Abstract
Hemophilia A is a congenital X-linked bleeding disorder caused by coagulation factor VIII (FVIII) deficiency. Routine infusion of factor replacement products is the current standard of care; however, the development of alloantibodies against FVIII remains a challenge. The treatment of hemophilia has undergone major advances over the past century to improve safety, effectiveness, manufacturing, and convenience of factor products. Major recent advances in the treatment of hemophilia A include the emergence of extended half-life products, factor VIII orthologs, and gene therapy products. Extended half-life products were designed to decrease the frequency of infusions, but only modest half-life extension is achieved. Factor VIII orthologs featuring lower cross-reactivity with anti-FVIII antibodies may be less susceptible to inactivation by inhibitors. Meanwhile, gene therapy may potentially provide a cure for hemophilia A, thus abrogating the need for protein-based factor replacement. This review aims to discuss current and emerging FVIII replacement products for hemophilia A.
Keywords: extended half-life FVIII, factor VIII, gene therapy, hemophilia A, inhibitor, ortholog, polyethylene glycol (PEG), replacement products
Introduction
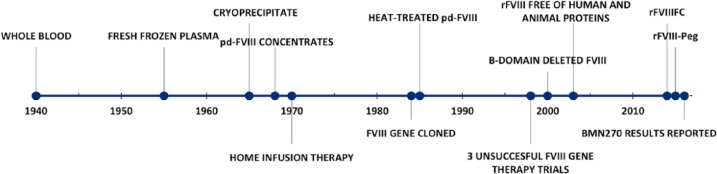
Hemophilia A (HA) and B (HB) are congenital X-linked bleeding disorders resulting from deficiency of coagulation factor VIII (FVIII) and IX (FIX), respectively. HA affects one in 5000 male births.1 It is a chronic disease characterized by spontaneous bleeding into muscles and joints, which can progress to debilitating arthropathy and significantly affect patients’ quality of life. Over the past century, the treatment of hemophilia has made major advances (Figure 1). Prior to the 1950s, whole-blood transfusion was the only available therapy. In the 1950s, while working at the Karolinska Institute, Margareta and Birger Blomback discovered that fraction 1-0 obtained when plasma was subjected to Cohn fractionation contained high concentrations of FVIII.2 Subsequently, in 1964, Judith Graham Pool discovered that the cryoprecipitate concentrated the FVIII further and opened the door to the possibility of home infusion.3 During the late 1960s and 1970s, the fractionation and purification process evolved, allowing patients to change from cryoprecipitate to plasma-derived concentrates, thus making home therapy more and more feasible.4–6 Unfortunately, the benefit from these purified and more efficient plasma-derived concentrates was halted by the transmission of HIV and hepatitis B and C through contaminated concentrates. Approximately 60–70% of severe hemophilia patients contracted HIV and almost 100% became infected with hepatitis C by the 1980s;6 a devastating tragedy. In 1984, the FVIII gene was cloned, which became one of the most promising advancements in the treatment of hemophilia, and led to the licensure of recombinant human FVIII (rFVIII) in 1992. Recombinant technology, along with enhanced blood product screening and viral attenuation protocol, made factor replacement products safe from viral transmission. Over the next several decades, with increased availability of safe and effective FVIII replacement therapies, prevention of joint disease through the use of routine infusions became the standard of care in most developed countries; however, crippling joint disease remains common in countries where factor products are less abundant.
Figure 1.
Timeline of events impacting the treatment of hemophilia A.
After several decades of safe factor products, there has been increasing attention paid to other complications of factor replacement therapy, such as neutralizing anti-FVIII antibody (inhibitor) development as well as the burden of care. An estimated 30% of severe HA patients develop an inhibitor;7 patients with inhibitors are at increased risk for severe hemorrhagic events and disability due to lack of available effective hemostatic therapies. Although immune tolerance induction can be performed to overcome this hurdle, its success rate is 59–86% in patients with severe HA.8 Since plasma-derived FVIII (pdFVIII) was introduced over 40 years ago, it has undergone generations of changes to increase the purity and safety of these products. However, observations surfaced that crude FVIII preparations with higher Von Willebrand factor (VWF) content are associated with less inhibitor development.9 Ex-vivo studies demonstrated a protective effect of VWF against endocytosis of FVIII by dendritic cells, thereby preventing the uptake of FVIII by antigen presenting cells.10 The C2 domain of FVIII is a binding epitope for a subset of inhibitors and VWF, and the competition for binding at this site between inhibitor and VWF may also contribute to a protective role of high VWF content against inhibitor development and inactivation of FVIII.11,12 The RODIN13 and CANAL7 cohort studies, as well as EUHASS European surveillance registry,14 failed to show any difference in the risk of inhibitor development between pdFVIII with large quantities of VWF content and rFVIII. However, in the French cohort study, rFVIII conferred a higher risk.15 The SIPPET study randomized previously untreated or minimally treated patients with severe HA to either pdFVIII/VWF or rFVIII.16 A lower incidence of new inhibitors was seen with pdFVIII (26.8%, 95% CI 18.4–35.2) relative to rFVIII (44.5%, 95% CI 34.7–54.3). However, the hazard ratio for high-titer inhibitors was 1.69 with a confidence interval of 0.96–2.98, which crosses 1, thus complicating the interpretation of these results. Therefore, the difference in risk of inhibitor development between pdFVIII and rFVIII, as well as the needed modifications to rFVIII that would reduce inhibitor development, remain a topic of debate.
After inhibitor development, the next major limitation of standard FVIII replacement products is the short half-life (8–12 h) as it requires patients to receive frequent IV injections (3–4 times per week). The burden of frequent infusions to maintain an FVIII level that prevents spontaneous bleeding events, preserves joint function, and allows for sporting activities that promote normal physical and emotional development is significant. In fact, inconvenience is one of the main barriers to treatment adherence.17,18 Lastly, although FVIII replacement therapy is readily available to developed countries, given the complexities of manufacturing rFVIII, having enough affordable supply for developing countries remains out of reach. It is with these limitations in mind that new FVIII replacement products have been and are being designed. This review aims to discuss current and emerging FVIII replacement products for HA. Of note, treatments for HA designed to modulate the coagulation system through mechanisms other than replacing FVIII, such as currently available bypassing agents or novel products in development (emicizumab, fitusiran, concizumab, etc.), will not be discussed.
