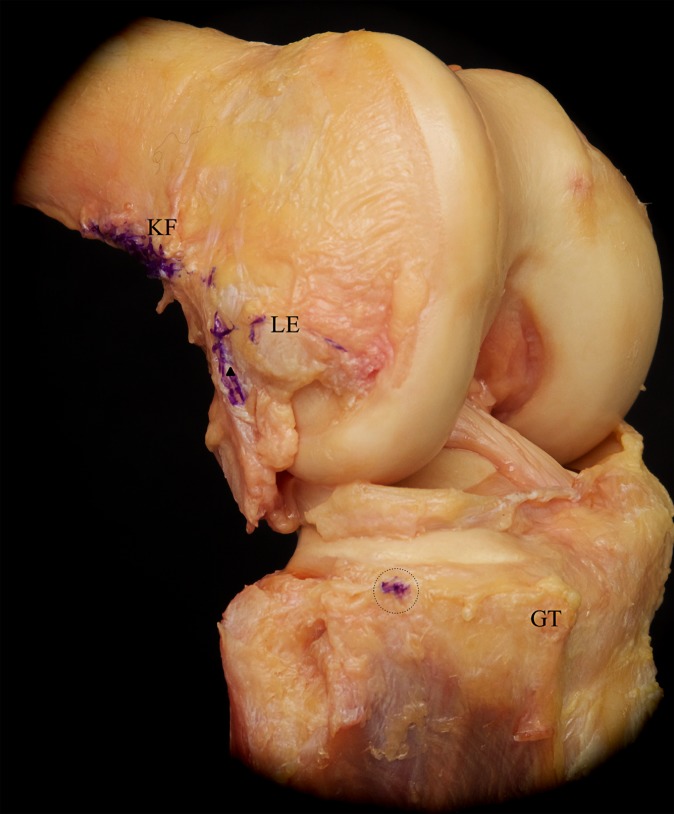
Reflection of the superficial iliotibial band (sITB) revealed its firm attachment to the distal femoral metaphysis via the Kaplan fibers (KF). The KF are in close proximity to the branches of the superior genicular artery (white arrowhead) and have accessory insertions (asterisk) proximal and anterior to the femoral epicondyle. Further, the superficial layer of the anterolateral capsule becomes visible.
TABLE 1.
Quantitative Anatomic Resultsa
| Kaplan fiber insertion | |
| Length | 20.6 ± 3.6 |
| Width | 4.7 ± 0.6 |
| Proximal distance from LFE | 17.1 ± 5.0 |
| Anterior distance from LFE | 7.4 ± 2.8 |
| Capsulo-osseous layer of the iliotibial band | |
| Length | 59.7 ± 8.4 |
| Width at LFE | 6.7 ± 1.9 |
| Width at joint line | 8.1 ± 1.4 |
| Length of insertion to lateral gastrocnemius fascia | 9.1 ± 2.0 |
| Lateral tibial tuberosity | |
| Distance from tip of the Gerdy tubercle | 23.4 ± 1.6 |
| Distance from posterior cortex of fibular head | 31.9 ± 5.7 |
| Distance from lateral cartilage margin | 6.6 ± 0.9 |
aMeasurements are reported in millimeters as mean ± SD. LFE, lateral femoral epicondyle.
Figure 4.
(A) With further posterior reflection of the superficial iliotibial band (sITB) and blunt separation from the deeper layers, the capsulo-osseous layer (black arrowhead) can be appreciated. The white arrowhead indicates the branches of the superior genicular artery. (B) Proximal, the longitudinally aligned fibers of the intermuscular septum (IS) can be differentiated from the Kaplan fibers (KF). Further, retraction of the sITB reveals the deep ITB (dITB), which merges with the sITB distally. No distinct anterolateral ligament could be observed. The asterisk highlights the accessory insertion of the KF.
