Abstract
Background:
Anticholinergic (AC) adverse drug events (ADEs) are caused by inhibition of muscarinic receptors as a result of designated or off-target drug–receptor interactions. In practice, AC toxicity is assessed primarily based on clinician experience. The goal of this study was to evaluate a novel concept of integrating big pharmacological and healthcare data to assess clinical AC toxicity risks.
Methods:
AC toxicity scores (ATSs) were computed using drug–receptor inhibitions identified through pharmacological data screening. A longitudinal retrospective cohort study using medical claims data was performed to quantify AC clinical risks. ATS was compared with two previously reported toxicity measures. A quantitative structure–activity relationship (QSAR) model was established for rapid assessment and prediction of AC clinical risks.
Results:
A total of 25 common medications, and 575,228 exposed and unexposed patients were analyzed. Our data indicated that ATS is more consistent with the trend of AC outcomes than other toxicity methods. Incorporating drug pharmacokinetic parameters to ATS yielded a QSAR model with excellent correlation to AC incident rate (R2 = 0.83) and predictive performance (cross validation Q2 = 0.64). Good correlation and predictive performance (R2 = 0.68/Q2 = 0.29) were also obtained for an M2 receptor-specific QSAR model and tachycardia, an M2 receptor-specific ADE.
Conclusions:
Albeit using a small medication sample size, our pilot data demonstrated the potential and feasibility of a new computational AC toxicity scoring approach driven by underlying pharmacology and big data analytics. Follow-up work is under way to further develop the ATS scoring approach and clinical toxicity predictive model using a large number of medications and clinical parameters.
Keywords: adverse drug reactions, anticholinergic toxicity, clinical toxicology, big data, drug safety, biomedical informatics
Introduction
Anticholinergics (ACs), as a drug class, provide significant therapeutic benefits in a variety of disease states. Many non-AC medications, however, may also elicit AC pharmacologic responses through off-target interactions with muscarinic receptors. The clinical consequences are well documented, ranging from subtle cognitive changes to acute symptoms such as tachycardia, delirium, hallucinations, etc.1 It has been reported that up to 60% of elderly patients in primary care settings receive at least one medication that may lead to AC toxicity.2,3 Given the widespread use of these medications, there is a critical need of a rational and efficient method for assessing AC risks.
A variety of AC toxicity scales have been proposed.4–9 The anticholinergic risk scale (ARS)6 and anticholinergic cognitive burden (ACB)5 assigned 0–3 toxicity scales to medications based on clinician opinion. Others measured drug–receptor dissociation constants from in vitro experiments.10 Attempts have been made to improve the scales by adding additional drugs,11 or combining several different scales.12 However, none of these approaches has emerged as a gold standard for reliable AC toxicity assessment.13–17 Furthermore, the plethora of AC toxicity measures attests to the need and challenges of developing a systematic, efficient, and clinically relevant AC toxicity scoring approach.
However, there is an ever-increasing wealth of freely available bioactivity and pharmacological data. The ChEMBL21 database,18 the largest bioactivity database in the world, contains more than 1.5 million small molecules, 10,000 receptors, and 14 million bioactivity records. Adopting established bioinformatics algorithms,19 the vast amount of pharmacological data can be mined to identify relevant drug–receptor inhibitions (including inhibitions as a result of off-target interactions) and form the basis of computational AC toxicity scores (ATSs). Similarly, AC clinical responses can be extracted from large healthcare data such as medical insurance claims. Thus, combining pharmacological and insurance claims data may afford an efficient and noninvasive method to assess drug-induced AC toxicity. Our goal in this work is not a complete computational solution, but rather to test the feasibility of the new big data-based approach.
Methods
Medications
To elucidate the complex nature of drug-induced toxicity, we need to capture the molecular-level information that entails drug actions at relevant receptors. There are five muscarinic (M) receptor subtypes that are involved in AC toxicity: M1, M4, and M5 are predominantly expressed in the central nervous system (CNS); M2 is mostly found in cardiac tissue; M3 is predominantly present in the gastrointestinal tract and genitourinary tissues.20 Due to funding limitations, a subset of 25 medications were selected for this pilot study (Table 1). The selection criteria include: (1) commonly prescribed; (2) representatives of eight major drug classes (antidepressants, antihistamines, anti-Parkinson’s agents, antipsychotics, benzodiazepines, gastrointestinal agents, muscle relaxants, and opioids);21 and (3) mostly consistently ranked by ACB and ARS scales. It is worth noting that many of the 25 medications are not considered as classic ACs or antimuscarinics.