Standard half-life FVIII products
Advances from the first recombinant full-length FVIII in 1992 to 2000 focused on removing animal and human proteins in the production process. In 2000, Moroctocog alfa (Refacto®), the first product with the 908 amino-acid B-domain (except a 14 amino-acid linker sequence) deleted, was approved by the FDA for use in hemophilia. The purpose of B-domain deletion is to improve production efficiency and has no impact on in-vivo pharmacokinetics or immunogenicity. Moroctocog alfa (Xyntha®), approved for use in 2008, is produced using an albumin-free cell culture process and is the currently available B-domain deleted (BDD) rFVIII product replacing Refacto®.
In 2013, Turoctocog alfa (NovoEight®), which contains a B-domain that is truncated to 10 amino-acids from the N-terminus and 11 amino-acids from the C-terminus, was approved by the FDA following two phase III trials, GuardianTM 1 and 3,19,20 demonstrating its safety and efficacy as prophylaxis and on-demand treatment (Table 1). Recently published interim results from GuardianTM 2 affirmed the long-term safety and efficacy of Turoctocog alfa in the prevention and treatment of bleeding events.21 No FVIII inhibitor developed over 4 years, which is a cumulative 451.6 patient-years exposure. Patients receiving prophylactic infusions had a median ABR of 1.88, 1.65, 1.57, and 1.22 among older children, adults, adolescents, and younger children, respectively. Among patients treated with prophylactic infusions, 90.5% of bleeds abated with ⩽2 infusions, while 100% of bleeding events in the on-demand group ceased with one infusion. In 2016, Octocog alfa (Kovaltry®), a full-length, unmodified rFVIII, was FDA-approved based on the LEOPOLD I,22 II,23 and Kids24 trials. It has the same amino-acid sequence as Kogenate® FS, but uses advanced purification techniques to enhance production.25 The changes in manufacturing, including co-expression of heat shock protein 70 in the new cell bank, leads to differences in sialylation of the rFVIII. It is believed that these changes in sialylation account for the 10% longer half-life of Kovaltry® when compared with Kogenate® FS in crossover pharmacokinetic studies.25 It features a low ABR when used as prophylaxis in previously treated adult and pediatric patients and is able to control bleeding episodes with 1–2 infusions.22–24
Table 1.
Standard half-life factor VIII products.
| Product | Approval year | FVIII protein | Cell line | Mean half-life, hours | Comparator mean half-life (product) | Prophylaxis regimen |
|---|---|---|---|---|---|---|
| Kogenate® FS26 | 1993 | Full length | BHK | 13.7 | – | 25 IU/kg 3× per week |
| Helixate® FS27 | 1993 | Full length | BHK | 13.7 | – | 25 IU/kg 3× per week |
| Advate®28 | 2003 | Full length | CHO | 12.0 | – | 20–40 IU/kg every other day or every 3 days to maintain trough >1% |
| Moroctocog alfa (Xyntha®)29 | 2008 | B-domain deleted | CHO | 11.2 | 13.3 (Advate®) |
30 IU/kg 3× per week |
| Turoctocog alfa (NovoEight®)19–21 | 2013 | B-domain truncated | CHO | 10.8 | 11 (Advate®) |
20–50 IU/kg 3× per week or 20–40 IU/kg every other day |
| Simoctocog alfa (Nuwiq®)32,33,35 | 2015 | Full length | HEK | 17.1* | Bioequivalent (Kogenate® FS) | 30–40 IU/kg every other day |
| Octocog alfa (Kovaltry®)22–25 | 2016 | Full length | BHK | 13.4 | 12.2 (Kogenate® FS) |
20–40 IU/kg 2–3× per week |
| rVIII-single chain (Afstyla®)37–39 | 2016 | B-domain and four amino-acids of a3 domain deleted | CHO | 14.5 | 13.3 (Advate®) |
20–50 IU/kg 2–3× per week |
BHK: baby hamster kidney; CHO: Chinese hamster ovary; FS: sucrose-formulated; HEK: human embryonic kidney; IU: international unit. *Median, lower/upper quartile: 13.7, 12.0/17.5.
From 1992 until 2014, approved rFVIII products, both full-length and BDD, were expressed in mammalian, non-human cell lines, such as Chinese hamster ovary (CHO) and baby hamster kidney (BHK). Recently, rFVIII protein was expressed in a human cell line (rhFVIII), and found to have higher VWF-binding affinity due to enhanced post-translational modifications (tyrosine sulfation).30,31 Simoctocog alfa (Nuwiq®) was approved for use by the FDA in 2015. Although, in early phase studies Simoctocog alfa demonstrated a mean half-life of 17.1 h, it is important to note that in the population studied, Simoctocog alfa was considered bioequivalent to its crossover comparator (Kogenate® FS).32 The median half-life for Nuwiq® is 13.7 h (Q1, Q3; 12.0, 17.5 h).33 Thus the impact of higher affinity VWF-binding on half-life is unclear and does not suffice for Simoctocog alfa (Nuwiq®) to be considered an extended half-life product. In previously treated adult patients with severe HA, Simoctocog alfa had a mean ABR of 2.28, 1.16, and 1.00 for all, spontaneous, and traumatic bleeds, respectively while receiving prophylactic infusions at 30–40 IU/kg every other day.32 Simoctocog alfa was effective in the management of bleeding and rated as ‘excellent or good’ for all 28 (100%) bleeding episodes that were treated with Simoctocog alfa in the study. Although a small number of surgical cases were evaluated in both studies (five in children35 and four in adults32), Simoctocog alfa demonstrated ‘excellent’ hemostatic efficacy during surgery. Inhibitor development or serious adverse events were not observed. Manufacturing of rFVIII in human cell lines eliminated the immunogenic glycoforms Galα1-3Galβ1-GlcNAc-R (α-Gal) and N-glycolylneuraminic acid (Neu5Gc), present in CHO and BHK-derived rFVIII products, which is hypothesized to reduce the risk of inhibitor formation to rhFVIII. The impact of these modifications on inhibitor development are being investigated in the NuProtect study [ClinicalTrials.gov identifier: NCT01992549], which is ongoing. Preliminary results from a preplanned interim analysis demonstrated that eight of 66 previously untreated patients (PUPs) who had received 20 exposure days developed a high-titer inhibitor, five developed a low-titer inhibitor, and four developed a transient inhibitor. Two patients developed an inhibitor after 20 exposure days. The cumulative incidence of all inhibitors was 20.8% (95% CI 10.68–30.95) and of high-titer inhibitor was 12.8% (95% CI 4.49–21.25).36 Whether these preliminary results will hold and can be compared with other clinical trial results remain to be seen. Final study results are expected in 2018.