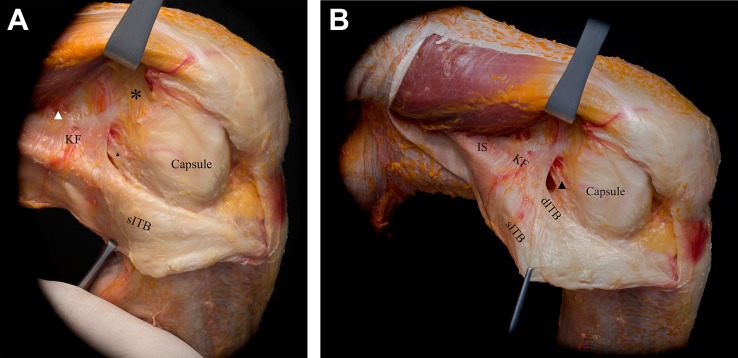
Capsulo-osseous Layer of the Iliotibial Band
Further release and posterior reflection of the superficial ITB revealed the capsulo-osseous layer of the ITB (Figure 4, Table 1). Morphologically, the capsulo-osseous layer had a triangular shape, with a slightly wider tibial insertion than femoral origin. Proximally, this structure was continuous with the fascia of the lateral gastrocnemius muscle. Additionally, multiple flimsy, variable, and indistinct attachments around the lateral femoral epicondyle were observed. The fascia of the biceps femoris muscle reinforced the capsulo-osseous layer posteriorly (Figure 4).
As the most medial and posterior portion of the ITB, the capsulo-osseous layer merged with the ITB in its distal segment; its insertion was continuous with the aponeurotic extension of the biceps femoris in an area approximately midway between the posterior aspect of the fibular head and the tip of the Gerdy tubercle. In the center of the insertion site, a small but well-defined tubercle was observed (see Table 1). The location of this tubercle was consistent with descriptions of the lateral tibial tuberosity by Terry and LaPrade,41 which was found in the present study to be 6.6 ± 0.9 mm distal to the lateral articular cartilage border (Figure 5).
Figure 5.
Removal of the anterolateral soft tissues showed the insertions and origins of the different layers of the iliotibial band (purple ink). The tibial insertion of the capsulo-osseous layer on the lateral tibial tuberosity (dotted circle) is located about halfway between the Gerdy tubercle (GT) and the fibular head. On the femoral side, the capsulo-osseous layer is continuous with the fascia of the lateral gastrocnemius tendon (white arrowhead). KF, Kaplan fiber insertion; LE, lateral epicondyle (after removal of the lateral collateral ligament).
Anterolateral Capsule
After removal of the ITB and its layers, the anterolateral joint capsule was dissected. The superficial layer of the capsule encompassed the LCL, whereas the deep layer passed deep to the LCL. Anterior to the LCL, the 2 layers were found to be fused into 1 contiguous layer. In the area where the 2 layers of the joint capsule merged, a capsular thickening, or mid-third capsular ligament as described by Hughston et al,14,15 was present in 35% (7/20) of the specimens (Figure 6). Furthermore, the coronary ligaments, consisting of meniscofemoral and meniscotibial ligaments, were observed in all specimens (Figure 7).
Figure 6.
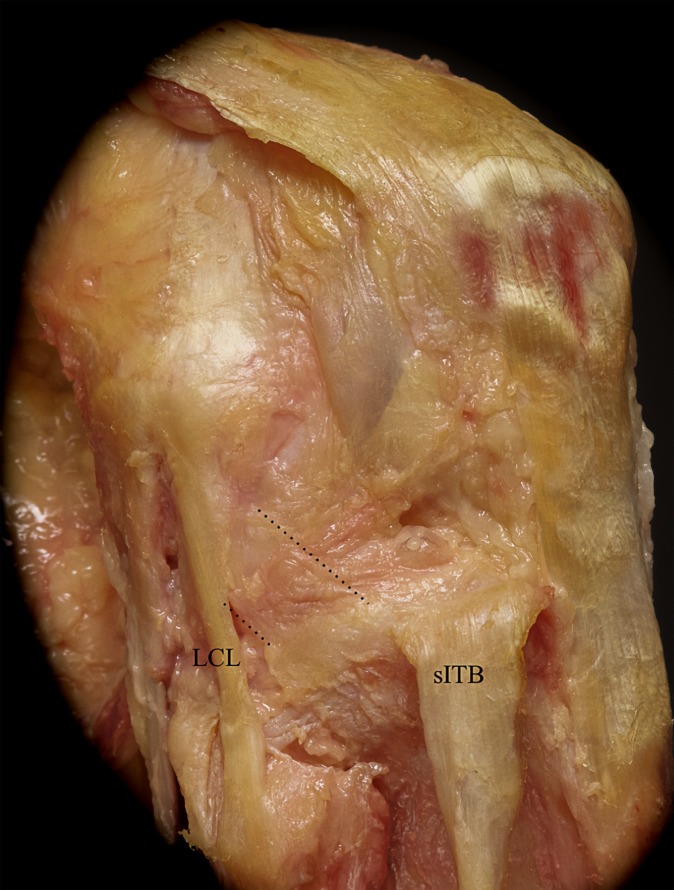
Reflection of the superficial iliotibial band (sITB) and its deep layers reveals the anterolateral joint capsule with a thickening (mid-third capsular ligament; area between the 2 dotted lines) anterior to the lateral collateral ligament (LCL). This mid-third capsular ligament was observed in 35% of the specimens.
