Abstract
The synergy and shared co-morbidity, certainly interplay between kidney and cardiovascular disease, where advanced renal failure influences on progression of cardiac disease in bi-direction relationship. Cardiovascular diseases are cause of death in almost 50% of uremic patients. Correction of uremia after successful renal transplantation leads to improved cardiovascular status in the maj ority of kidney transplanted patients. The aim of this study was an evaluation of the influence of renal allograft function on left ventricular remodelling in the first year after transplantation comparing echocardiographic findings before and twelve months after transplantation had been done. In retrospective-prospective study we followed up 30 patients with renal allograft in the first post transplant year. During the study values of serum creatinine and creatinine clearance were monthly monitored. Echocardiographic examination was done before transplantation and one year after the kidney transplantation. Results of our study showed that before transplantation 67% of patients had echocardiographic signs of left ventricular (LV) hypertrophy, while 33% of patients had normal echocardiographic findings. After first post transplant year, 63% of patients showed normal view of LV and 37% remained with LV hypertrophy. Diastolic dysfunction of LV till the end of study had been reduced from 70% to 40% of patients. The positive echocardio-graphic remodelling of LV significantly correlated with the rise in creatinine clearance and with the reduction of the serum creatinine. These results confirm positive correlation between renal allograft functional status and remodelling of left ventricular hypertrophy after successful renal transplantation.
Keywords: HNPCC, kidney transplantation, left ventricular hypertrophy, echocardiography
INTRODUCTION
Cardiovascular diseases are leading cause of morbidity and mortality in the uremic patients (1,2). Left ventricular hypertrophy (LVH) is a well-established marker of cardiovascular risk. The prevalence of LVH increases with progression of renal insufficiency. Left ventricular hypertrophy is frequently present among transplant patients (3). Many traditional and non-traditional factors appear to be the stimuli for left ventricular growth in renal failure patients. In renal transplant recipients, relatively little is known about the prognostic factors which influence on causes and consequences of LVH. The high prevalence of cardiovascular disease in renal transplant recipients could be attributing to both pretrans-plant and posttransplant risk factors. Cardiovascular mortality in the transplant population has been linked to reduced renal function. Some recently published studies emphasize cardiovascular consequences of improved kidney function in renal transplant patients (4). We hypothesized that renal allograft function could influence on course and outcome of left ventricular hypertrophy in the first posttransplant year. The objectives of this analysis were to describe the prevalence of LVH and prognostic impact of serum creatinine and creatinine clearance levels on LVH at first year after renal transplantation. Investigation was made by the comparison of echocardiographic findings made before and twelve months after transplantation.
MATERIALS AND METHODS
The retrospective-prospective clinical study of relationship between renal function and left ventricular remodelling in the renal transplant patients in the first year after transplantation was done at Clinic of Neph- rology, University of Sarajevo Clinics Centre. Thirty patients with kidney transplant were studied /19 male with the average of age 37,8 ±9,9 years and 11 female with the average of age 35,55±11 years/ in the first transplant year. All evaluated patients underwent renal transplantation between 2000 and 2007 (two cadavers and 28 living related kidney transplantation). Patients with severe vascular disease and heart failure, with acute renal rejection, chronic allograft nephropathy with progressively decreased renal function in the first three posttransplant months, and patients older than 55 years were not included in the study. All patients data were collected at clinic visits and by direct patient communication. Haematological and biochemical parameters were recorded just before renal transplantation and in one month interval after kidney transplantation in the first posttransplant year, and included control of serum creatinine and creatinine clearance values. Echocardiographic examination was done prior to kidney transplantation and one year after the kidney transplantation at Clinic of Cardiology, University of Sarajevo Clinics Centre. Echocardiographic analysis was performed on M mod, two-dimension and pulse Doppler apparatus. During evaluated period all patients were without signs of fluid overload, with good control of blood pressure. Immunosuppressive drugs /cy- closporine, mycophenolate mofetil, pronisone/ were prescribed by accepted protocol during follow-up. The criterion accepted for left ventricular hypertrophy was: left ventricular mass index (LVMI) for male >131 g/m2, for female LVMI > 100 g/m2 (5). Systolic left ventricular function was estimated by ejection function (EF), where systolic dysfunction was defined as EF<50%. Left ventricular diastolic function was observed through relationship between early and late atrial phase of rapid left ventricular filling (E/A). Left ventricular diastolic dysfunction was defined as E/A value <1,0.
