Abstract
Primary biliary cholangitis (PBC) is a chronic autoimmune liver disease. It has a varied course of progression ranging from being completely asymptomatic to aggressive disease leading to cirrhosis and resulting in liver transplantation. In addition, symptoms can be debilitating and can have a major impact on quality of life. For decades, there was only one anti-cholestatic agent available to target this disease and that was only effective in around half of patients, with little or no effect on symptoms. With increasing understanding of the pathogenic mechanisms of PBC and potential targets for drug treatment, pharmaceutical companies have shown a greater interest in this rare disease. A large number of novel therapeutic molecules have been developed and are currently being evaluated. In this review article all the novel molecules in use and in trials targeting cholestasis and symptoms in PBC are discussed.
Keywords: bile acids, obeticholic acid, primary biliary cholangitis/cirrhosis, ursodeoxycholic acid
Introduction
Primary biliary cholangitis (PBC) (formerly known as primary biliary cirrhosis)1 is a chronic autoimmune, cholestatic disease of the liver affecting predominantly women (9 women: 1 man).2 The characteristic laboratory findings include raised serum alkaline phosphatase (ALP) and positive antimitochondrial antibodies (AMA). With a specificity of over 95%, AMA are present in over 90% of patients with PBC.3 A liver biopsy can be performed to confirm the diagnosis, although this is no longer a routine practice. The current treatment guidelines on the management of PBC published by the European Association for the Study of the Liver (EASL) suggest that, in adult patients with cholestasis and no likelihood of systemic disease, a diagnosis of PBC can be made based on elevated ALP and the presence of AMA at a titre ⩾ 1:40.4
PBC is typically characterized by a combination of immune and cholestatic disease processes occurring simultaneously. Progressive damage to small and intermediate intrahepatic ducts is characteristic. If left untreated the inflammation and cholestasis can progress to liver fibrosis and cirrhosis with its characteristic complications. In such cases transplantation is the only treatment option left.2
Clinical presentation can be varied. The majority of patients are asymptomatic at presentation and are found incidentally to have abnormal liver chemistry on routine blood testing undertaken for reasons unrelated to their liver.5,6 Some patients present with typical symptoms of PBC, which include fatigue and pruritus. Occasionally, patients, in whom the diagnosis has not been made in the earlier stages of the disease, present when they have developed cirrhosis and related complications such as jaundice, variceal bleeding and hepatic encephalopathy.7
The pathogenesis of PBC, particularly the nature of the interrelationship between the immune and cholestatic processes, is still not clearly defined. Contrasting models suggest autoimmune reactivity as a cause or, alternatively a consequence, of chronic cholestasis.8 One proposed mechanism describes a complex cycle of damage to the epithelial cell lining of the small biliary ducts. This leads to neoantigen exposure and breakdown of immune tolerance to the dihydrolipoyl acetyltransferase (E2) component of the pyruvate dehydrogenase complex (PDC), resulting in cytokine and T cell-mediated inflammatory damage leading to progressive bile duct loss and cholestasis.9,10 At the core of the disease process, however, lies impairment of bile flow in the biliary ducts and change in the bile acid (BA) pool to a more hydrophobic profile. BAs are endproducts of cholesterol metabolism, which are significantly enriched in the liver and biliary tree. They play a key role in dietary fat absorption and, an increasingly appreciated role, regulation in cellular bioenergetics. A robust enterohepatic circulation mechanism operates in the human body to maintain homeostasis of BA pools in the liver and intestine. An excess of BAs in the liver (biliary tract) is toxic, particularly when the profile is hydrophobic, and has direct detrimental effects on the biliary duct.11–14 De Vries and colleagues described a model of a ‘biliary HCO3− umbrella’, which is protective of cholangiocytes and hepatocytes from toxic BAs. This biliary HCO3− umbrella is dependent upon adequate function of the Cl−/HCO3− anion exchange protein 2 (AE2), HCO3− exporter and an intact biliary glycocalyx on human cholangiocytes. A defective AE2 expression has been described in patients with PBC.15–18
Over the last few decades, extensive research into unfolding the mechanisms of BA metabolic pathways has shed light on previously less understood nuclear receptors (NRs): farnesoid X receptor (FXR), peroxisome proliferator-activated receptor alpha (PPAR-alpha), pregnane X receptor (PXR) and constitutive androstane receptor.19–22 NRs as transcription factors play a critical role in the synthesis, transport and metabolism of BAs. They have generated considerable interest as novel therapeutic targets for newer molecules. This review will outline the mechanism, biochemical alterations and therapeutic benefits of the first-generation therapy, ursodeoxycholic acid (UDCA), recently licensed second-line therapy, obeticholic acid (OCA) (Table 1), and additional therapies in clinical trials (Table 2). For the purpose of this article, only the novel treatment molecules being trialled in the management of PBC are described.
Table 1.
Therapies currently in use.
|
1. Management of cholestasis
|
| I. First-generation therapies:
|
| Ursodeoxycholic acid |
| II. Second-line therapies:
|
| Obeticholic acid (COBALT [ClinicalTrials.gov identifier: NCT02308111]) |
| Fibrates (unlicensed, BEZURSO trial [ClinicalTrials.gov identifier: NCT01654731]) |
|
2. Management of symptoms
|
| I. Pruritus
|
| Recommended therapies:
|
| Cholestyramine |
| Rifampicin |
| Naltrexone |
| Empirical therapies:
|
| Sertraline |
| Gabapentin |
Table 2.
Experimental therapies.
|
1. Management of cholestasis
|
| I. Additional anti-cholestatic drugs:
|
| LJN-452 [ClinicalTrials.gov identifier: NCT02516605] |
| NGM-282 [ClinicalTrials.gov identifiers: NCT02026401 and NCT02135536] |
| MBX-8025 [ClinicalTrials.gov identifier: NCT02609048] |
| GS-9674 (Gilead) [ClinicalTrials.gov identifier: NCT02943447] |
| Elafibranor [ClinicalTrials.gov identifier: NCT03124108] |
| II. Immunotherapy agent:
|
| FFP-104 [ClinicalTrials.gov identifier: NCT02193360] |
| III. Experimental therapies:
|
| Phototherapy |
| Plasmapheresis |
| Nasobiliary drainage |
| Albumin dialysis (Molecular Adsorbent Recirculating System) |
|
2. Management of symptoms
|
| I. Pruritus:
|
| Molecules in trials:
|
| GSK2330672 [ClinicalTrials.gov identifier: NCT01899703] |
| Lopixibat [ClinicalTrials.gov identifier: NCT01904058] |
| II. Fatigue:
|
| Rituximab (RITPBC trial) [ClinicalTrials.gov identifier: NCT02376335] |
Management of cholestasis in PBC
First-generation therapy
UDCA
UDCA was the first-line, and only available, licensed therapy for the management of PBC up until 2016. It is used at an optimal dose of 13–15 mg/kg body weight.4,23–26 UDCA is a physiological component of BAs in human beings but is only present in small quantities (~3%). In addition to exerting anti-apoptotic27–29 and anti-inflammatory effects,30–33 UDCA also exerts beneficial effects on cholestasis. It stimulates impaired hepatocellular secretion of hydrophobic BAs and stabilizes the biliary HCO3− umbrella, therefore protecting the cholangiocytes from the toxic effects of BAs. UDCA treatment has shown to improve liver biochemistry in responder patients with marked reduction of ALP, gamma-glutamyltransferase (GGT), cholesterol and immunoglobulin M (IgM).23,24,26 In addition, response to UDCA has also shown to delay histological progression and to improve transplant-free survival. A meta-analysis including 1038 patients from seven randomized controlled trials and six reports, who were treated with optimal dose of UDCA and followed for a minimum period of 24 months, showed a significant reduction in the need for liver transplantation (odds ratio = 0.65).34 Despite its beneficial effects, use of UDCA remains limited in around 40% of patients who show either no or only partial response to UDCA; this group of patients is classed as UDCA nonresponders.35,36 Over the decades, various models have been established and suggested to determine the UDCA nonresponse; these are summarized in Table 3.