Figure 7.
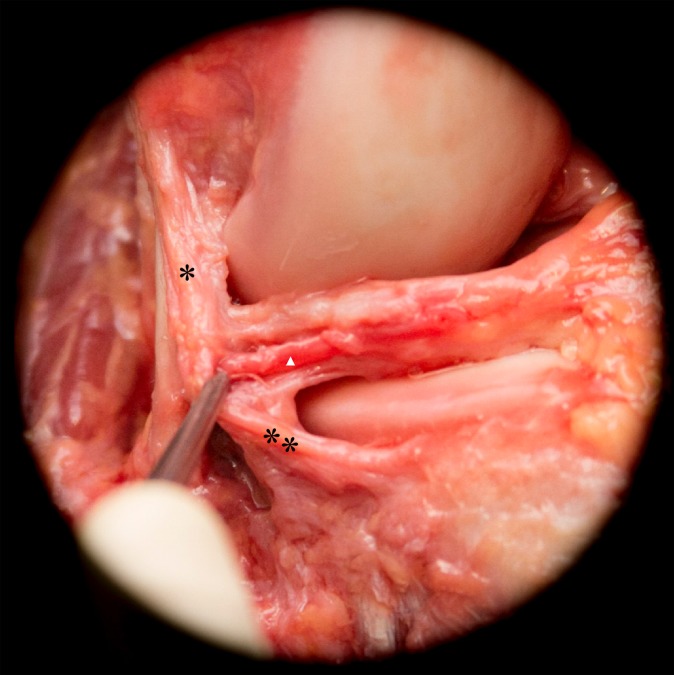
Removal of the anterolateral capsule revealed the coronary ligament, consisting of the meniscofemoral (*) and meniscotibial (**) ligaments. Medial to the coronary ligament, the inferior genicular artery (white arrowhead) runs from posterior to anterior.
Anterolateral Ligament
No discrete ligament completely matching the original published parameters5,7,19 of the ALL was observed. However, the capsulo-osseous layer of the ITB (observed in all specimens) and the mid-third capsular ligament (observed in 35% of specimens) most closely resembled the recent ALL descriptions and were in line with previous classic accounts of these structures.14,15
Discussion
The data from this study showed that the various components of the ITB are intricately and substantially associated with each other, resulting in multiple layers and discreet femoral and tibial attachments. In agreement with previous studies, these dissections demonstrated that the anterolateral knee is complex and contains several integrated structures, namely the ITB with its multiple layers and the anterolateral capsule.