Statistical analysis
The data were analyzed using the descriptive statistics for each parameter that was followed. Students’ t-test was used to compare arithmetic means of numeric variables of each parameters, with the acceptance of statistical significance at the level p<0,05. Logistic regression was used to establish the independent relationship between left ventricular mass index on echocardiographic findings, and renal function measured by creatinine clearance.
RESULTS
In the total group of patients, on baseline echocardiography before kidney transplantation, 10 of subjects (33%) showed normal mass of left ventricle, 67% had left ventricular hypertrophy, out of which fourteen (47%) had concentric and six (20%) had eccentric hypertrophy (Figure 1).
FIGURE 1.
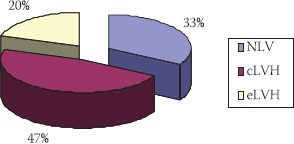
Echocardiographic findings before transplantation
NLV-normal mass of left ventricle; cLVH-concentric left ventricular hypertrophy; eLVH-eccentric left ventricular hypertrophy
Normal function of LV in the beginning of the study had 20% of patients, while 80% had dysfunction of left ventricle, out of which 70% had diastolic and 10% had systolic-diastolic dysfunction (Figure 2).
FIGURE 2.
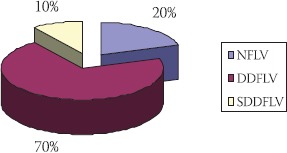
Functional status of LV in patients before transplantation
NFLV- normal function of LV; DDLV-diastolic dysfunction of LV; SDDLV systolic-diastolic dysfunction of LV
Ehocardiographic findings in patients first year after transplantation showed normal LV mass index in nineteen patients (63%), among them 79% were male (Figure 3). LV hypertrophy had 37% of patients, with dominant concentric type (90%). Among patients with LV hypertrophy, females were numerous.
FIGURE 3.
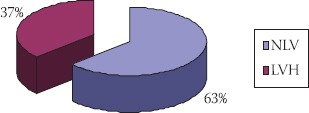
Ehocardiographic findings in transplant patients at the end of follow up
NLV normal mass of left ventricle LVH-left ventricular hypertrophy
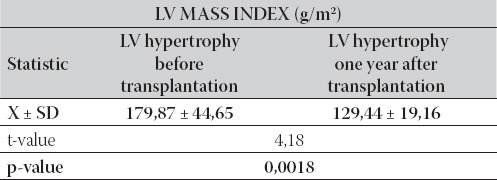
All patients with normal LV mass index in the beginning of this study remained with normal LV mass index till the end of this study. From the group of patients with LV hypertrophy (20 patients), nine patients had reached normal LV mass index till the end of this study. Patients with LV hypertrophy (11 patients) had shown significantly lower LV mass index compared with the start values (p=0,0018), which is presented in Table 1. In the same time, normal function of LV had been observed in 57% of patients, while 40% of patients were with diastolic dysfunction, and 3% of patients were with systolic-diastolic LV dysfunction.
TABLE 1.
Left ventricular mass index in group with LVH in the beginning and in the end of the study
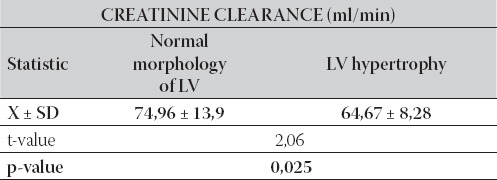
In the end of first year after the transplantation, the group with normal morphology of LV had higher values of creatinine clearance compared with LV hypertrophy group. The difference in mean values of creatinine clearance between the tested groups was statistically significant (p=0,025), as it is shown in Table 2.
TABLE 2.
Inter-group relationship between mean values of creatinine clearance compared to morphology status of LV in the end of follow
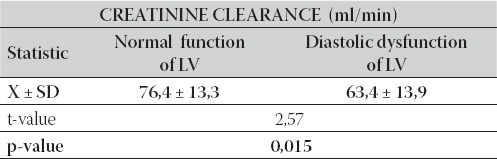
In respect of LV functional status, group of patients with normal LV function had significantly higher creatinine clearance (p=0,015) compared to the group of patients with diastolic dysfunction of LV (76,4 ml/min ± 13,3 vs. 63,4 ml/min ± 13,9), as it is presented in Table 3.
TABLE 3.
Inter-group relationship between mean values of creatinine clearance compared to functional status of LV
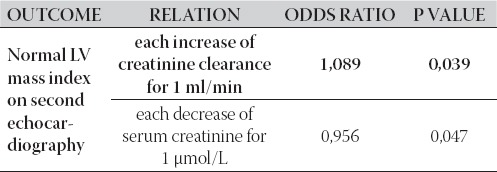
The logistic regression showed independent association between normalization of LV mass on the second echocardiography with each decrease in serum creatinine for 1 μmol/L (p=0,047), and with each increase of creatinine clearance for 1 ml/min (p=0,039), as it is shown in Table 4.
