Abstract
Background:
Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors show promising results in metastatic breast cancer. However, an increased incidence of adverse events is remarkable. Among others, gastrointestinal (GI) involvement is of momentous impact on patients and their quality of life.
Methods:
Our search included PubMed, ASCO, ESMO and SABCS databases. Randomized phase II/III trials in metastatic breast cancer receiving CDK4/6 inhibitors were identified and considered relevant based on providing a sufficient safety profile on the incidence of adverse GI effects.
Results:
Of the 999 records initially screened for relevance, 33 articles were found relevant and 4 studies were finally eligible for meta-analysis with a total of 2007 patients. The relative risk (RR) for all-grade nausea was 1.48 [95% confidence interval (CI): 1.12–1.93, p = 0.005], vomiting was 1.74 (95% CI: 1.09–2.76, p = 0.02), decreased appetite was 1.42 (95% CI: 1.07–1.88, p = 0.02), and for diarrhea it was 1.44 (95% CI: 1.19–1.74, p = 0.0002). Meanwhile, the RR for high-grade nausea was 1.10 (95% CI: 0.29–4.13, p = 0.89), vomiting was 1.38 (95% CI: 0.25–7.75, p = 0.72), decreased appetite was 4.00 (95% CI: 0.87–18.37, p = 0.07), and high-grade diarrhea was 1.19 (95% CI: 0.44–3.21, p = 0.73).
Conclusion:
Selective CDK4/6 inhibitors were not associated with higher-grade GI toxicities reflecting a well-tolerated safety profile. Regarding the increase in all-grade GI toxicities, it needs further caution with addition of cytotoxic chemotherapy.
Keywords: breast cancer, CDK4/6 inhibitors, gastrointestinal toxicity, palbociclib
Introduction
Targeting the cyclin D-CDK4/6-Rb pathway was an alluring task. Other targeted therapies used to tackle growth signals are usually compensated for by the redundancy of those pathways, hence developing resistance is very common.1,2 In contrast, CDK4/6 inhibitors interrupt the most crucial point in the life of the cell (known as the restriction point) when the cell decides to pass from G1 to S phase and pursue another cycle of cell division.3 This critical point is controlled by a well-defined, nonredundant, evolutionarily conserved pathway which is known as the cyclin D-CDK4/6-Rb pathway.4 Cyclin D complexes with CDK4/6 that in turn phosphorylates Rb protein and prevents it from stopping the cell cycle. In 2004, Fry and colleagues5 showed that the specific CDK4/6 inhibitor, palbociclib, has potent antiproliferative effects against Rb-proficient tumor cell lines and human tumor xenografts. Further reports in knockdown mice models revealed that either downregulation of CDK4 expression in mammary epithelial cells or mutant cyclin D1 caused resistance to tumorigenesis mediated by certain oncogenes.6,7 This reflects that the intact cyclin D1-CDK4-Rb pathway is instrumental for initiating breast cancer. In the following years, many clinical trials have been initiated to test efficacy and safety of CDK4/6 inhibitors in breast cancer. In 2014, results of the PALOMA-1 trial indicated double the rate of progression-free survival of metastatic breast cancer patients receiving palbociclib and letrozole versus those receiving letrozole only.8 This was further confirmed by the phase III PALOMA-2 trial.9 Both trials led to a United States Food and Drug Administration (US FDA) approval of a palbociclib/letrozole combination for metastatic breast cancer. Another combination of palbociclib/fulvestrant was also approved based on recent results from the PALOMA-3 trial.10
Nevertheless, the adverse effects associated with this newly introduced class of drugs were remarkable and might be troublesome in the palliative setting especially with long term use. Gastrointestinal (GI) adverse effects such as nausea, vomiting and diarrhea are universal events of most anticancer drugs so, it is essential to analyze the exact burden added from CDK4/6 inhibitors in this regard.