The single-chain FVIII molecule (rFVIII-single chain, Afstyla®), produced in CHO cells, is a novel recombinant product in which the coding sequences for most of the FVIII B-domain and four amino-acids of the adjacent acidic a3 domain were removed, resulting in a new N-glycosylation site at the junction of the heavy and light chain, which leads to expression of rFVIII as a single chain.34 Once activated, rFVIII-single chain has a sequence identical to that of FVIIIa from endogenous full-length FVIII. These modifications lead to a higher affinity for VWF and improved structural stability during manufacturing and storage.34 Although VWF can exert a protective effect on FVIII from degradation and clearance, the faster and tighter binding of rFVIII-single chain to VWF may mean a slight prolongation of its half-life when compared to Octocog alfa (14.5 versus 13.3 h).37,38 Since the half-life difference between rFVIII-single chain and Octocog alfa is minimal (~10%) and the modifications made were not intended to extend the half-life, we do not consider rFVIII-single chain an extended half-life product. Importantly, the activity of rFVIII-single chain is underestimated using a one-stage clot-based FVIII assay, therefore a chromogenic assay should be used to estimate its activity in patient plasma.43 In clinical trials, 93.8% of the 835 bleeding episodes achieved ‘excellent or good’ hemostatic effect as designated by the investigator. Meanwhile, the median spontaneous bleeding rate was 0.00 (Q1, Q3: 0.0, 2.4) and the median overall ABR was 1.14 (Q1, Q3: 0.0, 4.2) in patients who received rFVIII-single chain across all prophylactic regimens (20–40 IU/kg every second day or 20–50 IU/kg 2–3 times per week or at the investigator’s discretion).39 A subgroup analysis was also performed to evaluate the safety and efficacy of rFVIII-single chain during surgery. Of the 13 patients undergoing elective procedures, 100% reported to have excellent/good hemostasis. rFVIII-single chain was well tolerated and showed a favorable safety profile, without FVIII inhibitor development.
Extended half-life products
Extended half-life (EHL) products were developed, as the name implies, to extend the half-life of FVIII, resulting in longer infusion intervals. Although some of the previously discussed FVIII products have slightly longer half-lives and may be prescribed twice weekly, they were not designed with half-life extension in mind and thus are not included in the category of EHL products. To extend the time in circulation, FVIII can be fused with another molecule, either polyethylene glycol (PEG) or the Fc portion of immunoglobulin G (IgG).
Fusion of the Fc portion of human immunoglobulin G1 (IgG1) to clotting factors looks to harness the neonatal Fc receptor that recirculates immunoglobulins which have a half-life of 21 days.40 Efmoroctocog alfa (Eloctate®) is an FDA-approved BDD FVIII-fusion protein (rFVIIIFc) with a mean half-life of 19 h which was approximately 50% longer than Octocog alfa. Although a 50% longer half-life is an improvement, it is minimal compared to the five-fold half-life extension seen with FIX when fused with Fc. This is due in large part to the interaction between FVIII and VWF, which continues to be the main regulator of FVIII clearance.41 The use of this rFVIIIFc protein as prophylaxis with dosing tailored to the individual patient’s pharmacokinetics (25–65 IU/kg every 3–5 days) exhibited a median ABR of 1.6.42 Of 757 bleeding episodes that occurred during the study, 87.3% ceased after a single dose of rFVIIIFc, whereas 97.8% terminated following 1–2 doses. Plasma levels of FVIII activity after rFVIIIFc infusion can be assayed using either a chromogenic or one-stage assay, though both can vary significantly by reagent.43 The A-LONG, Kids A-LONG, and ASPIRE trials established the safety and hemostatic efficacy of rFVIIIFc as surgical prophylaxis.44 Patients with severe HA enrolled in these studies underwent a total of 22 major and 32 minor surgeries; the investigators ranked the hemostatic efficacy as ‘excellent or good’. A phase III trial [ClinicalTrials.gov identifier: NCT02234323] is currently accruing participants to evaluate the safety and efficacy of rFVIIIFc in previously untreated patients with severe HA. This study will elucidate whether we can anticipate a lower incidence of inhibitor development in children that receive rFVIIIFc; however, questions will likely remain given its traditional design as a single-arm open-label study. Nonetheless, these results will be much anticipated as it has been demonstrated in mouse models that rFVIIIFc is able to shift the immune response from immunogenic to tolerogenic.45
PEG, a polymer of ethylene oxide with a >20 kDa molecular weight, has been used to extend the half-life of other drugs, such as interferon and asparaginase.46 Many PEG–FVIII molecules did not reach clinical trials due to interference of PEG with the procoagulant activity of FVIII by altering its interaction with other molecules. However, three EHL products reached clinical trials (Table 2).
Table 2.