Table 3.
Criteria for defining nonresponse to ursodeoxycholic acid.
| Criterion | Definition of UDCA nonresponse with treatment duration | Authors | Study sample size (n) | |
|---|---|---|---|---|
| 1 | Rochester | ALP ⩾ 3× UNL or Mayo score ⩾ 4.5 at 6 months | Angulo et al. 199937 | 180 |
| 2 | Barcelona | ALP decline of ⩽ 40% and ALP > UNL at 12 months | Pares et al. 200638 | 192 |
| 3 | Paris-I | ALP > 3× UNL or AST > 2× UNL, or bilirubin > 17.1 µmol/L at 12 months | Corpechot et al. 200839 | 292 |
| 4 | Toronto | ALP > 1.67 UNL at 24 months | Kumagi et al. 201040 | 69 |
| 5 | Rotterdam | Bilirubin ⩾ 1× UNL and/or albumin < 1× UNL at 12 months | Kuiper et al. 200936 | 375 |
| 6 | Ehime | Decline in GGT ⩽ 70% and GGT ⩾ 1× UNL | Azemoto et al. 200941 | 83 |
| 7 | Paris-2 | ALP ⩾ 1.5× UNL or AST ⩾ 1.5× UNL, or bilirubin > 17.1 µmol/L at 12 months | Corpechot et al. 201135 | 165 |
ALP, alkaline phosphatase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase;
UDCA, ursodeoxycholic acid; UNL, upper normal limit.
In addition, a man with PBC and a woman diagnosed with PBC below the age of 55 years are described as independent risk factors predicting UDCA nonresponse.42 The newer prognostic models of GLOBE and UK-PBC continuous risk scores have been studied and validated in a much larger population from various centres across Europe and the USA. Using these prognostic models, UDCA response was shown to improve survival to a level comparable with the matched healthy population.43,44 Corpechot and colleagues in their analysis of 262 patients receiving 13–15 mg/kg UDCA daily for a mean of 8 years demonstrated, using a multistate modelling approach, a transplant-free survival of 84% and 66% at 10 and 20 years, respectively.45 UDCA response had also been demonstrated to reduce the risk of hepatocellular carcinoma (HCC). Trivedi and colleagues, in a cohort of 4656 patients, reported an incidence rate of 3.4 cases/1000 patient-years with a 12-month biochemical nonresponse.46
In summary, UDCA response is associated with improved liver biochemistry and long-term survival. All these findings highlight the importance of early intervention in patients with PBC. However, and critically for the optimal management of the disease, the response rate for UDCA is only of the order of 60%. Appreciation of the limitations of UDCA in underresponding patients and the resulting significant un-met need has led to the development of new therapies.
Second-line therapies
OCA
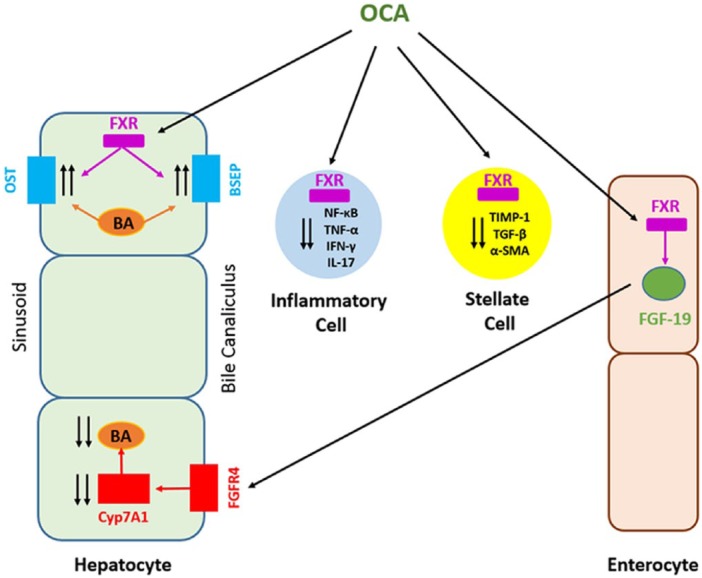
OCA (INT-747) is a novel semisynthetic derivative of the naturally occurring primary human BA, chenodeoxycholic acid (CA). This FXR ligand is approximately 100 times more potent than CA.47 FXR is an NR, predominantly expressed in the gastrointestinal tract, and plays an important role in the enterohepatic circulation of BAs. Activation of FXR inhibits de novo BA synthesis from cholesterol in hepatocytes and increased clearance of BAs from the hepatocytes. This results in the reduction of the overall BA pool, which protects against the toxic effects of the accumulation of BAs48 (Figure 1). In various preclinical and clinical studies, OCA has also been demonstrated to have FXR-mediated anti-cholestatic, anti-fibrotic and anti-inflammatory effects. A reduction in portal pressures in cirrhotic livers has been demonstrated in animal models.47,49
Figure 1.
Mechanism of action of obeticholic acid (OCA).
BA, bile acid; FGF-19, fibroblast growth factor 19; FXR, farnesoid X receptor; IFNɣ, interferon gamma; IL-17, interleukin 17; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; ɑ-SMA, alpha-smooth muscle actin; TGF-β, transforming growth factor beta; TIMP-1, tissue inhibitor of metalloproteinases-1, TNF-ɑ, tumor necrosis factor-alpha.