Table 1.
Selected medications and their ATS scores.
| Medication | Drug class | Muscarinic receptors |
Total ATS | ACB5 | ARS6 | ||||
|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | |||||
| Amitriptyline | Antidepressants | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Imipramine | Antidepressants | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Brompheniramine | Antihistamines | 0.75 | 0.43 | 0.43 | 0.75 | 0.75 | 3.11 | 3 | NA |
| Carbinoxamine | Antihistamines | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 3.25 | 3 | NA |
| Chlorpheniramine | Antihistamines | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Diphenhydramine | Antihistamines | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Benztropine | Anti-Parkinson agents | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Trihexyphenidyl | Anti-Parkinson agents | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | NA |
| Chlorpromazine | Antipsychotics | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Haloperidol | Antipsychotics | 1.00 | 0.39 | 0.39 | 1.00 | 1.00 | 3.78 | 1 | 1 |
| Perphenazine | Antipsychotics | 0.78 | 0.78 | 0.78 | 0.78 | 0.78 | 3.90 | 3 | 3 |
| Risperidone | Antipsychotics | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 1 | 1 |
| Thioridazine | Antipsychotics | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Trifluoperazine | Antipsychotics | 0.81 | 0.70 | 0.81 | 0.81 | 0.70 | 3.83 | 3 | 3 |
| Alprazolam | Benzodiazepines | 0.00 | 0.00 | 0.00 | 0.30 | 0.30 | 0.60 | 1 | NA |
| Clorazepate | Benzodiazepines | 0.34 | 0.34 | 0.34 | 0.34 | 0.34 | 1.70 | 1 | NA |
| Diazepam | Benzodiazepines | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 2.00 | 1 | NA |
| Atropine | Gastrointestinal agents | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Dicyclomine | Gastrointestinal agents | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Hyoscyamine | Gastrointestinal agents | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Loperamide | Gastrointestinal agents | 0.49 | 0.49 | 0.49 | 0.49 | 0.49 | 2.45 | 1 | 2 |
| Promethazine | Gastrointestinal agents | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | 3 |
| Ranitidine | Gastrointestinal agents | 0.65 | 0.65 | 0.00 | 0.00 | 0.00 | 1.30 | 1 | 1 |
| Orphenadrine | Muscle relaxants | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 | 3 | NA |
| Fentanyl | Opioids | 0.39 | 0.42 | 0.42 | 0.38 | 0.38 | 1.99 | 1 | NA |
ACB, anticholinergic cognitive burden; ARS, anticholinergic risk scale; ATS, anticholinergic toxicity score; NA, not available.
Computing ATS scores
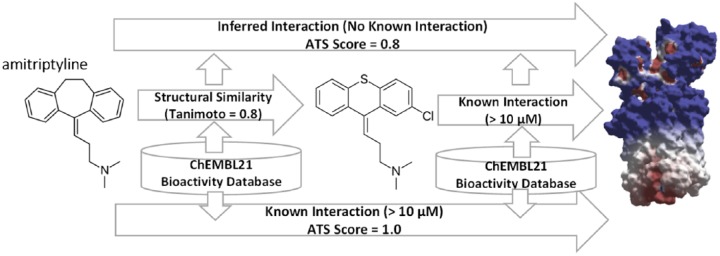
The molecular structures of the 25 medications were retrieved from DrugBank22 and used to query the ChEMBL21 bioactivity database using our in-house TargetSearch program (http://dxulab.pharmacy.isu.edu). ChEMBL21 was searched for either known bioactivity between a medication and five muscarinic receptor subtypes or unknown off-target interactions via inferred structure-bioactivity relationships, the cornerstone of our computational ATS approach. If a query medication was found to have similar chemical structure and features to a bioactive molecule in the database, and this bioactive molecule has known bioactivity data associated with any of the five muscarinic receptors, we can infer that the medication will share similar bioactivity on the same receptors. The widely used extended connectivity fingerprints (ECFPs) or Morgan algorithm23 was used in the TargetSearch program. A 10 µM inhibitory activity cutoff was used to ensure a higher level of confidence in identifying known and inferred relationships. When a hit is found, Tanimoto coefficients24 calculated by the Morgan algorithm23 yield receptor-specific ATS scores, representing the drug–receptor inhibition propensity. The receptor-specific ATS scores are on a continuous scale ranging 0–1. A receptor-specific ATS score of 1 indicates a medication has known bioactivity to a muscarinic receptor whereas a score of 0 means no known or inferred interaction is found. A score between 0–1 indicates an inferred interaction is identified. The individual receptor subtype ATS scores were summed to give the total ATS score of a medication. This computational approach, illustrated in Figure 1, essentially accounts for pharmacodynamic interactions of medications. It is fast, systematic, and has been shown to effectively capture off-target interactions.21
Figure 1.