Interestingly, a distinct ALL exactly matching original descriptions5,7,19 was not observed in any of the dissected specimens. While different authors have provided varying descriptions of the ALL, many of the recently published articles regarding ALL are consistent with the current study’s observations of the capsulo-osseous layer of the ITB and/or the mid-third capsular ligament.14,15,39,40,44 Furthermore, the current findings are in line with previous descriptions in the classic literature regarding the capsulo-osseous layer and the mid-third capsular ligament.14,15,40–42
While the original reports by Hughston and colleagues14,15 of the mid-third capsular ligament in 1976 did not describe the number of studied cadavers containing a mid-third capsular ligament, the 35% incidence of a capsular thickening in the present study, corresponding with the originally described mid-third capsular ligament,14,15,41 is similar to the incidence of an ALL described in some recent studies,31,37,47 lending support to the concept that the recently described ALL is synonymous with the mid-third capsular ligament.31,37,47 However, other recent studies have found the ALL in up to 100% of their dissections.4–6,19 The capsular thickening observed as the mid-third capsular ligament is likely due to the unique relationship between the LCL and the capsule. The superficial layer of the joint capsule crosses the LCL superficially, whereas the deep layer lies medial to the LCL.33 Anterior to the LCL, the 2 layers of the joint capsule merge into a single layer, which might lead to the morphological appearance of a thickening in some specimens.
Furthermore, the current study demonstrated that the capsulo-osseous layer of the ITB and the mid-third capsular ligament were spatially related to each other, and both occupied anatomic locations that are similar to recent descriptions of the ALL.6,7 In fact, Hughston et al15 reported that the mid-third capsular ligament and ITB worked synergistically.
The reasons for discrepant findings between the current study and some recently published studies is likely multifactorial. First, most recent anatomic studies have described the quantitative and qualitative anatomic characteristics of the ALL on embalmed specimens.5,6,31,37 However, it has been well established that the tissue quality in embalmed specimens is deteriorated and not ideal for detailed dissection compared with fresh-frozen cadavers (as used in the current study).2,35 As such, the dissections in the current study likely allowed for a more anatomic identification of native tissue planes than those performed on embalmed specimens. Additionally, in previous studies authors have described rotating the tibia internally to better visualize the ALL.5,6,19,31 It is possible that this technique inadvertently misrepresented the natural state of the anterolateral structures. In fact, biomechanical studies have suggested that the anterolateral capsule acts as a sheet of tissue rather than a ligament,11 which calls into question the appropriateness of isolating 1 strip of tissue. In the present study, a layer-by-layer dissection without rotational manipulation of the joint was performed, and native anatomic relationships were preserved. Further, the average age of the specimens in this study is notably lower than that reported in previous studies. It is likely that the tissue condition of these younger cadavers more accurately represented the anatomic characteristics of patients who typically have rotatory knee instability, who tend to be younger in age.4–7,19,31,37 Moreover, it is possible that the mid-third capsular thickening located at the confluence of capsular layers is more noticeable in some individuals than others, further contributing to the widely ranging prevalence of reported ALL and/or mid-third capsular ligament in published literature.4–8,13,19,31,37,45,47
Compared with the medial part of the knee,46 the lateral layer structure is more complex. Seebacher et al33 described 3 layers of the lateral knee. Layer 1 consists of the deep fascia, including the ITB and the fascia of the biceps femoris muscle. Layer 2 includes the retinacula and aponeurosis of the quadriceps muscle and the lateral patellofemoral ligaments. Thus, this layer is incomplete posteriorly and fuses with layer 1 anteriorly. Layer 3 is composed of the joint capsule. This layer-by-layer structure was confirmed by the current study; however, the retinacula and lateral patellofemoral ligaments were not the focus of these dissections.
The superficial ITB, which has been described as the most robust soft tissue structure of the anterolateral knee,29 inserts proximally via the deep layer onto the lateral distal femoral metaphysis.18,21,44 This insertion was described by Kaplan18 in 1958. These attachments, termed Kaplan fibers, are firm and distinct from the lateral intermuscular septum.18,21,44 A previous study confirmed the presence of these fibers in 93% of specimens,21 compared with 100% in the current study. Interestingly, Kaplan fibers exist in close proximity to branches of the superior genicular artery, and hemorrhage is frequently evident in this lateral supracondylar area on magnetic resonance images of patients with rotatory knee trauma.