Efficacy and tolerability of OCA has been evaluated in phase II and III trials.50 The first study to evaluate OCA was a phase II double-blind, placebo-controlled parallel group dose response study of 59 patients with PBC, who had a persistently raised ALP level of 1.5–10× upper normal limit (UNL) and were not on UDCA for a minimum of 6 months. They were randomized to receive placebo, OCA 10 mg or OCA 50 mg for 12 weeks. At 12 weeks, the 10 mg OCA group showed a decline in pretreatment ALP from 3.9× UNL to post-treatment ALP of 1.9× UNL. Pruritus was the most common side effect in all groups (placebo: 30%, 10 mg OCA: 70% and 50 mg OCA: 94%).51
Another double-blind phase II study of OCA recruited 165 patients with PBC with persistently elevated ALP of > 1.5–10× UNL, despite being on optimal dose of UDCA for a minimum of 6 months. They were randomized to receive 10 mg, 25 mg or 50 mg OCA or placebo for 3 months with a further open-label extension to 1 year. A statistically significant change in mean ALP from baseline to end of study was observed (defined as relative percentage change) in all OCA groups (21–25% change in OCA group compared with 3% change in the placebo group; p < 0.0001). Pruritus was again observed to be the most common side effect.52 The landmark phase III study (PBC OCA International Study of Efficacy [POISE])53 of 216 patients who were treated with OCA for 1 year, with an open-label extension for another 12 months, has been reported. The study recruited patients with inadequate or no biochemical response to a minimum of 12 months of UDCA therapy (defined by ALP of > 1.67× UNL), or UDCA intolerance. The eligibility criteria were defined biochemically as ALP of > 1.67× UNL or bilirubin levels of > 1× UNL but ⩽ 2× UNL. Subjects were randomized to 5 mg OCA with dose adjustment to 10 mg, 10 mg OCA or placebo. The primary endpoint was defined as ALP of < 1.67× UNL, with a minimum 15% reduction from baseline and a total bilirubin of ⩽ 1× UNL at 12 months. The primary endpoint was met in 47% and 46% in the 10 mg and 5–10 mg titration groups, compared with 10% in the placebo group (p < 0.0001). A minimum 15% reduction in ALP from baseline was seen in 77% of subjects in the 5–10 mg OCA titration group and 10 mg OCA group, compared with 29% in the placebo group. ALP reduction (secondary endpoint) was significantly greater in the OCA group compared with placebo from baseline to 1 year (least square mean [+/- standard error] reduction of -130 ± 15 U/L in the 10 mg group, -113 ± 14 U/L in the 5–10 mg titration group, compared with placebo -14 ± 15 U/L; p < 0.0001). Total bilirubin levels dropped significantly in both OCA groups, but showed a gradual increase in the placebo group (p < 0.0001). A total of 193 of the 198 subjects who continued to open-label extension showed a sustained decline in ALP and bilirubin levels. There was no significant change from baseline at 12 months in the noninvasive parameters of liver fibrosis (i.e. fibroscan/transient elastography, tissue inhibitor of metalloproteinases-1 and hyaluronic acid) between the two groups. OCA did not demonstrate any significant effect on the improvement of symptoms of PBC. A significantly worse score in the itch domain of the PBC-40 questionnaire was observed in the 10 mg treatment arm compared with placebo at 3 months. Pruritus was the main adverse event encountered in all the study groups (68% in 10 mg group, 56% in the 5–10 mg titration group and 38% in the placebo group). A further double-blind phase IIIb trial (COBALT, [ClinicalTrials.gov identifier: NCT02308111]) evaluating clinical outcomes (primary endpoints including Model for End-Stage Liver Disease score of ⩾ 15, refractory ascites, HCC, new onset or recurrence of variceal bleed, hepatic encephalopathy or spontaneous bacterial peritonitis needing hospitalization, liver transplantation and death) of patients with PBC treated with OCA is in the recruitment phase.
Based on the positive phase II and III evaluations, OCA is now approved in the USA and Europe for second-line use in patients showing an inadequate response to UDCA, or who are intolerant of UDCA. OCA also recently obtained National Institute for Health and Care Excellence approval for use in the UK. However, one needs to bear in mind that OCA is a relatively new agent. The data from phase II and III trials are limited over a relatively short period and primarily focused on biochemical endpoints. There are still no data available on the effect on quality of life and long- term clinical outcomes. Hence, approval of OCA is subject to demonstration of improvement in clinical outcomes from the ongoing COBALT study and, possibly, the postmarketing data.
Fibrates
Fibrates, mainly fenofibrate and bezafibrate, have been observed to normalize ALP and have long been proposed as adjuncts to UDCA in UDCA-nonresponsive patients with PBC. A meta-analysis of six trials,54 which included 84 patients, showed a significant improvement in ALP in patients treated with fenofibrate and UDCA combination compared with UDCA alone in UDCA-nonresponsive patients (mean difference: -90.44 IU/L, 95% confidence interval; p < 0.0000). However, there was no evidence to support any improvement in clinical symptoms. Furthermore, a retrospective study by Hegade and colleagues55 of 23 patients treated with fenofibrate and UDCA combination showed improvement in ALP but no overall improvement in predicted survival, as shown by UK-PBC risk score. A randomized-controlled trial on the efficacy and safety of fenofibrate combined with UDCA in patients with an incomplete biochemical response to UDCA is currently recruiting patients in China [ClinicalTrials.gov identifiers: NCT02965911 and NCT02823353]. The results from the long awaited phase III study of Bezafibrate in Combination with Ursodeoxycholic Acid in Primary Biliary Cirrhosis (BEZURSO) have recently been presented at the EASL 2017 international liver conference. A total of 100 patients with UDCA nonresponse, according to the Paris-2 criterion, were randomized to bezafibrate 400 mg or placebo in combination with UDCA (13–15 mg/kg/day). A significant change in ALP (described as percentage change from baseline to 24 months) was seen in the bezafibrate group compared with placebo (-60 compared with 0 respectively; p < 0.0001). Similar changes in total bilirubin (-14 compared with +14; p < 0.0001), alanine aminotransferase (ALT) (-36 compared with 0; p < 0.0001), cholesterol (-16 compared with 0; p < 0.0001), itch score (-75 compared with 0; p < 0.01) and median liver stiffness (-15 compared with +22; p < 0.01).
A critical question moving forward with regard to fibrates will be the relative positioning of these drugs in relation to OCA. Head-to-head comparison between OCA and fibrates would represent the ideal next step, although the authors are not aware of any such studies being planned. Mechanistically, there is also the important question as to whether the drugs are synergistic and would be a logical combination therapy. If efficacy of the drugs is similar (and, at present, there is no clear answer to the question), there is the governance challenge as to whether it is appropriate to use a drug ‘off-label’ (or even in some countries where PBC is a listed contra-indication) when there is an alternative licensed drug for the specific indication; something which may differ between jurisdictions. In terms of the evidence base, it could be concluded that fibrates offer an opportunity for patients with significant pruritus (given their neutral to beneficial itch profile compared with OCA) and should be avoided in patients with jaundice or renal impairment, given the potential to cause deterioration.