Material and methods
Literature search strategy
PubMed/MEDLINE searches were conducted using keywords ‘palbociclib’ OR ‘ribociclib’ OR ‘abemaciclib’ OR ‘CDK 4/6 inhibitor’ AND ‘breast cancer.’ Further search was performed in Google Scholar and databases of major oncology congresses from January 2010 to October 2016, including those of the American Society of Clinical Oncology, European Society of Medical Oncology and the San Antonio Breast Cancer Symposium. Clinical trials in English were retrieved and their bibliography was scanned for relevant articles. This was implemented according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.11
Inclusion and exclusion criteria
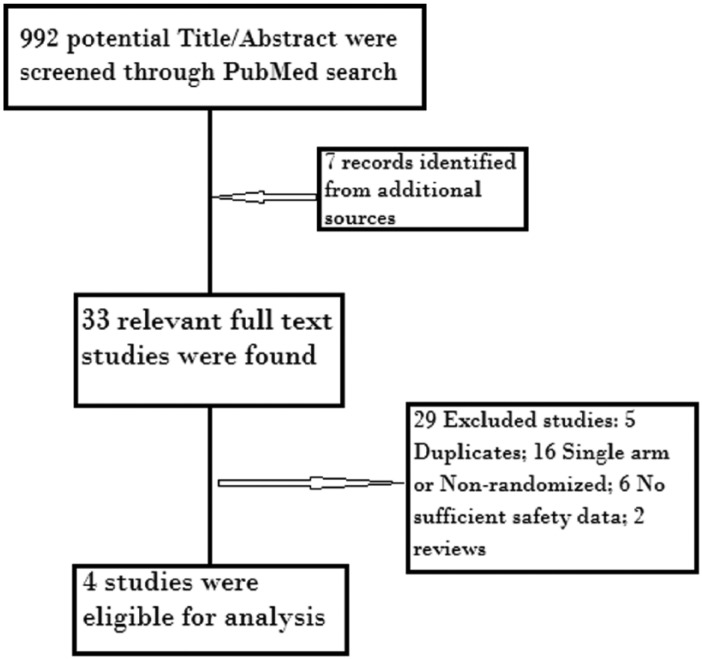
We included trials that met the following criteria: (1) phase II or III randomized clinical trials recruiting patients with breast cancer; (2) patients had to be randomly assigned to a CDK4/6 inhibitor (including palbociclib, ribociclib and abemaciclib) or control (placebo treatment); and (3) rate of GI toxicity was given along with an assessable sample size. The following were the exclusion criteria: (1) phase I trials; (2) nonrandomized trials; (3) duplicates of previous publications on the same population; and (4) insufficient reporting of the safety data. A flowchart of all the steps of the systematic review is depicted in Figure 1.
Figure 1.
Flowchart of the systematic review process.
Data extraction
A standardized protocol for data abstraction was used by two independent reviewers (LK, KS) to extract the following information from each study: surname of first author, year of publication, study phase, treatment arms, number of patients evaluable for analysis, number of patients that developed all-grade and high-grade (grade 3/4) nausea, vomiting, diarrhea and decreased appetite.
Statistical analysis
For each GI adverse event, relative risk (RR) and corresponding 95% confidence interval (CI) were the main effect measure. The number of events of each adverse effect was compared between participants assigned to the CDK4/6 inhibitors arm or control treatment arm in each eligible trial. Outcome heterogeneity among the studies in this analysis was checked by Cochrane’s Q test. To avoid the potential heterogeneity resulting from the use of two different CDK 4/6 inhibitors (palbociclib versus ribociclib) in the analysis, a random effect model was used in the subanalyses. Review Manager, version 5.3 (Nordic Cochrane Centre; Copenhagen, Denmark) was used for data analyses.
Results
Characteristics of the studies
A total of 992 records were identified through a PubMed search with 7 records from additional sources. After screening the title/abstract, only 33 articles were found relevant. Further data retrieval from relevant full-text articles yielded a further four studies that were eligible for meta-analysis (three were phase III and one was phase II). Causes of exclusion are outlined in Figure 1 along with the process of systematic review. A total of two studies9,12 compared a combination of palbociclib with letrozole versus letrozole alone in postmenopausal ER+/HER2-advanced breast cancer, one10 compared palbociclib with fulvestrant versus fulvestrant alone in pre- and postmenopausal women, the last one13 compared ribociclib with letrozole versus letrozole alone in post-menopausal women. Overall, two studies used abemaciclib14 and ribociclib15 in neoadjuvant settings and they were excluded because they did not report complete safety data and the period of drug intake did not exceed 14 days.