TABLE 4.
Interplay between LV mass index on second echocardiogra-phy and renal allograft function
DISCUSSION
Cardiovascular disease is the main cause of death in patients with end-stage renal disease. The prevalence of cardiovascular diseases, first of all left ventricular hypertrophy, coronary disease, congestive heart failure, among patients with end stage of renal disease (ESRD) on dialysis and transplant renal patients has been higher than in general population. Mortality rate is almost 52%, and it is 10 to 20 times higher than in general population (6). In the first 5 years after renal transplantation, half of all deaths are cardiac, often in the presence of a functional graft. Although improved survival of renal transplant recipients compared with patients undergoing dialysis has been shown, cardiovascular mortality remains twice that of the general population (7). A number of traditional and non-traditional risk factors in the pre-transplant and posttransplant periods contribute to cardiovascular morbidity and mortality in renal transplant patients, like as anaemia, hypertension, dyslipidemia, posttransplant diabetes mellitus and left ventricular hypertrophy. More non-traditional cardiovascular risk factors, such as CRP, homocysteine, advanced glycation end products may also be important. Recently, it has been shown that renal function in the first post-transplant year was good predictor not only graft survival but cardiovascular morbidity and death (8). Presence of left ventricular hypertrophy among dialysis patients, determined by echocardiography, reliable, non-invasive and reproducible method of examination, is from 60 % to 90%, and it is the most important factor for survival of this category of patients (9). By analyzed echocardiographic findings among our patients before renal transplantation, left ventricular hypertrophy is the most common alteration (67%). Compared with left ventricular functional status, only 20% of patients had normal function, diastolic dysfunction was predominated and had been found in 70% of cases. The left ventricular normal view till the end of first year after transplantation had been observed in 63% of patients compared with 33% on the start, while 37% of patients remained with left ventricular hypertrophy (67% of patients on the start), with LV mass index significantly lower compared with the start values (129,44 vs. 179,87, p=0,0018). In the end of this study, the reduction of diastolic dysfunction was significant (p=0,015). Hernandez et al. (10) reported that regression of left ventricular hypertrophy partially starts at first year after transplantation, is raised up to maximum value between first two years, persists between third and forth year, while long evolutions of this findings are still unknown. Rigatto et al. (11) followed up 473 kidney transplant patients in first year after transplantation, and showed left ventricular hypertrophy as the independent risk factor for mortality and congestive cardiac insufficiency.
Epidemiological studies show that about 60% of renal transplant patients have glomerular filtration rate lower then 60 ml/min, and about 15% of patients < 30ml/min (12). The renal function is measured by absolute value of serum creatinine and creatinine clearence. Lost renal function is established risk factor for the development of complications and death from cardiovascular disease and infection (13). The results of our study show that patients with LV hypertrophy before kidney transplantation had significantly higher creatinine serum values compared to patients with normal LV. Patients with LV hypertrophy at the end of the first post transplant year had significantly lower value of creatinine clearance than patients with normal LV mass index, by which the normalization of LV on follow up echocardiography significantly interplays with decrease in values of creatinine and increase in creatinine clearance rate. These findings are in the accordance with the results of other authors. The numerous variables influence on the allograft survival at the first post transplant year. Ten years long study of Hariharan et al. (14) which included 100 000 patients with cadaver or living related renal allograft, established significant independent correlation between the degree of renal dysfunction and risks of lost renal al-lograft function, with estimated relative allograft lost risk of 1,63 for every 88,4 μ^ια^ increased level of serum creatinine. Moreso and Grinyp (15) determined that renal allograft dysfunction is a powerful risk factor for the occurrence of cardiovascular complications.
Correlation between kidney failure and cardiovascular risk factors has important implications for the management of renal transplant recipients. Cardiac remodelling after successful renal transplantation could be based on uremia elimination, and modification of structural and functional characteristics of heart and blood vessels, especially in left ventricular hypertrophy. Good renal allograft function in the first posttransplant year by removed different traditional and non-traditional risk factors for cardiovascular disease confirms the existence of cardio-renal axis and interrelated functions of these two organic systems.
CONCLUSION
In our study we found that left ventricular hypertrophy is the most frequent cardiac abnormality, present immediately prior to renal transplantation in 67 % patients.