Extended half-life factor VIII products.
| Product | Approval year | Modification | Cell line | Mean half-life, hours | Comparator mean half-life (product) | Prophylaxis regimen |
|---|---|---|---|---|---|---|
| rFVIIIFc (Eloctate®)42 | 2014 | BDD rFVIII with fused Fc | HEK | 19 | 12.4 (Advate®) | 50 IU/kg every 4 days |
| rFVIII-pegylated (Adynovate®)48 | 2015 | Full-length rFVIII with 20 kDa non-specific pegylation | CHO | 14.3 | 10.4 (Advate®) | 40–50 IU/kg 2× per week |
| N8-GP51 | – | B-domain truncated rFVIII with 40 kDa site-specific pegylation | CHO | 19 | 11.7 (patients’ previous product) | 50 IU/kg every 4 days |
| BAY 94-902754 | – | BDD rFVIII with 60 kDa site-specific pegylation | BHK | 18.7 | 13 (Kogenate® FS) | 30–40 IU/kg 2 X per week or 45–60 IU/kg every 5 days |
BDD: B-domain deleted; BHK: baby hamster kidney; CHO: Chinese hamster ovary; Fc: fragment crystallizable; HEK: human embryonic kidney; IU: international unit; kDa: kilodalton; rFVIII: recombinant factor VIII.
Rurioctacog alfa pegol (Adynovate®) is composed of a 20 kDa branched PEG coupled to a full-length rFVIII (Advate®). The manufacturers utilized a low, controlled, non-specific site pegylation technique to produce approximately 2 moles of PEG per FVIII molecule to enhance the pharmacokinetic profile of rFVIII.47 Rurioctacog alfa pegol has lower binding affinity for the low-density lipoprotein-related protein (LRP) clearance receptor, which may also be a mechanism by which its half-life is extended 14–19.6 h, which demonstrates a 40% increased half-life when compared with Octocog alfa (Advate®). The one-stage clot assay and chromogenic assay can both be used to assay FVIII activity. No significant reagent-dependent differences between assays have been seen.43 As prophylaxis at 40–50 IU/kg twice weekly, a median ABR of 1.9 was observed and 36.9% of patients had no bleeding events.48 Likewise, 96.1% of patients enrolled in the study ranked its hemostatic efficacy as ‘good or excellent’. No incidence of inhibitor development was reported. A beneficial impact of pegylation on immunogenicity has been postulated as pegylation may interfere with FVIII interaction with antigen presenting cells.49 Studies in previously untreated patients are ongoing.
Turoctocog alfa pegol (N8-GP) is another novel agent featuring a 40 kDa PEG conjugated to a B-domain truncated FVIII via site-directed glycopegylation. It is not yet approved for use. N8-GP has a half-life of 19 h.50 With N8-GP, significant variation in the measured FVIII activity exists depending on the assay used, with some reagents leading to a significant underestimation.43 A phase III study of previously treated adolescent and adult patients with severe HA found a median ABR of 1.33, with mean ABR of 3.70 when N8-GP is used as prophylaxis at 50 IU/kg every fourth day.51 N8-GP demonstrated a hemostatic efficacy rate of 84.2%. Of the 968 documented bleeds, 83.6% of these episodes resolved following one injection of N8-GP and 95.5% after two injections. Inhibitor development (⩾0.6 Bethesda units) against FVIII was reported in one patient following N8-GP exposure for 93 days.
BAY 94-9027 is a BDD rFVIII with a cysteine codon introduced to allow for site-directed conjugation to a single 60-kDa PEG-maleimide molecule.52 A phase I trial compared BAY 94-9027 to sucrose-formulated rFVIII (Kogenate® FS) in previously treated adults with severe HA.53 BAY 94-9027 demonstrated a half-life of ~19 h (versus ~13 h for Kogenate® FS). Plasma FVIII activity can be measured accurately using a chromogenic assay and a one-stage assay that uses an ellagic acid-based reagent.43 Use of a silica-based reagent in the one-stage assay can lead to an underestimation of FVIII activity. In the PROTECT VIII phase II/III study, BAY 94-9027 showed a median ABR of 1.9 and 3.9 when given as prophylaxis every five (45–60 IU/kg) and seven (60 IU/kg) days, respectively, among previously treated patients with severe HA.54 BAY 94-9027 was also shown to effectively achieve hemostasis in 90% of bleeds with ⩽2 infusions, and was well tolerated without inhibitor development.
Based on these studies, the short-term safety of these pegylated molecules is clear. Supporting long-term safety, patients with other conditions have been exposed to pegylation for years without untoward consequences. However, raising concerns are the observations that at high doses of PEG (up to three times the dose used in hemophilia trials), renal, endothelial, and Kupffer cell vacuolation have been observed55 and that pegylated products rarely, if ever, been given to young children for a lifetime. Polyethylene glycol is used as a stabilizer in FVIII products; thus patients have been exposed to a type of PEG. The impact of PEG linked to FVIII and subject to cellular uptake versus PEG as a stabilizer is unknown. It will be difficult to confirm or disprove any risk associated with long-term use of pegylated products without long-term observational studies.
The integration of EHL products into routine clinical practice continues to be evaluated. Certain groups may derive more clinical benefit from EHL than others, including young children, those with an active lifestyle, difficult venous access, receiving routine prophylaxis and with fear of needles.56 However, the benefits of EHL FVIII remain controversial. Some argue that the benefits are not significant compared to those achieved by EHL FIX products, with modest half-life prolongation of only 1.4–1.6 fold and a persistent requirement for 100 or more infusions annually.56 Studies of health-related quality of life have demonstrated only modest benefits when changing from one prophylaxis regimen to an individualized regimen with rFVIIIFc, though more significant benefits were seen in those that changed from on-demand treatment to rFVIIIFc prophylactic regimen.57 Over time, as more information is gathered through post-marketing surveillance, the benefits and safety of EHL products will continue to be evaluated.