Additional anti-cholestatic therapies
The experience with OCA has clearly demonstrated the value of enhanced anti-cholestatic drug efficacy. This has led to attempts to identify further agents (potentially effective in the group who remain unresponsive to both UDCA and OCA and with improved side-effect profile), and to re-explore the potential for immunotherapy agents that have to date proved disappointing
Other FXR agonists
LJN-452 [ClinicalTrials.gov identifier: NCT02516605]
LJN-452 developed by Novartis (Sittingbourne, Kent, UK) is a non-BA FXR agonist. LJN-452 in in vitro pharmacological studies has shown to be a potent human FXR agonist with > 30,000-fold selectivity over other NRs. Preclinical data demonstrate a dose-dependent increase in fibroblast growth factor 19 (FGF-19) levels with LJN-452, in single-dose or multiple-dose studies, with its target engagement in enterocyte FXRs. LJN-452, in 69 healthy volunteers, with multiple doses of 0.1 mg for up to 2 weeks or a single dose up to 3 mg, has been shown to be well tolerated. It is orally administered in capsule form. A multi-part, randomized, double-blind, placebo-controlled study to assess the safety, tolerability and efficacy of LJN-452 in patients with PBC is currently in progress. The primary study outcomes include effects on markers of cholestasis and the safety and tolerability of daily dosing of LJN-452. Secondary objectives include evaluation of pharmacokinetics, overall disease-specific quality of life, changes in the itch domain of the PBC-40 questionnaire and 100 mm visual analogue score. Recruitment across Europe and the USA is ongoing.
GS-9674 [ClinicalTrials.gov identifier: NCT02943447]
GS-9674, developed by Gilead Sciences (Foster City, CA, USA) is an orally administered potent FXR agonist. It acts on the intestinal epithelium, resulting in the release of FGF-19, and in turn leading to a decrease in lipogenesis, gluconeogenesis, hepatic triglyceride accumulation and BA synthesis. A multicentre phase II, randomized, double-blind, placebo-controlled study evaluating the safety and tolerability of GS-9674 in patients with PBC without cirrhosis is underway and is currently recruiting at various sites across North America and Europe.
PPAR agonist
MBX-8025 [ClinicalTrials.gov identifier: NCT02609048]
MBX-8025 oral agent is a selective and potent PPAR-delta agonist developed by Cymabay Therapeutics (Newark, CA, USA). The results from their phase II double-blind placebo-controlled study of 26 patients, who received up to 12 weeks of treatment with MBX-8025, showed marked improvement in cholestasis markers. A mean decrease of ALP, from a baseline of 57% in the 50 mg dose group and 62% in the 200 mg dose group, was observed compared with 0.37% in the placebo group. The response rate in the placebo, 50 mg and 200 mg groups was 10%, 67% and 100%, respectively. The trial was however terminated early due to three cases of asymptomatic rise in ALT in the treatment arm (two in the 200 mg group and one in the 50 mg group). The liver injury, however, fully resolved on cessation of the drug. Another 8-week, dose-ranging, open-label, randomized, phase II study with an 18-week extension to evaluate the safety and efficacy of MBX-8025 (seladelpar) in patients with PBC has started recruitment in the USA. Patients with PBC, who are intolerant to or had an inadequate response to UDCA, will be enrolled to receive either 5 mg or 10 mg of seladelpar for 8 weeks. Based on 8-week data, further recruitment will be done where subjects will be given 25 mg seladelpar. The primary outcome measure is change in ALP levels.
Elafibranor [ClinicalTrials.gov identifier: NCT03124108]
Elafibranor, developed by GENFIT (Loos, France), is an orally administered PPAR-alpha/delta agonist. By activating PPAR-alpha, elafibranor decreases the synthesis, increases uptake and detoxification of BAs, in addition to anti-inflammatory effects through the NF-kappa-light-chain-enhancer of activated B cells and BCL6 pathways.56,57 Elafibranor has so far been evaluated in five phase II studies (in patients with Fredrickson type IIb dyslipidaemia, atherogenic dyslipidaemia, impaired glucose tolerance, haemostasis model assessment of insulin resistance and type 2 diabetes mellitus) and a phase IIb study in patients with nonalcoholic steatohepatitis. In addition to primary endpoints, significant improvement in ALP and GGT levels (-29% and -25%, respectively) were observed across all the studies. A multicentre, randomized, double-blind, placebo-controlled, phase II study to evaluate the efficacy and safety of elafibranor at doses of 80 mg and 120 mg, for 12 weeks of treatment, in patients with PBC with inadequate response to UDCA, is currently recruiting patients across the USA and Europe.
FGF-19 agonist
NGM-282 [ClinicalTrials.gov identifiers: NCT02026401 and NCT02135536]
NGM-282 developed by NGM Biopharmaceuticals (South San Francisco, CA, USA) is a recombinant protein identical to that of FGF-19. FGF-19 is a naturally occurring protein selectively secreted in the gastrointestinal tract and acts as an ileal hormone that directly regulates the classic pathway of BA synthesis. It works by altering the activity of the CYP7A1 enzyme through binding to the FGFR4-β-klotho co-receptor complex in the liver, release of which is promoted by FXR activity, and hence, essentially acting in a similar fashion to OCA but at a slightly different target point in the pathway.58 This interruption in the enterohepatic circulation pathway and resulting changes in the FGF-19 activity leads to significant alterations in both the size and composition of the BA pool. NGM-282 effectively mimics the actions of FGF-19 on BA synthesis through the binding of FGFR4c-β-klotho co-receptor. In a phase II double-blind, randomized-controlled trial of NGM-282, 45 patients with PBC with inadequate response to UDCA were recruited. Inclusion criteria were defined as ALP of ⩾ 1.67× UNL while on a therapeutic dose of UDCA for a minimum of 12 months. Subjects were randomized to receive either 0.3 mg or 3 mg of NGM-282 or placebo in addition to UDCA for 28 days. A statistically significant reduction in ALP (defined as percentage reduction in ALP from baseline to day 28 of treatment) was seen in both the NGM-282 groups (-15.8% in 0.3 mg group (p = 0.009) and -19.2% in 3 mg group (p = 0.003) compared with placebo. Common adverse effects included headache and lower gastrointestinal symptoms (mainly diarrhoea). Results from a recently completed phase IIb study evaluating three doses of NGM-282 administered in combination with UDCA are awaited.
Immunomodulators
FFP-104 [ClinicalTrials.gov identifier: NCT02193360]
FFP-104 (previously named as PG102) developed by the Dutch company, Fast Forward Pharmaceuticals (Utrecht, The Netherlands) is an anti-CD40 human monoclonal IgG4 antibody. It is derived by a process of conversion of a previously investigated chimeric monoclonal antibody (ch5D12) that specifically targets human CD40. The molecule was studied in a pilot phase II, open-label, multicentre, dose-escalation study to evaluate its safety, tolerability and pharmacodynamics in subjects diagnosed with PBC. The study is currently recruiting participants.