The meta-analysis included a total of 2007 patients; the majority of them had an Eastern Cooperative Oncology Group (ECOG) performance score ⩽1 (see Table 1). No exclusion criteria of patients with chronic GI diseases were found. The risk of bias was assessed using Cochrane risk of bias tool and results are shown in Figure 2.
Table 1.
The characteristics of the four included studies in the analysis.
| Study | Treatment regimen | Evaluable patients | Indication | Median age (year) case, control | ECOG-PS (%) |
Prior chemotherapy (%) case, control | Duration of anti-CDK 4/6 Treatment median in months | |
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | |||||||
| PALOMA-1, phase II1,7 | Palbociclib + letrozole versus letrozole alone | 165 (83 versus 77) |
Advanced breast cancer, no prior systemic treatment | 63, 64 | 55, 56 | 45, 44 | 40, 46 | 27.9, 29.6 |
| PALOMA-2, phase III8 | Palbociclib + letrozole | 666 (444 versus 222) |
advanced breast cancer, no prior systemic treatment | 62, 61 | 58%, 46% | 40%, 53% | n/a | n/a |
| PALOMA-3, Phase III9,20 | Fulvestrant + palbociclib or placebo | 517 (345 versus 172) |
endocrine-resistant MBC | 57, 56 | 207 (59.7), 115 (66.1) | 140 (40.3), 59 (33.9) | 251 (72.3%), 138 (79.3%) | 5.6 |
| MONALEESA-2, phase III12 | Ribociclib + letrozole or placebo + letrozole | 668 | recurrent or MBC, no prior systemic therapy | 62, 63 | 61.4, 60.5 | 38.6, 39.5 | 43.7, 43.4 | 15.3 |
ECOG-PS, Eastern Cooperative Oncology Group Performance Status; n/a, not applicable; MBC, Metastatic Breast Cancer.
Figure 2.
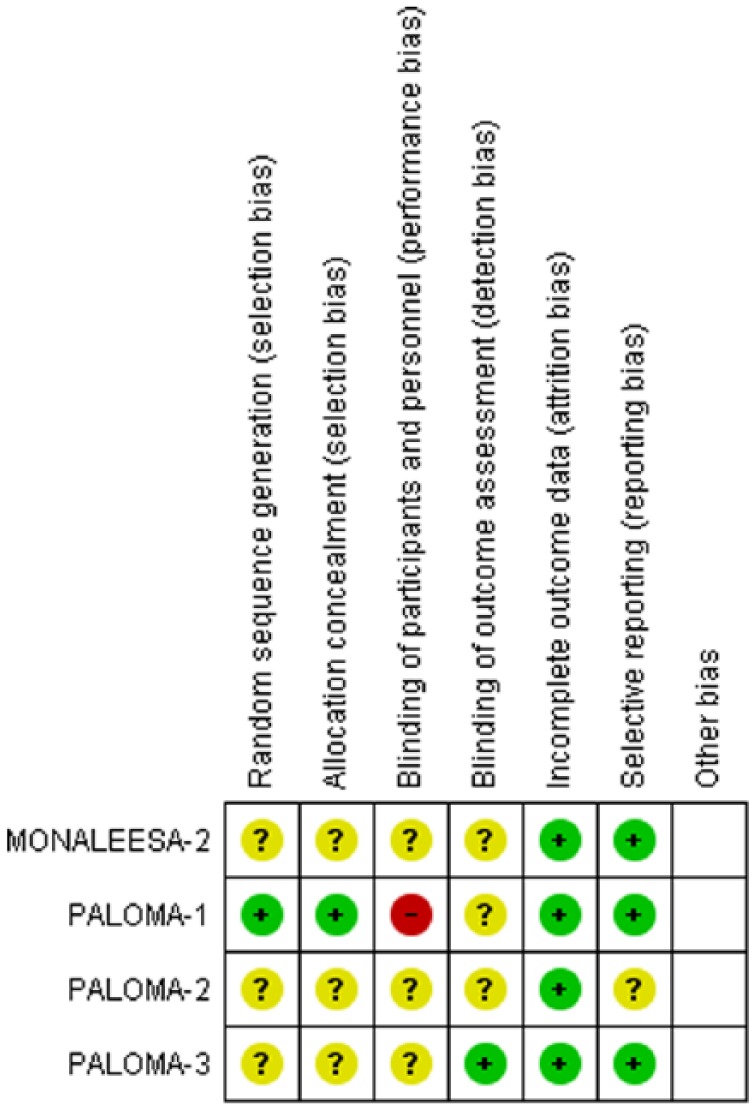
Risk of bias summary.