Significant association between regression of LV mass on the second echocardiography with improvement of renal al-lograft function showed that creatinine clearance in the first posttransplant year is an independent predictor of cardiovascular status and outcomes.
Mild renal insufficiency in renal transplant patients is the risk factor for diastolic dysfunction of LV.
List ofAbbreviations
LV - Left ventricle
LVH - Left ventricular hypertrophy
LVMI - Left ventricular mass index
EF - Ejection function
E/A - Diastolic function
NLV - Normal mass of left ventricle
cLVH - Concentric left ventricular hypertrophy
eLVH - Eccentric left ventricular hypertrophy
NFLV Normal function of left ventricle
DDLV - Diastolic dysfunction of left ventricle
SDDLV - Systolic-diastolic dysfunction of left ventricle
ESRD - End stage of renal disease
REFERENCES
- 1.Stack A.G, Saran R. Clinical correlates and mortality impact of left ventricular hypertrophy among new ESRD patients in United States. Am. J. Kidney Dis. 2002;40:1202–1210. doi: 10.1053/ajkd.2002.36881. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak M.J, Levey A.S, Schoolwerth A.C, et al. American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 3.Rigatto C, Parfrey P. Therapy insight: management of cardiovascular disease in the renal transplant recipient. Nat. Clin. Pract. Nephrol. 2006;2(9):514–526. doi: 10.1038/ncpneph0253. [DOI] [PubMed] [Google Scholar]
- 4.Rigatto C, Foley R, Kent G.M, Guttmann R, Parfrey P.S. Long-term changes in left ventricular hypertrophy after renal transplantation. Transplantation. 2000;70(4):570–575. doi: 10.1097/00007890-200008270-00006. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Savage D.D, Garrison R.J, Anderson K.M, Kannel W.B, Castelli W.P. Echocadiographic criteria for left ventricular hypertrophy: the Framingham Study. Am. J. Cardiol. 1987;59:956–960. doi: 10.1016/0002-9149(87)91133-7. [DOI] [PubMed] [Google Scholar]
- 6.London GM. Cardiovascular disease in chronic renal failure: pathophysiologic aspects. Semin. Dial. 2003;16(2):85–94. doi: 10.1046/j.1525-139x.2003.16023.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiner D.E, Sarnak M.J. Chronic Kidney Disease and Cardiovascular Disease: A Bi-Directional Relationship? Dialysis and Transplantation. 2007;36(3):113–118. [Google Scholar]
- 8.Meler-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75(8):1291–1295. doi: 10.1097/01.TP.0000061602.03327.E2. [DOI] [PubMed] [Google Scholar]
- 9.Rašić S, Kulenović I, Haračić A, Ćatović A. Left ventricular hypertrophy and risk factors for its development in uremic patients. Bosn. J. Basic Med. Sci. 2004;4(1):34–40. doi: 10.17305/bjbms.2004.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez D. Left ventricular hypertrophy after renal transplantation: new approach to a deadly disorder. Nephrol. Dial. Transplant. 2004;19:1682–1686. doi: 10.1093/ndt/gfh283. [DOI] [PubMed] [Google Scholar]
- 11.Rigatto C, Foley R, Jeffery J, Negrijn C, Tribula C, Parfrey P. Electrocardiographic left ventricular hypertrophy in renal transplant recipients: prognostic value and impact of blood pressure and anemia. J. Am. Soc. Nephrol. 2003;14:462–468. doi: 10.1097/01.asn.0000043141.67989.39. [DOI] [PubMed] [Google Scholar]
- 12.Gill J.S, Tonelli M, Mix C.H, Pereira B.J. The change in allograft function among long-term kidney transplant recipients. J. Am. Soc. Nephrol. 2003;14:136–142. doi: 10.1097/01.asn.0000070621.06264.86. [DOI] [PubMed] [Google Scholar]
- 13.Meier-Kriesche H.U, Baliga R, Kaplan B. Decreased renal function is strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75:1291–1295. doi: 10.1097/01.TP.0000061602.03327.E2. [DOI] [PubMed] [Google Scholar]
- 14.Hariharan S, McBr1de M.A, Cherikh W.S, Toller1s C.B, Bresnahan B.A, Johnson C.P. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62:311–318. doi: 10.1046/j.1523-1755.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 15.Moreso F, Grinyp J.M. Graft dysfunction and cardiovascular risk-an un-holy alliance. Nephrol. Dial. Transplant. 2007;22:699–702. doi: 10.1093/ndt/gfl657. [DOI] [PubMed] [Google Scholar]