Management of symptoms in PBC
It has been well established that symptom severity in PBC does not correlate with the degree of liver enzyme abnormality. Hence, improvement in cholestasis has little or no effect on PBC symptoms. A proportion of patients with pruritus do not benefit from the conventional available therapies, alone or in combination. This means that liver transplantation is left as the only option for intractable itch. Fatigue is another important symptom affecting patients with PBC. This can be a particularly debilitating symptom, affecting quality of life and resulting in social isolation. This demands a greater need to develop and validate therapeutic agents targeting symptoms in PBC in addition to cholestasis to improve the overall quality of life of patients with PBC.
Management of itch in PBC
Cholestyramine
Cholestyramine is a well-established and widely used first-line therapeutic agent for treating cholestatic itch.59,60 It is a nonabsorbable quaternary ammonium ion-exchange resin and works by combining with intestinal BAs, hence, reducing their reabsorption.61,62 The recommended dose of cholestyramine is 4–16 g/day in divided doses. Caution must be taken to take the medication 2–4 h apart from UDCA, as the drug interaction interferes with UDCA absorption.63 Often tolerability is an issue with cholestyramine, limiting its use. Unpleasant taste, bloating, diarrhoea and constipation are the commonest reported side effects.
Rifampicin
Rifampicin is a recommended second-line therapy in cholestatic pruritus.4 Its efficacy has been well established in randomized-controlled trials and meta-analysis.64–67 Rifampicin acts as a PXR agonist. The recommended maximum dose of rifampicin is 600 mg, usually started at a lower dose of 150 mg and gradually titrated up while carefully monitoring liver biochemistry.68,69 Adverse effects of rifampicin include hepatotoxicity, nephrotoxicity and drug interactions.
Naltrexone
Naltrexone is a recommended third-line agent for cholestatic pruritus.4 By virtue of its opioid antagonistic actions, it is believed to relieve pruritus in patients with PBC who are often described as having an increase in opioidergic tone.70–72 The recommended maximum daily dose of naltrexone is 50 mg/day, however, it is usually started at a lower dose of 12.5 mg/day and gradually titrated up. The commonest side effects include opioid withdrawal-like reaction during the initial few days of treatment.73,74
Sertraline
Sertraline, a commonly used antidepressant, is a selective serotonin reuptake inhibitor. Evidence though small in the form of randomized-controlled trials has shown sertraline to have some beneficial effects in resistant pruritus independent of its antidepressant effect.75,76 Side effects include dry mouth, dizziness and insomnia.
Gabapentin
Gabapentin, due to its effect of increasing nociceptive threshold, had been recommended as a potential treatment for cholestatic pruritus.77 A randomized, double-blind, placebo-controlled trial of 16 patients with cholestatic pruritus treated with 4 weeks of gabapentin failed to show any significant therapeutic advantage over placebo.78
Phototherapy
Phototherapy using ultraviolet B radiations has been suggested as therapy for cholestatic pruritus. Although the mechanism is unknown, results from an observational case series of 13 patients showed significant beneficial effects. There was a statistically significant improvement in pruritus with decrease in median visual analogue scale from 8.0 to 2.0 (p < 0.001), with the average duration of phototherapy being 8 weeks.79
Plasmapheresis
Plasmapheresis has been suggested as a treatment for resistant complications of PBC going as far back as the 1970s.80,81 However, its use in pruritus related to cholestasis is still in the experimental phase. A recent study of 17 patients with PBC with refractory pruritus and who received 129 sessions of plasmapheresis over 40 hospital admissions has been published.82 Refractory pruritus was defined as no response to therapy with both cholestyramine and titrated maximum dose of rifampicin (300 mg). Itch was quantified using a 10-point numeric rating scale before and after plasmapheresis and at the 30-day and 90-day time point. Mean pruritus score declined from 8.3 ± 1.4 to 3.1 ± 2.2 (p < 0.0001) in all patients and the antipruritic effect was maintained up to 90 days (p < 0.0001). A significant decrease in serum ALT, ALP, aspartate aminotransferase (AST), GGT (p < 0.001) and bilirubin (p < 0.002) was also noted. In summary, plasmapheresis offers a plausible strategy for treating refractory itch in PBC and other cholestatic conditions; however, further randomized-controlled studies are needed to establish its exact place in the treatment ladder for cholestatic itch.
Nasobiliary drainage
Nasobiliary drainage of BAs helps to reduce serum autotoxins, thereby exerting antipruritic effects. However, due to the nature of the therapy the effects are not sustainable over long periods of time.83,84 A multicentre retrospective study from Europe of 27 patients undergoing 29 nasobiliary drainage procedures reported improvement in pruritus in 89.6% of patients as measured on a visual analogue scale (score decreasing from 10.0 to 0.3; p < 0.0001). A significant improvement in serum bilirubin and ALP was also reported (p = 0.03 and 0.001, respectively).85 As the procedure involves performing an endoscopic retrograde cholangiopancreatography, potential for a high-risk adverse event of pancreatitis remains a concern.
Albumin dialysis
Albumin dialysis (molecular adsorbent recirculating system) using an adsorbent recirculating circuit is thought to exert its antipruritic effects by removing pruritogens from the circulation.86 In a study of 20 patients with cholestatic pruritus who underwent albumin dialysis, a significant improvement in pruritus as defined by visual analogue scale (scores decreased from 70.2 ± 4.8 to 20.1 ± 4.2; p < 0.001) has been demonstrated. No adverse effects were reported.87
GSK2330672 [ClinicalTrials.gov identifier: NCT01899703]
GSK2330672, developed by GlaxoSmithKline (London, UK), is an orally administered selective inhibitor of the human ileal bile acid transporter (IBAT) for treatment for pruritus associated with PBC.
In patients with PBC, inhibition of IBAT by GSK2330672 was anticipated to increase excretion of BAs and reduce BA concentrations in the liver and systemic circulation, resulting in reduced pruritus and associated symptoms. Results from a recent phase IIa, double-blind, randomized, placebo-controlled, crossover trial conducted by Hegade and colleagues88 in 21 patients who received 2 weeks of GSK2330672 or placebo, showed significant improvement in the pruritus symptom. Compared with placebo, itch intensity significantly improved from baseline with GSK2330672 using the PBC-40 itch domain (-14; p = 0.034), numeric rating scale (-23%; p = 0.037), and 5-D itch scale (-20%; p = 0.0045). Diarrhoea was the most common side effect associated with the GSK2330672 molecule. The drug was otherwise well tolerated with no adverse events reported. GLIMMER (GSK2330672 trial of IBAT inhibition with Multi-dose Measurement for Evaluation of Response) is a randomized, double-blind, multi-dose, placebo-controlled study to evaluate the efficacy, safety and tolerability of GSK2330672 administration for the treatment of pruritus in patients with PBC. The study is currently recruiting patients in the USA. The primary endpoint is defined as mean change in the mean worst daily itch score from baseline at week 16. Secondary endpoints include changes in PBC-40 itch score, ALP, ALT, AST, GGT, total bilirubin, albumin and prothrombin time.