Schematic workflow of the computational ATS scoring approach.
ATS, anticholinergic toxicity score.
ATS versus ACB and ARS
In order to qualitatively compare ATS with previously reported ACB and ARS scales, the ATS scores were converted from a continuous scale to an ordinal AC toxicity scale (1 = low, 2 = moderate, and 3 = high) used by ACB and ARS. Using ataxia, the most common ADE associated with AC toxicity (Table 2) as an example, the cumulative incidence rates were summed across medications at each severity category (1–3 ordinal scale). Correlations between cumulative incidence rates and three AC toxicity scales were analyzed.
Table 2.
Demographic and clinical characteristics of the retrospective cohort.
| Exposed |
Unexposed |
Exposed |
Unexposed |
||
|---|---|---|---|---|---|
| (N = 287,614) | (N = 287,614) | (N = 287,614) | (N = 287,614) | ||
| Demographic characteristics | Possible AC-related ADEs | ||||
| Age in years (mean, SD) | 37.97, 18.79 | 37.97, 18.79 | Ataxia | 20,104, 6.99% | 4465, 1.55%* |
| (median, range) | 39, 5–84 | 39, 5–84 | Constipation | 2969, 1.03% | 551, 0.19%* |
| <65 (N, %) | 268,414, 93.32 | 268,414, 93.32 | Agitation | 2027, 0.70% | 601, 0.21%* |
| ⩾65 (N, %) | 19,200, 6.68 | 19,200, 6.68 | Tachycardia | 1970, 0.68% | 798, 0.28%* |
| Male (N, %) | 126,376, 43.94% | 126,376, 43.94% | Pupil Dilation | 1436, 0.50% | 978, 0.34%* |
| Region (N, %) South | 124,538, 43.30% | 76,517, 26.6%* | Fractures | 1264, 0.44% | 820, 0.29%* |
| Midwest | 74,412, 25.87% | 106,246, 36.94% | Syncope | 979, 0.34% | 302, 0.11%* |
| West | 42,038, 14.62% | 39,496, 13.73% | Hallucinations | 900, 0.31% | 259, 0.09%* |
| East | 46,626, 16.21% | 65,355, 22.72% | Glaucoma | 745, 0.26% | 1219, 0.42%* |
| Medicare (N, %) | 10,779, 3.75% | 6688, 2.33%* | Urinary retention | 491, 0.17% | 173, 0.06%* |
| Clinical characteristics | Delirium | 277, 0.10% | 77, 0.03%* | ||
| CDI (mean, SD) | 1.39, 1.72 | 1.17, 1.59* | Dementia | 165, 0.06% | 124, 0.04%* |
| (median, range) | 1.0, 0–16 | 1.0, 0–18 | Irritability | 19, 0.01% | 13, 0.00%* |
| Charlson (mean, SD) | 0.38, 1.01 | 0.32, 0.88* | Hypotension | 137, 0.05% | 45, 0.02%* |
| (median, range) | 0, 0–17 | 0, 0–17 | Fractures Colles | 131, 0.05% | 64, 0.02%* |
| Length of follow-up in days (mean, SD) | 33.48, 108.8 | 33.48, 108.8 | Xerostomia | 41, 0.01% | 15, 0.01%* |
| (median, range) | 9.0, 1–4043 | 9.0, 1–4043 | Confusion | 16, 0.01% | 6, 0.00%* |
| Sum of Outcomes (mean) | 0.18 | 0.037* | Jerking | 15, 0.01% | 2, 0.00%* |
| (median, range) | 0, 0–8 | 0, 0–8 | Poisoning | 1, 0.00% | 0, 0.00%* |
p < 0.05.
AC, Anticholinergic; ADE, adverse drug event; CDI, Chronic Disease Indicator; SD, standard deviation.
Retrospective cohort study
The PharMetrics Legacy Health Plans Claims Data, a de-identified longitudinal database comprising medical and pharmacy insurance claims from over 102 managed care programs, is considered representative of the United States commercially insured population.25 A 10% random sample of the database (75 million unique patients) from January 2001 through December 2013 was used to identify an exposed cohort according to the following criteria: (1) filled at least one of 25 medications (Table 1); (2) had at least 1 year of continuous health plan enrollment prior to and 3 months of continuous health plan enrollment following their earliest medication fill; (3) had no AC-related ADEs during the 180 days prior to their earliest medication fill; and (4) were aged 5 years or older at the time of their earliest medication fill. Medication exposures were assessed during each patient’s follow-up period, which began with their earliest medication fill and ended when a patient had discontinued all medications (no fills within 45 days of the last fill + the last days supplied); had a gap of at least 1 month in their continuous health plan enrollment; or the data period ended). Indicator variables (yes/no) for each of the 25 medications were used to identify patients with one or more fills of each medication.