The aspect of the superficial and deep ITB that lies between the Kaplan fibers proximally and the insertion at the proximal tibia distally forms a functional unit that may contribute to rotatory knee stability.12,21 Similarly, in the classic literature, lengthening and tightening of this part of the superficial and deep ITB were observed with increased knee flexion.12,21 This increase in length is greatest at 60° of knee flexion, where the distance between the Kaplan fiber insertion and the Gerdy tubercle increases by 18%.21 Recently, a robotic study confirmed that both the superficial and deep ITB, including the Kaplan fiber insertion as well as the capsulo-osseous layer, are the greatest contributors to internal rotation restraint other than the ACL. The superficial and deep ITB were shown to supply more than 70% of the total internal rotation restraint at knee flexion angles greater than 60° in both ACL-intact and ACL-deficient knees.20
While the biomechanical functions of the capsulo-osseous layer and deep ITB have only been investigated in tandem, the capsulo-osseous layer is anatomically distinct from the deep ITB. However, distal to the lateral femoral epicondyle, the different components of the ITB merge together and function as a single unit.21,39,40 As seen in the dissections of the current study, the deep and capsulo-osseous layers of the ITB compose the medial- and posterior-most portions of the ITB and insert just posterior to the Gerdy tubercle. Interestingly, a small bony tubercle 23.4 mm posterior to the tip of the Gerdy tubercle and 6.6 mm distal to the lateral articular cartilage border was observed. The capsulo-osseous layer insertion is located at this point, termed the lateral tibial tuberosity by Terry and LaPrade41 in 1996.
The close topographical relationships of the anterolateral knee structures suggest that they act jointly to provide rotatory knee stability. Clinically, it may be most appropriate to refer to these various anterolateral knee structures as the anterolateral complex, consisting of the ITB with its superficial and deep layers as well as the anterolateral joint capsule. Importantly, biomechanical studies have shown that the components of the anterolateral complex form a cohesive functional unit that provides significant contributions to rotatory knee stability.20 Furthermore, studies have shown that high-grade injuries to either the Kaplan fibers or the anterolateral capsule might result in increased rotatory laxity.20,25
Conclusion
The anterolateral complex consists of the superficial and deep ITB, the capsulo-osseous layer of the ITB, and the anterolateral capsule. Distally, the different layers of the ITB merge into a single functional unit. In a subset of specimens (35%), a capsular thickening (which might refer to the mid-third capsular ligament) could be observed at the confluence of the superficial and deep capsule. A discrete ALL was not observed; however, the ALL described in recent studies likely refers to the capsulo-osseous layer and/or the mid-third capsular ligament described in the current study and classic literature. When considering rotatory knee stability, surgeons must thoroughly understand the structural and functional contributions of the anterolateral complex. Furthermore, researchers and clinicians must strive to use consistent terms and precise descriptions when referring to the complex anatomic characteristics of the anterolateral knee.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: Support for this research was received from the Department of Orthopaedic Surgery of the University of Pittsburgh.
Ethical approval for this research was received from the University of Pittsburgh Committee for Oversight of Research and Clinical Training Involving Decedents.
References
- 1. Bonanzinga T, Signorelli C, Grassi A, et al. Kinematics of ACL and anterolateral ligament, part I: combined lesion. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1055–1061. [DOI] [PubMed] [Google Scholar]
- 2. Brenner E. Human body preservation—old and new techniques. J Anat. 2014;224(3):316–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bull AM, Earnshaw PH, Smith A, Katchburian MV, Hassan AN, Amis AA. Intraoperative measurement of knee kinematics in reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 2002;84(7):1075–1081. [DOI] [PubMed] [Google Scholar]