Lopixibat (LUM001) [ClinicalTrials.gov identifier: NCT01904058]
Lopixibat is a novel apical sodium-dependent BA transporter inhibitor. Results from a phase II, randomized, double-blind, placebo-controlled trial (CLARITY study)89 in 61 patients with PBC with associated pruritus and randomized to lopixibat 10 mg or 20 mg or placebo in combination with UDCA showed significant reduction in pruritus within the treatment and placebo arms as measured by the adult itch reported outcome (Adult ItchRO) (26% with lopixibat and 23% with placebo; p ⩽ 0.0001). However, there was no significant difference in the comparison between the two arms (p = 0.47). Common side effects reported were diarrhoea (61%), nausea and abdominal pain.
Even though GSK2330672 and lopixibat have similar mechanisms of action, there was significant difference in the results from the two studies.
Management of fatigue in PBC
Rituximab
PDC is an enzyme complex linking glycolysis and the Kreb cycle and hence plays a crucial role in cellular bioenergetics. Patients with PBC have high titres of anti-PDC antibodies.90,91 The peripheral component of fatigue in patients with PBC was thought to be linked to their inability to sustain repeated muscle contractions. Using a novel magnetic resonance spectroscopy technique, it has been shown that fatigued patients have marked muscle acidosis related to mitochondrial dysfunction and prolonged recovery time, which in turn was related to serum anti-PDC levels. Rituximab, with its B cell-depleting monoclonal antibody mechanism, was believed to be potentially of benefit in treating fatigued patients.92 We carried out a study of rituximab as a treatment for fatigue in PBC at clinical trial units in Newcastle, UK. This has concluded and the results from the study will be published separately.
Conclusion
For decades, the prospect of PBC management was limited to UDCA. With a large cohort of patients nonresponsive to UDCA, there has been a huge un-met need. The recently approved OCA has compelling data from phase II and III trials on its efficacy in improving liver biochemistry with the normalization of ALP in nearly 90% of patients. However, concerns about pruritus will limit its use in larger subsections of patients with PBC affected by itch. Furthermore, there is no evidence that OCA improves quality of life. Even though OCA is a huge stepping stone in the landscape of PBC management, there is still a wider need to develop and explore new agents in the management of cholestasis and symptoms in patients, not only to improve biochemistry and clinical endpoints, but also to target improvements in the overall quality of life of these patients. The pharmaceutical industry has shown huge interest in rare liver disease and, with the advent of these new molecules currently being evaluated, we can hope to have better tolerated and more effective therapies in the near future for PBC.
Acknowledgments
AK and DEJ were involved in the literature search and review, and drafting this manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Amardeep Khanna, Clinical Research Associate, Institute of Cellular Medicine, 4th Floor William Leech Building, Medical School, Framlington Place, Newcastle University, Newcastle upon Tyne NE2 4HH, UK.
David E. Jones, Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, UK
References
- 1. Beuers U, Gershwin ME, Gish RG, et al. Changing nomenclature for PBC: from ‘cirrhosis’ to ‘cholangitis’. Gastroenterology 2015; 149: 1627–1629. [DOI] [PubMed] [Google Scholar]
- 2. Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet 2003; 362: 53–61. [DOI] [PubMed] [Google Scholar]
- 3. Invernizzi P, Lleo A, Podda M. Interpreting serological tests in diagnosing autoimmune liver diseases. Semin Liver Dis 2007; 27: 161–172. [DOI] [PubMed] [Google Scholar]
- 4. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017; 67: 145–172. [DOI] [PubMed] [Google Scholar]
- 5. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet 2015; 386: 1565–1575. [DOI] [PubMed] [Google Scholar]
- 6. Parés A, Rodés J. Natural history of primary biliary cirrhosis. Clin Liver Dis 2003; 7: 779–794. [DOI] [PubMed] [Google Scholar]
- 7. Ali AH, Sinakos E, Silveira MG, et al. Varices in early histological stage primary biliary cirrhosis. J Clin Gastroenterol 2011; 45: e66–e71. [DOI] [PubMed] [Google Scholar]
- 8. Liaskou E, Hirschfield GM, Gershwin ME. Mechanisms of tissue injury in autoimmune liver diseases. Semin Immunopathol 2014; 36: 553–568. [DOI] [PubMed] [Google Scholar]
- 9. Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol 2013; 8: 303–330. [DOI] [PubMed] [Google Scholar]
- 10. Jones DE. Pathogenesis of primary biliary cirrhosis. Postgrad Med J 2008; 84: 23–33. [DOI] [PubMed] [Google Scholar]
- 11. de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab 2013; 17: 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res 2014; 55: 1553–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap – bile acids in metabolic control. Nat Rev Endocrinol 2014; 10: 488–498. [DOI] [PubMed] [Google Scholar]
- 14. Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 2003; 72: 137–174. [DOI] [PubMed] [Google Scholar]
- 15. Banales JM, Sáez E, Úriz M, et al. Up-regulation of microRNA 506 leads to decreased Cl−/HCO3− anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology 2012; 56: 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Medina JF, Martínez-Ansó E, Vázquez JJ, et al. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology 1997; 25: 12–17. [DOI] [PubMed] [Google Scholar]
- 17. Prieto J, García N, Martí-Climent JM, et al. Assessment of biliary bicarbonate secretion in humans by positron emission tomography. Gastroenterology 1999; 117: 167–172. [DOI] [PubMed] [Google Scholar]
- 18. Vries E, Beuers U. Management of cholestatic disease in 2017. Liver Int 2017; 37(Suppl. 1): 123–129. [DOI] [PubMed] [Google Scholar]
- 19. Kovacs P, Kress R, Rocha J, et al. Variation of the gene encoding the nuclear bile salt receptor FXR and gallstone susceptibility in mice and humans. J Hepatol 2008; 48: 116–124. [DOI] [PubMed] [Google Scholar]
- 20. Sinal CJ, Tohkin M, Miyata M, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000; 102: 731–744. [DOI] [PubMed] [Google Scholar]
- 21. Uppal H, Toma D, Saini SPS, et al. Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice. Hepatology 2005; 41: 168–176. [DOI] [PubMed] [Google Scholar]
- 22. Wittenburg H, Lyons MA, Li R, et al. FXR and ABCG5/ABCG8 as determinants of cholesterol gallstone formation from quantitative trait locus mapping in mice. Gastroenterology 2003; 125: 868–881. [DOI] [PubMed] [Google Scholar]