An unexposed control cohort was extracted according to the following criteria: (1) never filled any of the 25 medications; (2) matched to patients in the exposed cohort on age, sex, and length of continuous health plan enrollment.
A total of 19 common AC-related ADEs were collected from clinical literature and compendial references.1 It is worth noting that these 19 ADEs are not meant to be an exhaustive list of all potential AC-related ADEs, but represent a majority of well accepted ADEs. ICD-9-CM diagnosis codes were used to identify patients with suspected AC toxicity during the follow-up period. The first occurrence of each ADE was captured. For each patient, indicator (yes/no) variables were created for each of the 19 ADEs. The total number of distinct ADEs per patient was also calculated. To account for confounding by indication, agitation, irritability, and hallucinations were not included as eligible ADEs if a patient had any fills for antipsychotics or benzodiazepines.
Mean, standard deviation, median, and range were calculated to describe continuous clinical and demographic characteristics, while percentages were used to describe categorical characteristics. Comorbidities were assessed using the Charlson Comorbidity Index (CCI) and Chronic Disease Indicator (CDI) scores.26,27 The CCI score uses medical claims to assign a comorbidity score based on the number and severity of 19 comorbid conditions. The CDI score uses medication claims to determine the number of chronic diseases. This study received institutional review board exempt status from the Idaho State University and Colorado Multiple Institutional Review Boards.
Calculation of AC clinical risks
For each of the 25 medications, the number of exposed patients and the number of ICD-9 diagnosis codes representing potential AC-related ADEs were determined. To account for potential confounding effects due to exposure to nonstudy medications and other clinical factors, the number of ADEs in the exposed cohort was adjusted by subtracting the baseline number of ADEs in the unexposed cohort. The clinical risks of overall AC-related ADEs and tachycardia for each medication were calculated by dividing the adjusted number of ADEs from the number of exposed patients, and reported as the cumulative incidence rates per patient and per 100 patients, respectively. SAS version 9.4 and Microsoft Excel 2013 were used in data analysis.
Calculation of drug absorption and distribution
For each of the 25 medications, a set of 7 bioavailability-related parameters [molecular volume, hydrogen bond acceptors, polarizability, polar surface area, Caco-2 cell model (human epithelial colorectal adenocarcinoma cells used to model the gut–blood barrier), central nervous system activity, human serum albumin binding] were calculated using the QikProp program.28 These descriptors account for various aspects of drug absorption and distribution such as solubility, membrane permeability, bioavailability in serum, gut–blood barrier permeability, and brain–blood barrier (BBB) permeability (see Supplementary Information).
Quantitative Structure–Activity Relationship (QSAR) model
Multiple linear regression (MLR) was carried out using the Strike statistical program28 to search for the best QSAR predictive model. MLR was performed on the 25 medications, represented by their ATS scores, 7 QSAR parameters, and ADE incident rates. The QSAR models were analyzed using leave-1-out cross validation. Model quality (goodness of regression and prediction) was determined by R2 (coefficient of determination) and Q2 (cross-validated or predictive R2).
Results
The 25 medications, their corresponding drug classes, receptor-specific/total ATS scores, and ACB/ARS scores, are listed in Table 1. Antihistamines, antipsychotics, and gastrointestinal agents account for a majority of the 25 medications. Receptor-specific and total ATS scores range 0–1 and 0.60–5.00, respectively. Overall, 14 out of the 25 medications have receptor-specific ATS scores of 1.0 for all five muscarinic receptors. The other 11 drugs have variable degrees of receptor inhibitions with total ATS scores ranging 0.60–3.90.
Figure 2 shows the cumulative incidence rate of ataxia, the most common form of AC-related ADE identified in the retrospective cohort study (Table 2), computed across all 25 medications on an ordinal scale for direct comparison of ATS against previously reported ACB and ARS methods.
Figure 2.