Gene therapy
The cloning of the FVIII gene in the 1980s opened the door for the development of gene therapy, wherein cure is the goal of treatment. The realization that gene therapy could have important benefits for patients was further supported by the observation that modest improvements in factor levels (by 1–2%) can produce significant reduction in the risk of spontaneous bleeding events and reduce the need for factor replacement infusions.58 In addition, gene therapy has a wide therapeutic range wherein gene expression does not need to be tightly regulated,67 as well as an easily quantifiable therapeutic endpoint (FVIII plasma levels). These benefits have made gene therapy a promising treatment for hemophilia. Furthermore, the allure of one-time treatment as opposed to frequent lifelong prophylactic infusions is appealing to many patients. In the late 1990s several phase I gene therapy studies in patients with hemophilia A were started.60,61 These studies used different approaches; transfected dermal fibroblasts, retroviral vector, and adenoviral vector; however, all had disappointing results without sustained FVIII activity. Following these failed trials, investigators returned to the laboratory. Driven by the success of gene therapy for factor IX,59,62 and advances that facilitate FVIII packaging in viral vectors, there is a surge of interest in gene therapy for HA. The non-enveloped, non-integrating adeno-associated virus (AAV) has emerged as the leading vector based on preclinical studies. Wild-type AAV is non-pathogenic for acute disease, with low immunogenic properties.63 However, it has been implicated in hepatocellular carcinoma,64 raising concern for long-term consequences of AAV-based gene therapy. AAV demonstrates broad tropism for mitotically quiescent tissues such as hepatocytes, neurons, and muscles with a decreased risk of insertional mutagenesis because the recombinant plasmid is episomally retained and therefore does not integrate in the host DNA.65
There are several AAV-based FVIII gene therapy products in development, though only one has completed early phase human data. A phase I/II trial of BMN 270 [ClinicalTrials.gov identifier: NCT02576795], an AAV5–FVIII gene product for severe HA, recently released preliminary data supporting its proof of concept in gene therapy.66 Although the sample size is small, all seven participants who received high-dose (6 × 10e13 vg/kg) BMN 270 showed sustained FVIII activity (>10 IU/dl). In fact, six of seven patients demonstrated FVIII activity >50 IU/dl; two of which were >150 IU/dl. Lower bleeding events occurred in these patients, especially greater than 8 weeks. BMN 270 was shown to be well tolerated with no significant adverse events (SAEs), although mildly elevated liver enzymes occurred without significant effect on FVIII activity. BMN 270 has been designated as an Orphan Drug by the FDA in February 2016 and by the European Commission in March 2016. Additional gene therapy trials are expected from manufacturers such as Shire, Dimension Therapeutics, Sangamo, and Spark Therapeutics. Spark Therapeutics recently began recruiting patients for their phase I/II trial of SPK-8011 [ClinicalTrials.gov identifier: NCT03003533], an AAV capsid containing a codon-optimized BDD FVIII transgene. Meanwhile, Sangamo Therapeutics is expected to begin accruing patients in their phase I/II trial [ClinicalTrials.gov identifier: NCT03061201] of SB-525, an AAV2/6 human FVIII gene product.
Although the episomal location of AAV vectors reduces the risk of insertional mutagenesis, it also results in decreased transgene expression as the transduced cells divide due to loss of proviral DNA with each cell division.67 Accordingly, AAV-based gene therapy is not anticipated to provide a lifelong cure. Sustained levels for approximately 10 years are anticipated, but further longitudinal studies are needed. To address the potential for loss of FVIII activity over time, hematopoietic stem cells (HSCs) as vehicles for gene therapy has been pursued.68 Lentiviral vectors have been extensively used in gene therapy clinical trials, hence it has a favorable safety and efficacy profile for use in transduction of HSCs. So far, insertional mutagenesis has not been reported in patients treated with HSC-LV in clinical trials in various diseases, including HIV.69 Likewise, preclinical studies have demonstrated that HSC gene therapy is effective in mice, including mice with pre-existing inhibitors to human FVIII.70,71 Gene therapy with HSC may likely require a more intensive conditioning regimen in the presence of inhibitors prior to HSC transplant to reach therapeutic efficacy.70–72
Bioengineered FVIII products
Orthologs are genes from different species that evolved from a common ancestral gene. Differences in amino-acid sequences of FVIII gene products across different species can result in reduced antigenicity of ortholog proteins, making them less susceptible to inactivation by anti-human FVIII inhibitors. Porcine FVIII derived from plasma has been used since the 1960s. Because anti-human FVIII alloantibodies have <30% cross-reactivity against porcine FVIII,73 it was used in patients with FVIII inhibitors despite thrombocytopenia and hypersensitivity reactions as common side effects. Likewise, porcine FVIII has been shown to be effective in treating hemorrhagic events in patients with acquired hemophilia A (AHA) due to development of FVIII autoantibodies. Based on the clinical trial results of 28 subjects with AHA who had 86% of bleeds successfully controlled, BDD recombinant porcine FVIII (r-pFVIII, Obizur®) was FDA-approved for treatment of acute bleeds in patients with AHA. Meanwhile, a phase II study in patients with congenital HA and FVIII inhibitors presenting with non-life-/non-limb-threatening bleeding showed that hemostasis was achieved in 25 hemorrhagic events with administration of r-pFVIII with eight or fewer injections.74 In fact, one injection was sufficient to control 80% of the reported bleeding events. Patients tolerated the product well without significant SAEs. A trial evaluating the use of r-pFVIII in patients with congenital HA and inhibitors undergoing surgery is currently ongoing [ClinicalTrials.gov identifier: NCT02895945].
Other strategies are currently being developed to enhance FVIII gene expression as well, including a hybrid human–porcine FVIII molecule called ET3, which has shown promising results from preclinical gene therapy studies.75,76
Conclusion
The treatment of HA continues to evolve since the use of whole-blood transfusion in the first half of the 20th century, and novel therapeutic products are currently being developed with the goal of enhanced safety and effectiveness and lower risk of inhibitor incidence. Recombinant factor replacement remains the cornerstone of hemophilia treatment, although inhibitor development, cost, and frequent intravenous injections due to its short half-life continue to fuel the search for other treatment options for hemophilia. EHL products promised longer dosing intervals; however, study results are disappointing, with only modest half-life extension of 1.4–1.6-fold in patients with HA. Gene therapy offers a long-term and potentially curative treatment option, but it is limited by large-scale manufacturing, inefficient FVIII expression, host immune response, the nature of liver cell turnover, and safety concerns regarding hepatic toxicity and insertional mutagenesis. Ultimately, a variety of these novel products each will find a role within a diverse HA community, thus supporting individualized treatment for each patient.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: LC has no conflict of interest to report. CK has received honoraria for participation on advisory boards from Shire and Genentech, as a consultant from Novo Nordisk, and research funding from Novo Nordisk.