Incidence of GI adverse effects
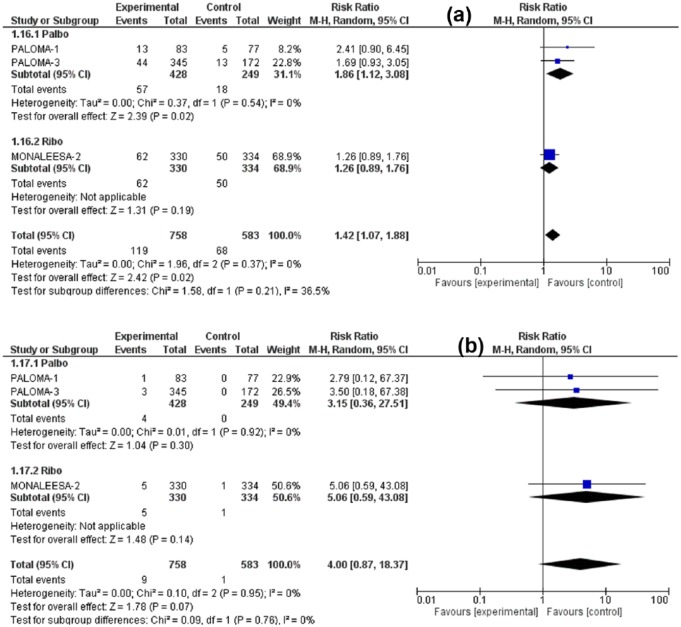
In the intervention arm (either palbociclib or ribociclib), the incidence of all-grade GI toxicities ranged for nausea 24.5–51.5%, vomiting 14–29.3%, diarrhea 19.1–35%, and for decreased appetite 12.8–18.6%. On the other hand, the incidence of high-grade (3–4) nausea was 1–2.4%, vomiting 0–3.6%, diarrhea 0–4%, and decreased appetite 0–1.5% (See Table 2).
Table 2.
The incidence of gastrointestinal adverse events among the four eligible studies.
| Toxicity Profile | PALOMA-1 |
PALOMA-2 |
PALOMA-3 |
MONALEESA-2 |
||||
|---|---|---|---|---|---|---|---|---|
| Casen = 83 (%) | Controln = 77 (%) | Casen = 444 (%) | Controln = 222 (%) | Casen = 345 (%) | Controln = 172 (%) | Case n = 334 (%) |
Controln = 330 (%) | |
| Any adverse effect | 82 (98.8) | 65 (84.4) | 440 (99) | 211 (95) | 337 (97.7) | 153 (89) | 329 (98.5) | 320 (97) |
| Serious AE | 7 (8.4) | 0 | 87 (19.6) | 28 (12.6) | 33 (9.6) | 24 (14) | 71 (21.3) | 39 (11.8) |
| Nausea all G | 21 (25.3) | 10 (13) | 155 (35) | 58 (26) | 100 (29) | 45 (26.2) | 172 (51.5) | 94 (28.5) |
| Nausea G 3–4 | 2 (2) | 1 (1) | 4 (<1) | 4 (2) | 0 | 1 (0.6) | 8 (2.4) | 2 (0.6) |
| Vomiting all G | 12 (14) | 3 (3.9) | N/A | N/A | 50 (14.5) | 21 (12.2) | 98 (29.3) | 51 (15.5) |
| Vomiting G 3–4 | 0 | 1 (1) | N/A | N/A | 1 (0.3) | 1 (0.6) | 12 (3.6) | 3 (0.9) |
| Diarrhea all G | 17 (20.5) | 8 (10) | 115 (26) | 42 (19) | 66 (19.1) | 30 (17.4) | 117 (35) | 73 (22.1) |
| Diarrhea G 3–4 | 3 (4) | 0 | 4 (1) | 2 (1) | 0 | 1 (0.6) | 4 (1.2) | 3 (0.9) |
| Decreased appetite all G | 13 (15.7) | 5 (6) | N/A | N/A | 44 (12.8) | 13 (7.6) | 62 (18.6) | 50 (15.2) |
| Decreased appetite G 3–4 | 1 (1) | 0 | N/A | N/A | 3 (0.9) | 0 | 5 (1.5) | 1 (0.3) |
| Treatment discontinuation due to toxicity | 11 (13.3) | 2 (2.6) | 43 (9.7) | 13 (5.9) | 9 (2.6) | 3 (0.9) | 25 (7.50) | 25 (7.50) |
| Treatment-related deaths | 0 | 0 | 2.3 | 1.8 | 0 | 0 | 1 | 0 |
AE, adverse event; G, grade; N/A, not applicable.