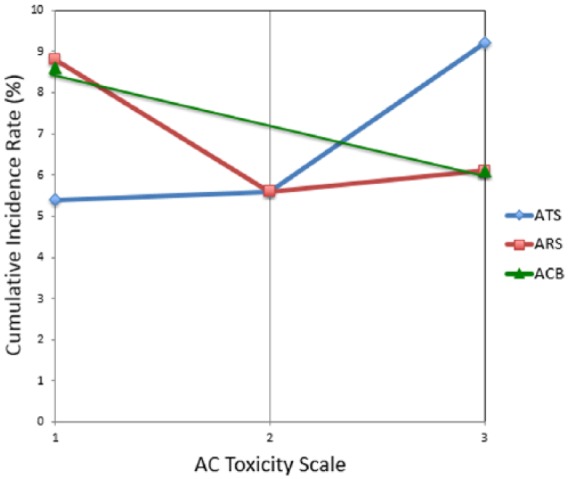
Correlations between ATS/ARS/ACB scales to cumulative incidence rate of ataxia.
ACB, anticholinergic cognitive burden; ARS, anticholinergic risk scale; ATS, anticholinergic toxicity score.
The retrospective cohort study included 287,614 exposed and 287,614 unexposed patients. Table 2 shows that mean age was 38 years (median = 39 years, range = 5–84 years); less than half (44%) patient population were men; the mean length of follow up was 33 days (median = 9 days). The exposed cohort had significantly higher level of comorbidities (CCI 0.38 versus 0.32, p < 0.05) than the unexposed cohort. Ataxia was the most common ADE (7%). Among the 25 medications, promethazine was the most prescribed (received by 36% patients).
Table 3 shows the number of ADEs in the exposed cohort and adjusted for baseline ADEs in the unexposed cohort for each of the 25 medications. The number of ADEs were used to calculate the clinical risks (i.e. cumulative incidence rates) of overall AC-related ADEs and tachycardia.
Table 3.
AC-related ADEs and ATS scores by medication.
| Number of exposed patients | Percent of exposed patients | Number of all ADEsb | ADE incidence rate (per patient) |
Number of tachycardiab | Tachycardia incidence rate (per 100 patients) |
|
|---|---|---|---|---|---|---|
| Promethazine | 103,960 | 36.15 | 7148 | 0.07 | 319 | 0.31 |
| Chlorpheniramine | 42,277 | 14.70 | 2268 | 0.05 | 42 | 0.10 |
| Alprazolama | 36,969 | 12.85 | 5292 | 0.14 | 399 | 1.08 |
| Diazepama | 29,615 | 10.3 | 2112 | 0.07 | 124 | 0.42 |
| Ranitidine | 17,962 | 6.25 | 2977 | 0.17 | 162 | 0.90 |
| Brompheniramine | 17,238 | 5.99 | 1030 | 0.06 | 24 | 0.14 |
| Amitriptyline | 10,357 | 3.60 | 2706 | 0.26 | 204 | 1.97 |
| Atropine | 10,334 | 3.59 | 1140 | 0.11 | 43 | 0.42 |
| Dicyclomine | 9499 | 3.30 | 1252 | 0.13 | 48 | 0.51 |
| Hyoscyamine | 8566 | 2.98 | 1044 | 0.12 | 41 | 0.48 |
| Diphenhydramine | 5956 | 2.07 | 481 | 0.08 | 16 | 0.27 |
| Orphenadrine | 4316 | 1.50 | 308 | 0.07 | 15 | 0.36 |
| Risperidonea | 2854 | 0.99 | 626 | 0.22 | 42 | 1.46 |
| Carbinoxamine | 1803 | 0.63 | 89 | 0.05 | 2 | 0.10 |
| Fentanyl | 1646 | 0.57 | 828 | 0.50 | 54 | 3.29 |
| Imipramine | 1280 | 0.45 | 290 | 0.23 | 15 | 1.16 |
| Loperamide | 1078 | 0.37 | 190 | 0.18 | 11 | 1.05 |
| Clorazepatea | 735 | 0.26 | 112 | 0.15 | 10 | 1.30 |
| Benztropine | 322 | 0.11 | 159 | 0.49 | 12 | 3.88 |
| Chlorpromazinea | 272 | 0.09 | 60 | 0.22 | 5 | 1.97 |
| Haloperidola | 203 | 0.07 | 82 | 0.40 | 8 | 3.81 |
| Trihexyphenidyl | 118 | 0.04 | 52 | 0.44 | 3 | 2.52 |
| Perphenazinea | 76 | 0.03 | 27 | 0.36 | 3 | 3.91 |
| Thioridazinea | 24 | 0.01 | 7 | 0.30 | 1 | 2.48 |
| Trifluoperazinea | 19 | 0.01 | 14 | 0.76 | 1 | 3.13 |
Agitation, irritability, and hallucinations were not included as eligible ADEs if a patient had any fills for antipsychotics or benzodiazepines.