Contributor Information
Lorraine A. Cafuir, Department of Hematology and Medical Oncology, Emory University School of Medicine, USA
Christine L. Kempton, Department of Hematology and Medical Oncology, Emory University, School of Medicine, 550 Peachtree Street NE, Medical Office Tower, Suite 1035, Atlanta, GA 30308, USA.
References
- 1. Mannucci PM, Franchini M. Haematology clinic: haemophilia A. Hematology. 2014; 19: 181–182. [DOI] [PubMed] [Google Scholar]
- 2. Nilsson IM, Blomback M, Blomback B, et al. The use of human AHF (Fraction I-0) in haemophilia A. Blut 1962; 8: 92–101. [DOI] [PubMed] [Google Scholar]
- 3. Kasper CK. Judith Graham Pool and the discovery of cryoprecipitate. Haemophilia 2012; 18: 833–835. [DOI] [PubMed] [Google Scholar]
- 4. Pool JG. Cryoprecipitate in the treatment of hemophilia. Calif Med 1970; 113: 66–67. [PMC free article] [PubMed] [Google Scholar]
- 5. Webster WP, Roberts HR, Thelin GM, et al. Clinical use of a new glycine-precipitated antihemophilic fraction. Am J Med Sci 1965; 250: 643–651. [DOI] [PubMed] [Google Scholar]
- 6. Mannucci PM. Hemophilia and related bleeding disorders: a story of dismay and success. Hematology Am Soc Hematol Educ Program 2002; 1–9. [DOI] [PubMed] [Google Scholar]
- 7. Gouw SC, van der Bom JG, Auerswald G, et al. Recombinant versus plasma-derived factor VIII products and the development of inhibitors in previously untreated patients with severe hemophilia A: the CANAL cohort study. Blood 2007; 109: 4693–4697. [DOI] [PubMed] [Google Scholar]
- 8. Holstein K, Batorova A, Carvalho M, et al. Current view and outcome of ITI therapy: a change over time? Thromb Res. 2016; 148: 38–44. [DOI] [PubMed] [Google Scholar]
- 9. Berntorp E, Ekman M, Gunnarsson M, et al. Variation in factor VIII inhibitor reactivity with different commercial factor VIII preparations. Haemophilia 1996; 2: 95–99. [DOI] [PubMed] [Google Scholar]
- 10. Dasgupta S, Repesse Y, Bayry J, et al. VWF protects FVIII from endocytosis by dendritic cells and subsequent presentation to immune effectors. Blood 2007; 109: 610–612. [DOI] [PubMed] [Google Scholar]
- 11. Salvagno GL, Astermark J, Ekman M, et al. Impact of different inhibitor reactivities with commercial factor VIII concentrates on thrombin generation. Haemophilia 2007; 13: 51–56. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki T, Arai M, Amano K, et al. Factor VIII inhibitor antibodies with C2 domain specificity are less inhibitory to factor VIII complexed with von Willebrand factor. Thromb Haemost 1996; 76: 749–754. [PubMed] [Google Scholar]
- 13. Gouw SC, van der Bom JG, Ljung R, et al. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med 2013; 368: 231–239. [DOI] [PubMed] [Google Scholar]
- 14. Fischer K, Lassila R, Peyvandi F, et al. Inhibitor development in haemophilia according to concentrate: four-year results from the European HAemophilia Safety Surveillance (EUHASS) project. Thromb Haemost 2015; 113: 968–975. [DOI] [PubMed] [Google Scholar]
- 15. Goudemand J, Rothschild C, Demiguel V, et al. Influence of the type of factor VIII concentrate on the incidence of factor VIII inhibitors in previously untreated patients with severe hemophilia A. Blood 2006; 107: 46–51. [DOI] [PubMed] [Google Scholar]
- 16. Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med 2016; 374: 2054–2064. [DOI] [PubMed] [Google Scholar]
- 17. Saxena K. Barriers and perceived limitations to early treatment of hemophilia. J Blood Med 2013; 4: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zappa S, McDaniel M, Marandola J, et al. Treatment trends for haemophilia A and haemophilia B in the United States: results from the 2010 practice patterns survey. Haemophilia 2012; 18: e140–e153. [DOI] [PubMed] [Google Scholar]
- 19. Lentz SR, Misgav M, Ozelo M, et al. Results from a large multinational clinical trial (Guardian1) using prophylactic treatment with turoctocog alfa in adolescent and adult patients with severe haemophilia A: safety and efficacy. Haemophilia 2013; 19: 691–697. [DOI] [PubMed] [Google Scholar]
- 20. Kulkarni R, Karim FA, Glamocanin S, et al. Results from a large multinational clinical trial (Guardian3) using prophylactic treatment with turoctocog alfa in paediatric patients with severe haemophilia A: safety, efficacy and pharmacokinetics. Haemophilia 2013; 19: 698–705. [DOI] [PubMed] [Google Scholar]
- 21. Lentz SR, Cerqueira M, Janic D, et al. Interim results from a large multinational extension trial (Guardian2) using turoctocog alfa for prophylaxis and treatment of bleeding in patients with severe haemophilia A. Haemophilia 2016; 22: e445–e449. [DOI] [PubMed] [Google Scholar]
- 22. Saxena K, Lalezari S, Oldenburg J, et al. Efficacy and safety of BAY 81-8973, a full-length recombinant factor VIII: results from the LEOPOLD I trial. Haemophilia 2016; 22: 706–712. [DOI] [PubMed] [Google Scholar]
- 23. Kavakli K, Yang R, Rusen L, et al. Prophylaxis vs. on-demand treatment with BAY 81-8973, a full-length plasma protein-free recombinant factor VIII product: results from a randomized trial (LEOPOLD II). J Thromb Haemost 2015; 13: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ljung R, Kenet G, Mancuso ME, et al. BAY 81-8973 safety and efficacy for prophylaxis and treatment of bleeds in previously treated children with severe haemophilia A: results of the LEOPOLD Kids Trial. Haemophilia 2016; 22: 354–360. [DOI] [PubMed] [Google Scholar]