RR of GI toxicities
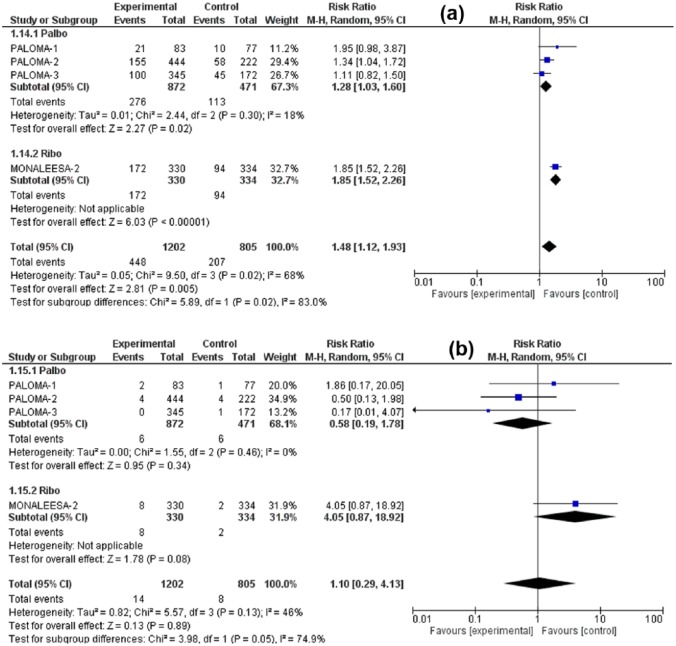
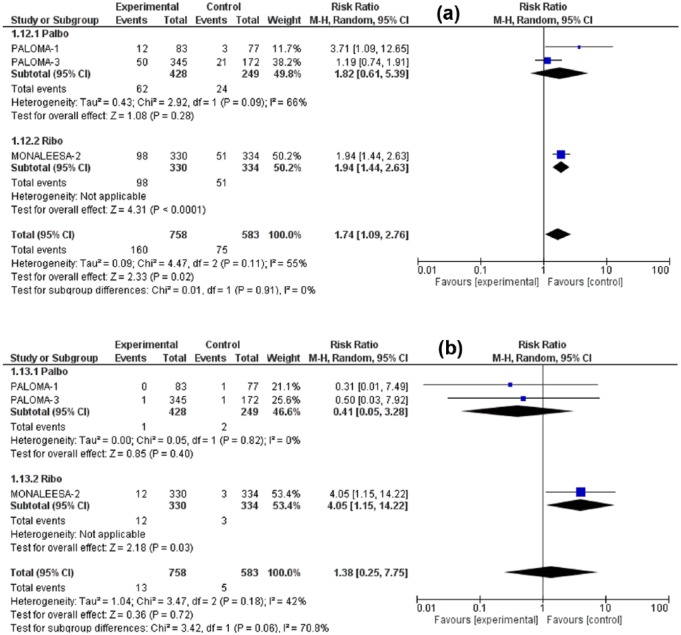
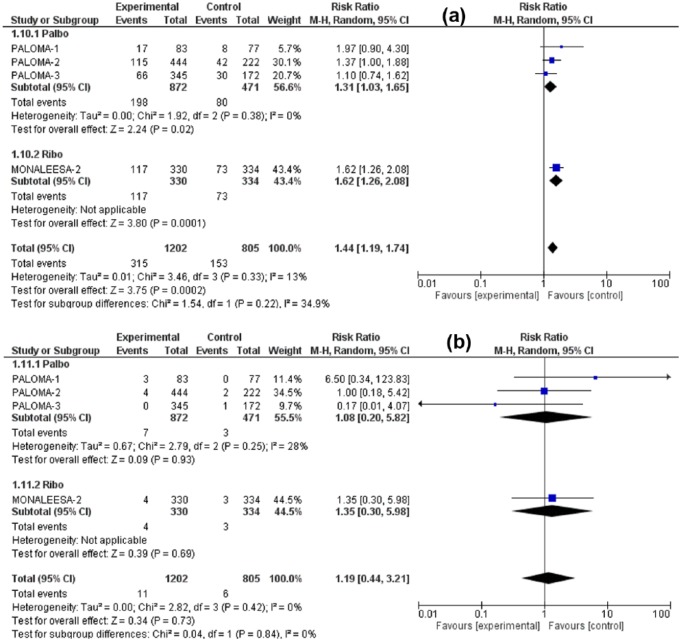
The RR for all-grade nausea was 1.48 (95% CI: 1.12–1.93, p = 0.005), for all-grade vomiting was 1.74 (95% CI: 1.09–2.76, p = 0.02), for all-grade decreased appetite 1.42 (95% CI: 1.07–1.88, p = 0.02) for all-grade diarrhea 1.44 (95% CI: 1.19–1.74, p = 0.0002). Meanwhile, the RR for high-grade (grade 3/4) nausea was 1.10 (95% CI: 0.29–4.13, p = 0.89), for high-grade vomiting 1.38 (95% CI: 0.25–7.75, p = 0.72) for high-grade diarrhea 1.19 (95% CI: 0.44–3.21, p = 0.73), and for high-grade decreased appetite 4.00 (95% CI: 0.87–18.37, p = 0.07). Although all-grade GI toxicities were associated with significantly higher RR in the CDK4/6 inhibitors arm versus control treatment, the high-grade toxicities were nonsignificantly higher. Figures 3–6 illustrate the forest plots for all-grade and high-grade GI adverse effects for palbociclib and ribociclib versus the control treatment.
Figure 3.
Forest plot of all-grade (a) and high-grade (b) nausea.
CI, confidence interval
Figure 4.
Forest plot of all-grade (a) and high-grade (b) vomiting.
Figure 5.
Forest plot of all-grade (a) and high-grade (b) diarrhea.
Figure 6.
Forest plot of all-grade (a) and high-grade (b) decreased appetite.
Given that the analysis comprises two different drugs in the intervention arm, a subgroup analysis was performed (palbociclib versus ribociclib). The variation in the mean effects in the two subgroups was significant in all-grade vomiting, high-grade vomiting and high-grade decreased appetite otherwise no significant subgroup difference was found in remaining toxicities. Interestingly, the ribociclib subgroup was associated with increased risk of all-grade and high-grade vomiting, but with lower risk of high-grade decreased appetite in comparison with the palbociclib subgroup. However, it has to be noted that the total number of studies in these analyses is too small to get a confident conclusion about differences in toxicities between the two agents. Furthermore, it is not clear if any GI toxicities were contributors to dose modifications or discontinuation of treatment. There was no significant increase in treatment-related deaths due to GI toxicities.
Discussion
To the best of our knowledge, this is the first analysis to assess the risk of GI toxicities associated with CDK 4/6 inhibitors. Our analysis suggests that adding a CDK 4/6 inhibitor to hormonal therapy marginally increased the incidence of any-grade decreased appetite, nausea, vomiting and diarrhea with no significant increase in the risk of high-grade GI toxicities compared with control.