Adjusted using baseline ADEs in the unexposed cohort.
ADE, adverse drug event; ATS, anticholinergic toxicity score.
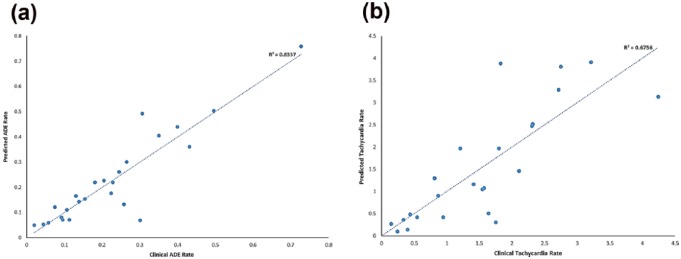
MLR using ATS and drug bioavailability-related parameters yielded a QSAR model with excellent correlation to overall AC incident rates (R2 = 0.83) and predictive performance [cross validation Q2 = 0.64; Figure 3(a) and Supplementary Information]. Good correlation and predictive performance (R2 = 0.67/Q2 = 0.29) were also obtained for an M2 receptor-specific QSAR model and tachycardia, an M2 receptor-specific ADE [Figure 3(b) and Supplementary Information].
Figure 3.
(a) QSAR model for overall ADE risk assessment and prediction (R2 = 0.83, Q2 = 0.64, F = 10.0, p < .00001); (b) QSAR model for M2 receptor-specific tachycardia risk assessment and prediction (R2 = 0.68, Q2 = 0.29, F = 4.2, p < .001).
ADE, adverse drug event; QSAR, quantitative structure–activity relationship.
Discussion
ATS versus other toxicity scales
Previously published AC toxicity scales employed one of two techniques for classifying drugs as mild, moderate, or high AC activity: (1) measurement of serum radio-receptor anticholinergic activity (SAA) or in vitro affinity of drug binding to muscarinic receptors, and (2) classification of AC activity by an expert panel of clinicians (i.e. Delphi method). However, SAA only measures peripheral AC activity and may not represent CNS AC effects. Thus, an association between cognitive impairment and SAA has not been consistently demonstrated.29,30 In vitro binding studies have not been reported for many medications and would be expensive to undertake given the large number of commercially available drugs. Finally, expert opinion, although widely employed, is subjective as demonstrated by differences in ranking between specific drugs (Table 1) and the wide variability of drugs selected in current scales. Duran and colleagues have shown considerable variability across current toxicity scales when a comprehensive list of over 120 medications is compared.19
Our exploratory work yielded a novel toxicity scoring system that begins to address some of the weaknesses of other published scales, such as: (1) AC toxicity is unlikely to conform to an ordinal scale; (2) different scales evaluated different sets of medications; (3) many medications were not scored consistently across different scales; and (4) off-target drug interactions and muscarinic receptor subtype specificity were not considered. Despite these limitations, attempts to predict AC-related ADEs have shown generally positive associations,17,30 suggesting that a further improved method may lead to clinical utility.
Figure 3 shows that ATS is better suited for differentiating increasing severity of ADE risks than ACB and ARS. The cumulative AC incidence rates generally rise with increasing ATS scores whereas ACB and ARS deviate from AC incidence trend. Our approach is intrinsically different from all previous reported methods and offers the following advantages: (1) pharmacologically driven by large and diverse bioactivity databases; (2) receptor subtype-based scoring which may provide greater sensitivity; (3) greater resolution on a continuous toxicity scale; and (4) it is objective, systematic, efficient, and scalable to a large number of medications.
Receptor subtypes
Table 1 demonstrates a strong similarity among many medications that are similarly active across all five muscarinic subtypes (i.e. similar ATS scores across all receptor subtypes). However, for some drugs such as alprazolam (active only on M4 and M5) and ranitidine (active only on M1 and M2), interactions at specific muscarinic receptors could be clinically important. Our QSAR model using M2-only ATS scores suggests that ATS receptor subtype-specific scores may have a potential utility for assessing receptor subtype-specific ADEs [Figure 3(b) and Supplementary Information].