- 4. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3186–3195. [DOI] [PubMed] [Google Scholar]
- 5. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daggett M, Ockuly AC, Cullen M, et al. Femoral origin of the anterolateral ligament: an anatomic analysis. Arthroscopy. 2016;32(5):835–841. [DOI] [PubMed] [Google Scholar]
- 7. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: anatomy, length changes and association with the Segond fracture. Bone Joint J. 2014;96-B(3):325–331. [DOI] [PubMed] [Google Scholar]
- 8. Dombrowski ME, Costello JM, Ohashi B, et al. Macroscopic anatomical, histological and magnetic resonance imaging correlation of the lateral capsule of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2854–2860. [DOI] [PubMed] [Google Scholar]
- 9. Ellison AE, Berg EE. Embryology, anatomy, and function of the anterior cruciate ligament. Orthop Clin North Am. 1985;16(1):3–14. [PubMed] [Google Scholar]
- 10. Guenther D, Griffith C, Lesniak B, et al. Anterolateral rotatory instability of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2909–2917. [DOI] [PubMed] [Google Scholar]
- 11. Guenther D, Rahnemai-Azar A, Bell K, et al. The anterolateral capsule of the knee behaves like a sheet of fibrous tissue. Am J Sports Med. 2017;45(4):849–855. [DOI] [PubMed] [Google Scholar]
- 12. Hassler H, Jakob RP. On the cause of the anterolateral instability of the knee joint: a study on 20 cadaver knee joints with special regard to the tractus iliotibialis [in German]. Arch Orthop Trauma Surg. 1981;98(1):45–50. [DOI] [PubMed] [Google Scholar]
- 13. Helito CP, Demange MK, Bonadio MB, et al. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med. 2014;42(10):2356–2362. [DOI] [PubMed] [Google Scholar]
- 14. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities, part I: the medial compartment and cruciate ligaments. J Bone Joint Surg Am. 1976;58(2):159–172. [PubMed] [Google Scholar]
- 15. Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities, part II: the lateral compartment. J Bone Joint Surg Am. 1976;58(2):173–179. [PubMed] [Google Scholar]
- 16. Hussein M, van Eck CF, Cretnik A, Dinevski D, Fu FH. Individualized anterior cruciate ligament surgery: a prospective study comparing anatomic single- and double-bundle reconstruction. Am J Sports Med. 2012;40(8):1781–1788. [DOI] [PubMed] [Google Scholar]
- 17. Hussein M, van Eck CF, Cretnik A, Dinevski D, Fu FH. Prospective randomized clinical evaluation of conventional single-bundle, anatomic single-bundle, and anatomic double-bundle anterior cruciate ligament reconstruction: 281 cases with 3- to 5-year follow-up. Am J Sports Med. 2012;40(3):512–520. [DOI] [PubMed] [Google Scholar]
- 18. Kaplan EB. The iliotibial tract; clinical and morphological significance. J Bone Joint Surg Am. 1958;40-A(4):817–832. [PubMed] [Google Scholar]
- 19. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: an anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606–1615. [DOI] [PubMed] [Google Scholar]
- 20. Kittl C, El-Daou H, Athwal KK, et al. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med. 2016;44(2):345–354. [DOI] [PubMed] [Google Scholar]
- 21. Lobenhoffer P, Posel P, Witt S, Piehler J, Wirth CJ. Distal femoral fixation of the iliotibial tract. Arch Orthop Trauma Surg. 1987;106(5):285–290. [DOI] [PubMed] [Google Scholar]
- 22. Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee—the contributions of the supporting structures: a quantitative in vitro study. J Bone Joint Surg Am. 1976;58(5):583–594. [PubMed] [Google Scholar]
- 23. Musahl V, Ayeni OR, Citak M, Irrgang JJ, Pearle AD, Wickiewicz TL. The influence of bony morphology on the magnitude of the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2010;18(9):1232–1238. [DOI] [PubMed] [Google Scholar]
- 24. Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2010;38(8):1591–1597. [DOI] [PubMed] [Google Scholar]
- 25. Musahl V, Rahnemai-Azar AA, Costello J, et al. The influence of meniscal and anterolateral capsular injury on knee laxity in patients with anterior cruciate ligament injuries. Am J Sports Med. 2016;44(12):3126–3131. [DOI] [PubMed] [Google Scholar]
- 26. Nitri M, Rasmussen MT, Williams BT, et al. An in vitro robotic assessment of the anterolateral ligament, part 2: anterolateral ligament reconstruction combined with anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(3):593–601. [DOI] [PubMed] [Google Scholar]