Chemotherapeutics usually exert their GI adverse effects through their toxic effect on the rapidly proliferating GI mucosa. In addition, other antineoplastic agents may cause nausea and vomiting by directly acting on the vomiting center in the brain. CDK 4/6 inhibitors act by releasing the inhibition of the Rb protein so that it can exert its role as a G1-S cell cycle check point. As a result, cell cycle arrest in quiescent phases occurs with marked inhibition of cancer cells and also normal cell proliferation.16 Of the normal tissues, one of the most vulnerable tissues to this antiproliferative action is the GI epithelium. In mouse models, it was evident that the binding of cyclin D3 to CDK4 and 6 is essential for intestinal epithelial cell proliferation.17 It is noteworthy that the effect of the cyclin D-CDK4/6 pathway in the GI epithelial cells is complex. GI symptoms do not only develop due to the direct toxic effect on mucosa, but also due to functional defects in certain brush-border enzymes, disturbances in cellular response to injury and interplay with other pathways.17–19 Of those pathways interacting with CDK4/6, Wnt/β-catenin, mitogen-activated protein kinase and nuclear factor (NF)-kB contribute largely to physiology of Gut.3,20
Despite the increase in all-grade GI toxicities, there was no significant increase in the grade 3–4 toxicities; this is quite reassuring with the expanding use of such drugs in patients with metastatic breast cancer. As suggested in preclinical studies, the antiproliferative effect of CDK4/6 inhibitors may differ between malignant and normal cells with more profound and longer inhibition of the malignant clones than with normal cells, which recover rapidly with minimal permanent damage.21 In the phase I trial of palbociclib,22 the most common dose-limiting toxicity was neutropenia and surprisingly it was not associated with concomitant diarrhea. Furthermore, the increased side effects associated with addition of palbociclib in the PALOMA3 study did not lead to deterioration in the patient-reported quality of life outcomes published by Harbeck and colleagues.23
The management of GI symptoms induced by CDK 4/6 inhibitors is not yet well established. It should also be noted that palbociclib has (as with other targeted therapies) serious drug interactions with several medications; of interest here are those drugs commonly used to treat GI symptoms. For example, in an early phase drug-interaction study on healthy participants, administration of multiple doses of the proton pump inhibitor rabeprazole, decreased serum concentration of palbociclib by 41%; such interference is expected to be minimal with the use of histamine 2 blockers and local antacids.24
Moreover, two of the most common drugs used to treat chemotherapy-induced nausea and vomiting should not be used to treat palbociclib-induced nausea and vomiting: dexamethasone and aprepitant. By either inducing or inhibiting the CYP3A4 enzyme, dexamethasone and aprepitant may decrease or increase palbociclib serum levels respectively.24 In that aspect, metoclopramide and domperidone appear to be well tolerated with CDK 4/6 inhibitors. These interaction patterns should be taken into account in future studies combining CDK 4/6 inhibitors with cytotoxic chemotherapy.
During the peer review process of this article, the results of the MONARCH 2 study were published. In this phase III study, patients progressing on firstline hormonal therapy were randomized to either fulvestrant alone or fulvestrant and abemaciclib. In concordance with our findings, addition of abemaciclib was associated with significant increase in the risk of all-grade diarrhea, nausea and vomiting.25
However, our analysis has some potential weaknesses and results should be taken cautiously. First, the GI toxicities might be caused by some of the concomitant medications/diseases that patients with metastatic breast cancer might use/suffer from. Such medications/diseases were not accounted for in our analysis and may confound the results. Second, our study included two different CDK 4/6 inhibitors (palbociclib and ribociclib) and two different hormonal backbones (letrozole and fulvestrant) and such heterogeneity may influence the outcome. Finally, this was not an individual data level analysis26, meaning that individual confounders were not taken into account.
In conclusion, our analysis shows that the use of CDK 4/6 inhibitors causes an increased risk of any-grade decreased appetite, nausea, vomiting and diarrhea with no significant increase in the higher-grade toxicities compared with control arms. These results should be taken seriously in the ongoing trials combining CDK 4/6 inhibitors with cytotoxic chemotherapy. Furthermore, choice of proper anti-emetics and antidiarrheals should be revised according to the drug-interaction profile of each CDK4/6 inhibitor separately.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: LK received a research grant from Novartis oncology. Other authors report no conflicts of interest.