- 23. Combes B, Carithers RL, Maddrey WC, et al. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1995; 22: 759–766. [PubMed] [Google Scholar]
- 24. Heathcote EJ, Cauch-Dudek K, Walker V, et al. The Canadian multicenter double-blind randomized controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 1994; 19: 1149–1156. [PubMed] [Google Scholar]
- 25. Lindor KD, Dickson ER, Baldus WP, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology 1994; 106: 1284–1290. [DOI] [PubMed] [Google Scholar]
- 26. Poupon RE, Balkau B, Eschwège E, et al. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. N Engl J Med 1991; 324: 1548–1554. [DOI] [PubMed] [Google Scholar]
- 27. Benz C, Angermuller S, Otto G, et al. Effect of tauroursodeoxycholic acid on bile acid-induced apoptosis in primary human hepatocytes. Eur J Clin Invest 2000; 30: 203–209. [DOI] [PubMed] [Google Scholar]
- 28. Beuers U. Drug insight: mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol 2006; 3: 318–328. [DOI] [PubMed] [Google Scholar]
- 29. Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. J Hepatol 2001; 35: 134–146. [DOI] [PubMed] [Google Scholar]
- 30. Miura T, Ouchida R, Yoshikawa N, et al. Functional modulation of the glucocorticoid receptor and suppression of NF-κB-dependent transcription by ursodeoxycholic acid. J Biol Chem 2001; 276: 47371–47378. [DOI] [PubMed] [Google Scholar]
- 31. Takigawa T, Miyazaki H, Kinoshita M, et al. Glucocorticoid receptor-dependent immunomodulatory effect of ursodeoxycholic acid on liver lymphocytes in mice. Am J Physiol Gastrointest Liver Physiol 2013; 305: G427–G438. [DOI] [PubMed] [Google Scholar]
- 32. Tanaka H, Makino I. Ursodeoxycholic acid-dependent activation of the glucocorticoid receptor. Biochem Biophys Res Commun 1992; 188: 942–948. [DOI] [PubMed] [Google Scholar]
- 33. Tanaka H, Makino Y, Miura T, et al. Ligand-independent activation of the glucocorticoid receptor by ursodeoxycholic acid. Repression of IFN-gamma-induced MHC class II gene expression via a glucocorticoid receptor-dependent pathway. J Immunol 1996; 156: 1601–1608. [PubMed] [Google Scholar]
- 34. Shi J, Wu C, Lin Y, et al. Long-term effects of mid-dose ursodeoxycholic acid in primary biliary cirrhosis: a meta-analysis of randomized controlled trials. Am J Gastroenterol 2006; 101: 1529–1538. [DOI] [PubMed] [Google Scholar]
- 35. Corpechot C, Chazouillères O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol 2011; 55: 1361–1367. [DOI] [PubMed] [Google Scholar]
- 36. Kuiper EMM, Hansen BE, de Vries RA, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 2009; 136: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 37. Angulo P, Lindor KD, Therneau TM, et al. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver Int 1999; 19: 115–121. [DOI] [PubMed] [Google Scholar]
- 38. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology 2006; 130: 715–720. [DOI] [PubMed] [Google Scholar]
- 39. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 2008; 48: 871–877. [DOI] [PubMed] [Google Scholar]
- 40. Kumagi T, Guindi M, Fischer SE, et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol 2010; 105: 2186–2194. [DOI] [PubMed] [Google Scholar]
- 41. Azemoto N, Abe M, Murata Y, et al. Early biochemical response to ursodeoxycholic acid predicts symptom development in patients with asymptomatic primary biliary cirrhosis. J Gastroenterol 2009; 44: 630–634. [DOI] [PubMed] [Google Scholar]
- 42. Carbone M, Mells GF, Pells G, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology 2013; 144: 560–569.e7. [DOI] [PubMed] [Google Scholar]
- 43. Carbone M, Sharp SJ, Flack S, et al. The UK-PBC risk scores: derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology 2016; 63: 930–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology 2015; 149: 1804–1812.e4. [DOI] [PubMed] [Google Scholar]
- 45. Corpechot C, Carrat F, Bahr A, et al. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology 2005; 128: 297–303. [DOI] [PubMed] [Google Scholar]
- 46. Trivedi PJ, Lammers WJ, Van Buuren HR, et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gut 2016; 65: 321–329. [DOI] [PubMed] [Google Scholar]
- 47. Fiorucci S, Rizzo G, Antonelli E, et al. A farnesoid X receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharm Exp Ther 2005; 314: 584–595. [DOI] [PubMed] [Google Scholar]
- 48. Ananthanarayanan M, Balasubramanian N, Makishima M, et al. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem 2001; 276: 28857–28865. [DOI] [PubMed] [Google Scholar]
- 49. Fiorucci S, Rizzo G, Antonelli E, et al. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor γ contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharm Exp Ther 2005; 315: 58–68. [DOI] [PubMed] [Google Scholar]
- 50. Kowdley KV, Jones D, Luketic V, et al. An international study evaluating the farnesoid X receptor agonist obeticholic acid as monotherapy in PBC. J Hepatol 2011; 54(Suppl. 1): S13. [Google Scholar]
- 51. Jones D, Kowdley K, Chapman R, et al. The first new monotherapy therapeutic PBC study in a decade? An international study evaluating the farnesoid X receptor agonist obeticholic acid in PBC. Gut 2011; 60(Suppl. 2): A50. [Google Scholar]
- 52. Hirschfield GM, Mason A, Luketic V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 2015; 148: 751–761.e8. [DOI] [PubMed] [Google Scholar]
- 53. Gerken G, Nitschmann S. Obeticholic acid in primary biliary cholangitis: PBC OCA international study of efficacy (POISE). Internist 2017; 58: 202–204. [DOI] [PubMed] [Google Scholar]
- 54. Zhang Y, Li S, He L, et al. Combination therapy of fenofibrate and ursodeoxycholic acid in patients with primary biliary cirrhosis who respond incompletely to UDCA monotherapy: a meta-analysis. Drug Des Devel Ther 2015; 9: 2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hegade VS, Khanna A, Walker LJ, et al. Long-term fenofibrate treatment in primary biliary cholangitis improves biochemistry but not the UK-PBC risk score. Dig Dis Sci 2016; 61: 3037–3044. [DOI] [PubMed] [Google Scholar]
- 56. Sookoian S, Pirola CJ. Elafibranor for the treatment of NAFLD: one pill, two molecular targets and multiple effects in a complex phenotype. Ann Hepatol 2016; 15: 604–609. [PubMed] [Google Scholar]
- 57. Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator− activated receptor− α and− δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016; 150: 1147–1159.e5. [DOI] [PubMed] [Google Scholar]
- 58. Goetz R, Ohnishi M, Ding X, et al. Klotho coreceptors inhibit signaling by paracrine fibroblast growth factor 8 subfamily ligands. Mol Cell Biol 2012; 32: 1944–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lindor KD, Gershwin ME, Poupon R, et al. Primary biliary cirrhosis. Hepatology 2009; 50: 291–308. [DOI] [PubMed] [Google Scholar]