- 27. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(3):669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahnemai-Azar AA, Abebe ES, Johnson P, et al. Increased lateral tibial slope predicts high-grade rotatory knee laxity pre-operatively in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1170–1176. [DOI] [PubMed] [Google Scholar]
- 29. Rahnemai-Azar AA, Miller RM, Guenther D, et al. Structural properties of the anterolateral capsule and iliotibial band of the knee. Am J Sports Med. 2016;44(4):892–897. [DOI] [PubMed] [Google Scholar]
- 30. Rasmussen MT, Nitri M, Williams BT, et al. An in vitro robotic assessment of the anterolateral ligament, part 1: secondary role of the anterolateral ligament in the setting of an anterior cruciate ligament injury. Am J Sports Med. 2016;44(3):585–592. [DOI] [PubMed] [Google Scholar]
- 31. Runer A, Birkmaier S, Pamminger M, et al. The anterolateral ligament of the knee: a dissection study. Knee. 2016;23(1):8–12. [DOI] [PubMed] [Google Scholar]
- 32. Saiegh YA, Suero EM, Guenther D, et al. Sectioning the anterolateral ligament did not increase tibiofemoral translation or rotation in an ACL-deficient cadaveric model. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1086–1092. [DOI] [PubMed] [Google Scholar]
- 33. Seebacher JR, Inglis AE, Marshall JL, Warren RF. The structure of the posterolateral aspect of the knee. J Bone Joint Surg Am. 1982;64(4):536–541. [PubMed] [Google Scholar]
- 34. Siebold R, Branch TP, Freedberg HI, Jacobs CA. A matched pairs comparison of single- versus double-bundle anterior cruciate ligament reconstructions, clinical results and manual laxity testing. Knee Surg Sports Traumatol Arthrosc. 2011;19(suppl 1):S4–S11. [DOI] [PubMed] [Google Scholar]
- 35. Silva RM, Matera JM, Ribeiro AA. New alternative methods to teach surgical techniques for veterinary medicine students despite the absence of living animals: is that an academic paradox? Anat Histol Embryol. 2007;36(3):220–224. [DOI] [PubMed] [Google Scholar]
- 36. Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BH, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598–1605. [DOI] [PubMed] [Google Scholar]
- 37. Stijak L, Bumbasirevic M, Radonjic V, et al. Anatomic description of the anterolateral ligament of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2083–2088. [DOI] [PubMed] [Google Scholar]
- 38. Tanaka M, Vyas D, Moloney G, Bedi A, Pearle AD, Musahl V. What does it take to have a high-grade pivot shift? Knee Surg Sports Traumatol Arthrosc. 2012;20(4):737–742. [DOI] [PubMed] [Google Scholar]
- 39. Terry GC, Hughston JC, Norwood LA. The anatomy of the iliopatellar band and iliotibial tract. Am J Sports Med. 1986;14(1):39–45. [DOI] [PubMed] [Google Scholar]
- 40. Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee: its anatomy and injury patterns associated with acute anterolateral-anteromedial rotatory instability. Am J Sports Med. 1996;24(1):2–8. [DOI] [PubMed] [Google Scholar]
- 41. Terry GC, LaPrade RF. The posterolateral aspect of the knee: anatomy and surgical approach. Am J Sports Med. 1996;24(6):732–739. [DOI] [PubMed] [Google Scholar]
- 42. Terry GC, Norwood LA, Hughston JC, Caldwell KM. How iliotibial tract injuries of the knee combine with acute anterior cruciate ligament tears to influence abnormal anterior tibial displacement. Am J Sports Med. 1993;21(1):55–60. [DOI] [PubMed] [Google Scholar]
- 43. Thein R, Boorman-Padgett J, Stone K, Wickiewicz TL, Imhauser CW, Pearle AD. Biomechanical assessment of the anterolateral ligament of the knee: a secondary restraint in simulated tests of the pivot shift and of anterior stability. J Bone Joint Surg Am. 2016;98(11):937–943. [DOI] [PubMed] [Google Scholar]
- 44. Vieira EL, Vieira EA, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269–274. [DOI] [PubMed] [Google Scholar]
- 45. Vincent JP, Magnussen RA, Gezmez F, et al. The anterolateral ligament of the human knee: an anatomic and histologic study. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):147–152. [DOI] [PubMed] [Google Scholar]
- 46. Warren LF, Marshall JL. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979;61(1):56–62. [PubMed] [Google Scholar]
- 47. Watanabe J, Suzuki D, Mizoguchi S, Yoshida S, Fujimiya M. The anterolateral ligament in a Japanese population: study on prevalence and morphology. J Orthop Sci. 2016;21(5):647–651. [DOI] [PubMed] [Google Scholar]