Contributor Information
Kyrillus S. Shohdy, Clinical Oncology Department, Kasr Alainy School of Medicine, Cairo University, Al-Saray St. El-Maniel, 11451, Cairo, Egypt.
Shaimaa Lasheen, Clinical Oncology Department, Kasr Alainy School of Medicine, Cairo University, Cairo, Egypt.
Loay Kassem, Clinical Oncology Department, Kasr Alainy School of Medicine, Cairo University, Cairo, Egypt.
Omar Abdel-Rahman, Clinical Oncology Department, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
References
- 1. Murphy CG, Dickler MN. The role of CDK4/6 inhibition in breast cancer. Oncologist 2015; 20: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garber K. The cancer drug that almost wasn’t. Science 2014; 345: 865–867. [DOI] [PubMed] [Google Scholar]
- 3. Xu H, Yu S, Liu Q, et al. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J Hematol Oncol 2017; 10: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rocca A, Farolfi A, Bravaccini S, et al. Palbociclib (PD 0332991): targeting the cell cycle machinery in breast cancer. Expert Opin Pharmacother 2014; 15: 407–420. [DOI] [PubMed] [Google Scholar]
- 5. Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004; 3: 1427–1438. [PubMed] [Google Scholar]
- 6. Landis MW, Pawlyk BS, Li T, et al. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 2006; 9: 13–22. [DOI] [PubMed] [Google Scholar]
- 7. Yu Q, Sicinska E, Geng Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 2006; 9: 23–32. [DOI] [PubMed] [Google Scholar]
- 8. Finn RS, Crown JP, Ettl J, et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res 2016; 18: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finn R, Martin M, Rugo HS, et al. PALOMA-2: primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2 - advanced breast cancer (ABC). J Clin Oncol 2016; 34(Suppl. 15): 507. [Google Scholar]
- 10. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015; 373: 209–219. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16: 25–35. [DOI] [PubMed] [Google Scholar]
- 13. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 14. Hurvitz S, Abad MF, Rostorfer R, et al. Breast cancer, early stageInterim results from neoMONARCH: A neoadjuvant phase II study of abemaciclib in postmenopausal women with HR + /HER2- breast cancer (BC). Ann Oncol 1 October 2016; 27(Suppl. 6). [Google Scholar]
- 15. Curigliano G, Gómez Pardo P, Meric-Bernstam F, et al. Ribociclib plus letrozole in early breast cancer: a presurgical, window-of-opportunity study. Breast 2016; 28: 191–198. [DOI] [PubMed] [Google Scholar]
- 16. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ko TC, Pan F, Sheng H, et al. Cyclin D3 is essential for intestinal epithelial cell proliferation. World J Surg 2002; 26: 812–818. [DOI] [PubMed] [Google Scholar]
- 18. Wei L, Leibowitz BJ, Wang X, et al. Inhibition of CDK4/6 protects against radiation- induced intestinal injury in mice. J Clin Invest 2016; 126: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ding QM, Ko TC, Evers BM. Caco-2 intestinal cell differentiation is associated with G1 arrest and suppression of CDK2 and CDK4. Am J Physiol 1998; 275: C1193– C1200. [DOI] [PubMed] [Google Scholar]
- 20. Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal 2014; 26: 570–579. [DOI] [PubMed] [Google Scholar]
- 21. Hu W, Sung T, Jessen BA, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res 2016; 22: 2000–2008. [DOI] [PubMed] [Google Scholar]
- 22. Flaherty KT, LoRusso PM, DeMichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res 2012; 18: 568–576. [DOI] [PubMed] [Google Scholar]
- 23. Harbeck N, Iyer S, Turner N, et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: patient-reported outcomes from the PALOMA-3 trial. Ann Oncol 2016; 27: 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palbociclib. Product Information.Ibrance (palbociclib). Pfizer US Pharmaceuticals Group, New York, NY: Pfizer, https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207103s000lbl.pdf (accessed 11 November 2016). [Google Scholar]
- 25. Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35: 2875–2884 [DOI] [PubMed] [Google Scholar]
- 26. Verma S, Huang Bartlett C, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist 2016; 21: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]