- 60. Datta DV, Sherlock S. Cholestyramine for long term relief of the pruritus complicating intrahepatic cholestasis. Gastroenterology 1966; 50: 323–332. [PubMed] [Google Scholar]
- 61. Trivedi HD, Lizaola B, Tapper EB, et al. Management of pruritus in primary biliary cholangitis: a narrative review. Am J Med 2017; 130: 744.e1–744.e7. [DOI] [PubMed] [Google Scholar]
- 62. Hong-Bin C, Yue H, Chun H, et al. Randomized controlled trial of cholestyramine and hydrotalcite to eliminate bile for capsule endoscopy. Saudi J Gastroenterol 2016; 22: 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rust C, Sauter GH, Oswald M, et al. Effect of cholestyramine on bile acid pattern and synthesis during administration of ursodeoxycholic acid in man. Eur J Clin Invest 2000; 30: 135–139. [DOI] [PubMed] [Google Scholar]
- 64. Bachs L, Elena M, Parés A, et al. Comparison of rifampicin with phenobarbitone for treatment of pruritus in biliary cirrhosis. Lancet 1989; 333: 574–576. [DOI] [PubMed] [Google Scholar]
- 65. Podesta A, Lopez P, Terg R, et al. Treatment of pruritus of primary biliary cirrhosis with rifampin. Dig Dis Sci 1991; 36: 216–220. [DOI] [PubMed] [Google Scholar]
- 66. Ghent CN, Carruthers SG. Treatment of pruritus in primary biliary cirrhosis with rifampin: results of a double-blind, crossover, randomized trial. Gastroenterology 1988; 94: 488–493. [DOI] [PubMed] [Google Scholar]
- 67. Khurana S, Singh P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta-analysis of prospective randomized-controlled trials. Liver Int 2006; 26: 943–948. [DOI] [PubMed] [Google Scholar]
- 68. Prince MI, Burt AD, Jones DEJ. Hepatitis and liver dysfunction with rifampicin therapy for pruritus in primary biliary cirrhosis. Gut 2002; 50: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sampaziotis F, Griffiths WJH. Severe coagulopathy caused by rifampicin in patients with primary sclerosing cholangitis and refractory pruritus. Br J Clin Pharmacol 2012; 73: 826–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ballantyne JC, Loach AB, Carr DB. The incidence of pruritus after epidural morphine. Anaesthesia 1989; 44: 863. [DOI] [PubMed] [Google Scholar]
- 71. Bergasa NV, Talbot TL, Alling DW, et al. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology 1992; 102: 544–549. [DOI] [PubMed] [Google Scholar]
- 72. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol 2002; 37: 717–722. [DOI] [PubMed] [Google Scholar]
- 73. Jones EA, Neuberger J, Bergasa NV. Opiate antagonist therapy for the pruritus of cholestasis: the avoidance of opioid withdrawal-like reactions. QJM 2002; 95: 547–552. [DOI] [PubMed] [Google Scholar]
- 74. Thornton JR, Losowsky MS. Opioid peptides and primary biliary cirrhosis. BMJ 1988; 297: 1501–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Browning J, Combes B, Mayo MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol 2003; 98: 2736–2741. [DOI] [PubMed] [Google Scholar]
- 76. Mayo MJ, Handem I, Saldana S, et al. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology 2007; 45: 666–674. [DOI] [PubMed] [Google Scholar]
- 77. Anand S. Gabapentin for pruritus in palliative care. Am J Hosp Palliat Med 2013; 30: 192–196. [DOI] [PubMed] [Google Scholar]
- 78. Bergasa NV, McGee M, Ginsburg IH, et al. Gabapentin in patients with the pruritus of cholestasis: a double-blind, randomized, placebo-controlled trial. Hepatology 2006; 44: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 79. Decock S, Roelandts R, Van Steenbergen W, et al. Cholestasis-induced pruritus treated with ultraviolet B phototherapy: an observational case series study. J Hepatol 2012; 57: 637–641. [DOI] [PubMed] [Google Scholar]
- 80. Turnberg LA, Mahoney MP, Gleeson MH, et al. Plasmaphoresis and plasma exchange in the treatment of hyperlipaemia and xanthomatous neuropathy in patients with primary biliary cirrhosis. Gut 1972; 13: 976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Alallam A, Barth D, Heathcote EJ. Role of plasmapheresis in the treatment of severe pruritus in pregnant patients with primary biliary cirrhosis. Can J Gastroenterol Hepatol 2008; 22: 505–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Krawczyk M, Liebe R, Wasilewicz M, et al. Plasmapheresis exerts a long-lasting antipruritic effect in severe cholestatic itch. Liver Int 2017; 37: 743–747. [DOI] [PubMed] [Google Scholar]
- 83. Beuers U, Gerken G, Pusl T. Biliary drainage transiently relieves intractable pruritus in primary biliary cirrhosis. Hepatology 2006; 44: 280–281. [DOI] [PubMed] [Google Scholar]
- 84. Hofmann AF, Huet PM. Nasobiliary drainage for cholestatic pruritus. Hepatology 2006; 43: 1170–1171. [DOI] [PubMed] [Google Scholar]
- 85. Hegade VS, Krawczyk M, Kremer AE, et al. The safety and efficacy of nasobiliary drainage in the treatment of refractory cholestatic pruritus: a multicentre European study. Aliment Pharmacol Ther 2016; 43: 294–302. [DOI] [PubMed] [Google Scholar]
- 86. Leckie P, Tritto G, Mookerjee R, et al. ‘Out-patient’ albumin dialysis for cholestatic patients with intractable pruritus. Aliment Pharmacol Ther 2012; 35: 696–704. [DOI] [PubMed] [Google Scholar]
- 87. Parés A, Herrera M, Avilés J, et al. Treatment of resistant pruritus from cholestasis with albumin dialysis: combined analysis of patients from three centers. J Hepatol 2010; 53: 307–312. [DOI] [PubMed] [Google Scholar]
- 88. Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet 2017; 389: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 89. Mayo MJ, Pockros P, Jones D, et al. CLARITY: a phase 2, randomized, double-blind, placebo-controlled study of lopixibat chloride (formerly Lum001), a novel apical sodium-dependent bile acid transporter inhibitor, in the treatment of primary biliary cirrhosis associated with itching. J Hepatol 2016; 64: S197. [Google Scholar]
- 90. Myers RP, Shaheen AA, Swain MG, et al. Rituximab for primary biliary cirrhosis (PBC) refractory to ursodeoxycholic acid (UDCA). Hepatology 2007; 46: 550. [Google Scholar]
- 91. Robe AJ, Kirby JA, Jones DEJ, et al. A key role for autoreactive B cells in the breakdown of T-cell tolerance to pyruvate dehydrogenase complex in the mouse. Hepatology 2005; 41: 1106–1112. [DOI] [PubMed] [Google Scholar]
- 92. Hollingsworth KG, Newton JL, Taylor R, et al. Pilot study of peripheral muscle function in primary biliary cirrhosis: potential implications for fatigue pathogenesis. Clin Gastroenterol Hepatol 2008; 6: 1041–1048. [DOI] [PubMed] [Google Scholar]