ATS-based toxicity predictive model and future work
The strong association between our ATS-based QSAR model and ADE incidence rates provides encouraging hope for the development of a clinically useful toxicity prediction scale [Figure 3(a) and Supplementary Information]. Currently, ATS scores only represent the pharmacodynamic interactions of medications with muscarinic receptors. Drug pharmacokinetic parameters also must be considered to assess AC clinical risks. For example, despite having a maximum total ATS score of 5, the pharmacokinetics of atropine and its low dosage present in the prescription product Lomotil® yielded relatively low ADE incidence rate (i.e. insignificant ADE risks). Adding additional parameters to adjust for dose and pharmacokinetic effects will be important for clinical utility. To test this hypothesis, we used an in silico pharmacokinetic program to calculate several key absorption and distribution parameters for the 25 medications. The excellent correlation to overall AC incident rates (R2 = 0.83) and predictive performance (cross validation Q2 = 0.64) demonstrates the importance of combining ATS scores and drug pharmacokinetic parameters for developing a clinically significant predictive model [Figure 3(a) and Supplementary Information]. We also combined the same set of pharmacokinetic parameters with receptor subtype-specific ATS scores to assess ADEs originating from inhibition of specific muscarinic receptor subtypes. As an example, tachycardia is considered primarily related to M2 receptor inhibition. The good correlation and predictive performance of the M2 only QSAR model (R2 = 0.67/Q2 = 0.29) support that ATS can be potentially used to characterize the receptor subtype specificity of ADEs [Figure 3(b) and Supplementary Information]. Although dosage information was not included in the current study, future work will include dose and parameters to address drug clearance and elimination, etc. Our main goal in this pilot study is to obtain the best overall ADE (CNS and peripheral) model for the general population. The same computational protocol can be followed to build models specifically for subpopulations such as elderly patients (age 65+) or patients with ‘compromised BBB’ if the cohort can be properly defined by age and medical conditions.
Study limitations
The current ATS approach, while offering a foundation for objective and systematic AC toxicity evaluation, can be further improved by incorporating the following areas: (1) clinical pharmacokinetic and bioavailability data; (2) dosage and duration of treatment; (3) patient genomic variability in drug metabolism and pharmacodynamics; and (4) perhaps most importantly, investigation of other polypharmacologic drug actions contributing to similar ADEs. Additionally, confounding by indication and under reporting of ADEs in claims data are potentially limiting factors that may lead to inaccurate determination of clinical outcomes. Nevertheless, the ATS scoring approach and ATS-based predictive models highlight opportunities for further improvements while avoiding patient safety concerns. After further refining the ATS scoring system, clinical outcome determination and validation can be undertaken using more robust randomized controlled clinical studies.
Conclusions
To our knowledge, our ATS scoring method represents the first application of big data-driven bioinformatics and biomedical informatics aimed at direct translation to patient care. Pilot results from this test-of-concept work demonstrate the potential and feasibility of the ATS approach in improving clinical AC toxicity assessment and that further development and refinements are warranted. Optimistically, ATS may provide a rational path forward for assessing and predicting cumulative AC-related ADEs resulting from concomitant AC medication use. The ATS computational methodology may even be adapted to facilitate rapid rational assessment of other types of drug-induced toxicity.
Supplementary Material
Footnotes
Funding: This work is supported in part by ALSAM Foundation Skaggs Scholars Award to D.X., H.A., R.V. and V.C., NIGMS IDeA Mountain West Clinical and Translational Infrastructure Network (CTR-IN) Mini and Pilot Grants (1U54GM104944-02/15-746Q-ISU-MG5-00, 5U54GM104944-03/16-746Q-ISU-PG44-00) to D.X. and V.C., and Idaho State University Faculty Seed Grant to D.X. and V.C.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Dong Xu, Department of Biomedical and Pharmaceutical Sciences, College of Pharmacy, Idaho State University, 1311 East Central Drive, Meridian, ID 83642, USA.
Heather D. Anderson, Department of Clinical Pharmacy, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO, USA School of Public Health, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Aoxiang Tao, Department of Biomedical and Pharmaceutical Sciences, College of Pharmacy, Idaho State University, Meridian, ID, USA.