- 25. Shah A, Delesen H, Garger S, et al. Pharmacokinetic properties of BAY 81-8973, a full-length recombinant factor VIII. Haemophilia 2015; 21: 766–771. [DOI] [PubMed] [Google Scholar]
- 26. Bayer Healthcare LLC. Kogenate FS (Package Insert). Whippany, NJ: Bayer Healthcare LLC, 2016. [Google Scholar]
- 27. CSL Behring LLC. Helixate FS (Package Insert). Kankakee, Il: CSL Behring LLC, 2016. [Google Scholar]
- 28. Baxter Health Corporation, Advate (Package Insert). Westlake, CA: Baxter Health Corporation, 2015. [Google Scholar]
- 29. Wyeth Pharmaceuticals, Inc. Xyntha (Package Insert). Philadelphia, PA: Wyeth Pharmaceuticals, Inc; 2014. [Google Scholar]
- 30. Kannicht C, Ramstrom M, Kohla G, et al. Characterisation of the post-translational modifications of a novel, human cell line-derived recombinant human factor VIII. Thromb Res 2013; 131: 78–88. [DOI] [PubMed] [Google Scholar]
- 31. Sandberg H, Kannicht C, Stenlund P, et al. Functional characteristics of the novel, human-derived recombinant FVIII protein product, human-cl rhFVIII. Thromb Res 2012; 130: 808–817. [DOI] [PubMed] [Google Scholar]
- 32. Lissitchkov T, Hampton K, von Depka M, et al. Novel, human cell line-derived recombinant factor VIII (human-cl rhFVIII; Nuwiq®) in adults with severe haemophilia A: efficacy and safety. Haemophilia 2016; 22: 225–231. [DOI] [PubMed] [Google Scholar]
- 33. Octapharma USA. Nuwiq® [package insert]. Hoboken, NJ; Octapharma USA, Inc, 2015. [Google Scholar]
- 34. Schmidbauer S, Witzel R, Robbel L, et al. Physicochemical characterisation of rVIII-SingleChain, a novel recombinant single-chain factor VIII. Thromb Res 2015; 136: 388–395. [DOI] [PubMed] [Google Scholar]
- 35. Klukowska A, Szczepanski T, Vdovin V, et al. Novel, human cell line-derived recombinant factor VIII (Human-cl rhFVIII, Nuwiq®) in children with severe haemophilia A: efficacy, safety and pharmacokinetics. Haemophilia 2016; 22: 232–239. [DOI] [PubMed] [Google Scholar]
- 36. Liesner R, Abashidze M, Aleinikova O, et al. Inhibitor development in previously untreated patients with severe haemophilia A treated with Nuwiq, a new generation recombinant FVIII of human origin. In: ASH 58th annual meeting & exposition, San Diego, CA, 4 December 2016 San Diego Convention Center. [Google Scholar]
- 37. Klamroth R, Simpson M, von Depka-Prondzinski M, et al. Comparative pharmacokinetics of rVIII-SingleChain and octocog alfa (Advate®) in patients with severe haemophilia A. Haemophilia 2016; 22: 730–738. [DOI] [PubMed] [Google Scholar]
- 38. Zollner S, Raquet E, Claar P, et al. Non-clinical pharmacokinetics and pharmacodynamics of rVIII-SingleChain, a novel recombinant single-chain factor VIII. Thromb Res 2014; 134: 125–131. [DOI] [PubMed] [Google Scholar]
- 39. Mahlangu J, Kuliczkowski K, Karim FA, et al. Efficacy and safety of rVIII-SingleChain: results of a phase 1/3 multicenter clinical trial in severe hemophilia A. Blood 2016; 128: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest 1970; 49: 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berntorp E. Haemophilia treatment in 2030. Haemophilia 2016; 22(Suppl. 5): 15–19. [DOI] [PubMed] [Google Scholar]
- 42. Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood 2014; 123: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kitchen S, Tiefenbacher S, Gosselin R. Factor activity assays for monitoring extended half-life FVIII and factor IX replacement therapies. Semin Thromb Hemost 2017; 43: 331–337. [DOI] [PubMed] [Google Scholar]
- 44. Mahlangu JN, Ragni M, Gupta N, et al. Long-acting recombinant factor VIII Fc fusion protein (rFVIIIFc) for perioperative haemostatic management in severe haemophilia A. Thromb Haemost 2016; 116: 1–8. [DOI] [PubMed] [Google Scholar]
- 45. Krishnamoorthy S, Liu T, Drager D, et al. Recombinant factor VIII Fc (rFVIIIFc) fusion protein reduces immunogenicity and induces tolerance in hemophilia A mice. Cell Immunol 2016; 301: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laffan M. New products for the treatment of haemophilia. Br J Haematol 2016; 172: 23–31. [DOI] [PubMed] [Google Scholar]
- 47. Turecek PL, Bossard MJ, Graninger M, et al. BAX 855, a PEGylated rFVIII product with prolonged half-life. Development, functional and structural characterisation. Hamostaseologie 2012; 32(Suppl. 1): S29–S38. [PubMed] [Google Scholar]
- 48. Konkle BA, Stasyshyn O, Chowdary P, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood 2015; 126: 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stennicke HR, Kjalke M, Karpf DM, et al. A novel B-domain O-glycoPEGylated FVIII (N8-GP) demonstrates full efficacy and prolonged effect in hemophilic mice models. Blood 2013; 121: 2108–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tiede A, Brand B, Fischer R, et al. Enhancing the pharmacokinetic properties of recombinant factor VIII: first-in-human trial of glycoPEGylated recombinant factor VIII in patients with hemophilia A. J Thromb Haemost 2013; 11: 670–678. [DOI] [PubMed] [Google Scholar]
- 51. Giangrande P, Andreeva T, Chowdary P, et al. Clinical evaluation of glycoPEGylated recombinant FVIII: efficacy and safety in severe haemophilia A. Thromb Haemost 2016; 117: 252–261. [DOI] [PubMed] [Google Scholar]
- 52. Mei B, Pan C, Jiang H, et al. Rational design of a fully active, long-acting PEGylated factor VIII for hemophilia A treatment. Blood 2010; 116: 270–279. [DOI] [PubMed] [Google Scholar]
- 53. Coyle TE, Reding MT, Lin JC, et al. Phase I study of BAY 94-9027, a PEGylated B-domain-deleted recombinant factor VIII with an extended half-life, in subjects with hemophilia A. J Thromb Haemost 2014; 12: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reding MT, Ng HJ, Poulsen LH, et al. Safety and efficacy of BAY 94-9027, a prolonged-half-life factor VIII. J Thromb Haemost 2016; 15: 411–419. [DOI] [PubMed] [Google Scholar]
- 55. Ivens IA, Baumann A, McDonald TA, et al. PEGylated therapeutic proteins for haemophilia treatment: a review for haemophilia caregivers. Haemophilia 2013; 19: 11–20. [DOI] [PubMed] [Google Scholar]
- 56. Giangrande P. The future of hemophilia treatment: longer-acting factor concentrates versus gene therapy. Semin Thromb Hemost 2016; 42: 513–517. [DOI] [PubMed] [Google Scholar]
- 57. Wyrwich KW, Krishnan S, Auguste P, et al. Changes in health-related quality of life with treatment of longer-acting clotting factors: results in the A-LONG and B-LONG clinical studies. Haemophilia 2016; 22: 866–872. [DOI] [PubMed] [Google Scholar]
- 58. Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007; 357: 535–544. [DOI] [PubMed] [Google Scholar]
- 59. George LA, Sullivan SK, Giermasz A, et al. Spk-9001: Adeno-associated virus mediated gene transfer for Hemophilia B achieves sustained mean factor IX activity levels of >30% without immunosuppression. Blood 2016; 128: 3. [Google Scholar]
- 60. Powell JS, Ragni MV, White GC, II, et al. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood 2003; 102: 2038–2045. [DOI] [PubMed] [Google Scholar]
- 61. Chuah MK, Collen D, VandenDriessche T. Clinical gene transfer studies for hemophilia A. Semin Thromb Hemost 2004; 30: 249–256. [DOI] [PubMed] [Google Scholar]
- 62. Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014; 371: 1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 2003; 4: 346–358. [DOI] [PubMed] [Google Scholar]
- 64. Nault JC, Datta S, Imbeaud S, et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat Genet 2015; 47: 1187–1193. [DOI] [PubMed] [Google Scholar]
- 65. Chuah MK, Evens H, VandenDriessche T. Gene therapy for hemophilia. J Thromb Haemost 2013; 11(Suppl. 1): 99–110. [DOI] [PubMed] [Google Scholar]
- 66. Pas J, Rangarajan S, Wilde J, et al. Interim results of an open-label, phase 1/2 study of BMN 270, an AAV5-FVIII gene transfer in severe hemophilia A. Haemophilia 2016; 22: 151–152. [Google Scholar]
- 67. Lheriteau E, Davidoff AM, Nathwani AC. Haemophilia gene therapy: progress and challenges. Blood Reviews 2015; 29: 321–328. [DOI] [PubMed] [Google Scholar]
- 68. Lytle AM, Brown HC, Paik NY, et al. Effects of FVIII immunity on hepatocyte and hematopoietic stem cell-directed gene therapy of murine hemophilia A. Mol Ther Methods Clin Dev 2016; 3: 15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McGarrity GJ, Hoyah G, Winemiller A, et al. Patient monitoring and follow-up in lentiviral clinical trials. J Gene Med 2013; 15: 78–82. [DOI] [PubMed] [Google Scholar]
- 70. Doering CB, Gangadharan B, Dukart HZ, et al. Hematopoietic stem cells encoding porcine factor VIII induce pro-coagulant activity in hemophilia A mice with pre-existing factor VIII immunity. Mol Ther 2007; 15: 1093–1099. [DOI] [PubMed] [Google Scholar]
- 71. Ide LM, Iwakoshi NN, Gangadharan B, et al. Functional aspects of factor VIII expression after transplantation of genetically-modified hematopoietic stem cells for hemophilia A. J Gene Med 2010; 12: 333–344. [DOI] [PubMed] [Google Scholar]
- 72. Kuether EL, Schroeder JA, Fahs SA, et al. Lentivirus-mediated platelet gene therapy of murine hemophilia A with pre-existing anti-factor VIII immunity. J Thromb Haemost 2012; 10: 1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mannucci PM, Franchini M. Porcine recombinant factor VIII: an additional weapon to handle anti-factor VIII antibodies. Blood Transfus 2016: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mahlangu JN, Andreeva TA, Macfarlane DE, et al. Recombinant B-domain-deleted porcine sequence factor VIII (r-pFVIII) for the treatment of bleeding in patients with congenital haemophilia A and inhibitors. Haemophilia 2017; 23: 33–41. [DOI] [PubMed] [Google Scholar]
- 75. McIntosh J, Lenting PJ, Rosales C, et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood 2013; 121: 3335–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brown HC, Wright JF, Zhou S, et al. Bioengineered coagulation factor VIII enables long-term correction of murine hemophilia A following liver-directed adeno-associated viral vector delivery. Mol Ther Methods Clin Dev 2014; 1: 14036. [DOI] [PMC free article] [PubMed] [Google Scholar]