Katia L. Hannah, School of Public Health, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Sunny A. Linnebur, Department of Clinical Pharmacy, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Robert J. Valuck, Department of Clinical Pharmacy, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado Anschutz Medical Campus, Aurora, CO, USA School of Public Health, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Vaughn L. Culbertson, Department of Pharmacy Practice and Administrative Sciences, College of Pharmacy, Idaho State University, 1311 East Central Drive, Meridian, ID 83642, USA
References
- 1. Tune LE. Anticholinergic effects of medication in elderly patients. J Clin Psychiatry 2001; 62(Suppl. 21): 11–14. [PubMed] [Google Scholar]
- 2. Schubert CC, Boustani M, Callahan CM, et al. Comorbidity profile of dementia patients in primary care: are they sicker? J Am Geriatr Soc 2006; 54: 104–109. [DOI] [PubMed] [Google Scholar]
- 3. Boustani M, Schubert C, Sennour Y. The challenge of supporting care for dementia in primary care. Clin Interv Aging 2007; 2: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carnahan RM, Lund BC, Perry PJ, et al. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol 2006; 46: 1481–1486. [DOI] [PubMed] [Google Scholar]
- 5. Boustani M, Campbell N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health 2008; 4: 311–320. [Google Scholar]
- 6. Rudolph JL, Salow MJ, Angelini MC, et al. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168: 508–513. [DOI] [PubMed] [Google Scholar]
- 7. Han L, McCusker J, Cole M, et al. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med 2001; 161: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 8. Ehrt U, Broich K, Larsen JP, et al. Use of drugs with anticholinergic effect and impact on cognition in Parkinson’s disease: a cohort study. J Neurol Neurosurg Psychiatry 2010; 81: 160–165. [DOI] [PubMed] [Google Scholar]
- 9. Sittironnarit G, Ames D, Bush AI, et al. Effects of anticholinergic drugs on cognitive function in older Australians: results from the AIBL study. Dement Geriatr Cogn Disord 2011; 31: 173–178. [DOI] [PubMed] [Google Scholar]
- 10. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc 2008; 56: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 11. Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167: 781–787. [DOI] [PubMed] [Google Scholar]
- 12. Ancelin ML, Artero S, Portet F, et al. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ 2006; 332: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pont LG, Nielen JTH, McLachlan AJ, et al. Measuring anticholinergic drug exposure in older community-dwelling Australian men: a comparison of four different measures. Br J Clin Pharmacol 2015; 80: 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kashyap M, Belleville S, Mulsant BH, et al. Methodological challenges in determining longitudinal associations between anticholinergic drug use and incident cognitive decline. J Am Geriatr Soc 2014; 62: 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lertxundi U, Domingo-Echaburu S, Hernandez R, et al. Expert-based drug lists to measure anticholinergic burden: similar names, different results. Psychogeriatrics 2013; 13: 17–24. [DOI] [PubMed] [Google Scholar]
- 16. Woehrling EK, Parri HR, Tse EHY, et al. A predictive in vitro model of the impact of drugs with anticholinergic properties on human neuronal and astrocytic systems. PLoS ONE 2015; 10: e0118786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Durán CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol 2013; 69: 1485–1496. [DOI] [PubMed] [Google Scholar]
- 18. Gaulton A, Bellis LJ, Bento AP, et al. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res 2012; 40: D1100–D1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keiser MJ, Setola V, Irwin JJ, et al. Predicting new molecular targets for known drugs. Nature 2009; 462: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uchiyama T, Chess-Williams R. Muscarinic receptor subtypes of the bladder and gastrointestinal tract. J Smooth Muscle Res 2004; 40: 237–247. [DOI] [PubMed] [Google Scholar]
- 21. Hester SA. Drugs with anticholinergic activity. Prescriber’s Letter 2011; 18: 271223. [Google Scholar]
- 22. Knox C, Law V, Jewison T, et al. DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res 2011; 39: D1035–D1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yildirim MA, Goh K-I, Cusick ME, et al. Drug-target network. Nat Biotech 2007; 25: 1119–1126. [DOI] [PubMed] [Google Scholar]
- 24. Willett P. Similarity-based virtual screening using 2D fingerprints. Drug Discov Today 2006; 11: 1046–1053. [DOI] [PubMed] [Google Scholar]
- 25. IMS Health Inc. PharMetrics health plan claims data user guide & data dictionary, 2014. [Google Scholar]
- 26. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 27. Malone DC, Billups SJ, Valuck RJ, et al. Development of a chronic disease indicator score using a veterans affairs medical center medication database. J Clin Epidemiol 1999; 52: 551–557. [DOI] [PubMed] [Google Scholar]
- 28. Small-Molecule Drug Discovery Suite 2016-4. New York, NY: Schrödinger, LLC, 2016. [Google Scholar]
- 29. Mulsant BH, Pollock BG, Kirshner M, et al. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry 2003; 60: 198–203. [DOI] [PubMed] [Google Scholar]
- 30. Lampela P, Lavikainen P, Garcia-Horsman JA, et al. Anticholinergic drug use, serum anticholinergic activity, and adverse drug events among older people: a population-based study. Drugs Aging 2013; 30: 321–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.