Abstract
Actions of acetylcholine (ACh), histamines, serotonins (5-HT) and prostaglandins (PGF2-alfa) in concentrations of 10-4, 10-3, 10-1 and 10-1 mol/dm3 were analyzed in vitro conditions in isolated specimens of tracheas of 24 pigs, 7 guinea pigs, and dead persons for different reasons (8), in the presence and without presence of propranolol. Whilst, research regarding actions of aerosolized histamines (10 mg, 1%, 2 min), in the presence and without the presence of aerosolized propranolol (20 mg, 2%, 2 min) was done in vivo in 6 healthy persons. Study results show that propranolol does not emphasize contraction of the airways smooth musculature as induced by ACh, histamine, 5-HT and PGF2-alfa in vitro conditions (p>0,1). Also, in vivo we found a non-significance of tracheal smooth musculature constriction (p>0,1).
Keywords: Trachea, ACh, propranolol, histamine, 5-HT and PGF2-alfa
INTRODUCTION
Interaction of substances with adrenergic receptors induces a series of changes in the cell, with characteristic physiologic effects. Sympathetic nerves release noradrenalin in ganglions. This mediator inhibits excitatory parasympathetic ways, as it was found in intestine (1). There are data, based on the pharmaco-logic and electrophysiological experiments, showing that in tracheal musculature of the guinea pigs and in human’s airways a non-adrenergic - non-cholinergic nervous system is present (NANC) (2). It seems that the neurotransmitter of these nerves is a vasoactive intestinal polypeptide (VIP) even though purines can also be incorporated here. Role of this system in a physiologic and pathologic condition is not well known (3). Recognition of sympathetic stimulation effects, which develops through beta-adrenergic receptors, enables anticipation of adrenergic beta-blockers actions. These effects are very powerful if sympathetic control is strengthened. In addition, some blockers stimulate, by itself, beta-receptors, the so called agonist intrinsic effect, thus along with the beta-blockage, registration of beta-stimulation effects might happen. Propranolol inhibits action of beta-agonist substances in isolated trachea of guinea pigs (4,5). Activation of the cholinergic nerve releases ACh that connects with muscarinic receptors M3 in smooth muscles by causing contraction. ACh also connects with the muscarinic receptors M2 that are situated in postganglionic cholinergic nerve. Stimulation of these receptors limits the release of Ach, thus mus-carinic receptors M2 acts as auto-receptor (6). Loss of the M2 receptors function appears in some models of hyperactive animals and in some patients with asthma, leading towards an increase of vagal hyper-reactivity. Some authors assume that histamine and 5-HT causes the release of the ACh in vivo and in vitro, and that these chemical mediators cause direct release of the ACh from terminal postganglionic nerves. There is a possibility that activation of receptors with 5-HT causes depolarization of terminal postsynaptic nerves. 5-HT cause depolarization of terminal cholinergic nerves of the airways through receptors with 5-HT3 (an ionic channel connected for ligands); this was concluded in humans’ airways and in guinea pigs (7). Nevertheless, other studies show that a selective ligand for 5-HT3 does not have a contractile effect in isolated tracheas in mice’s. By using a combination of pharmacologic and immunehys- tochemical, it was concluded that 5-HT cause release of the ACh from epithelium and not from nerves (8). Aim of the experiment was in vitro and in vivo evaluation of the possible action of the beta-blockers in modulating a response of the smooth musculature of the airways against ACh, hista- mines, 5-HT and PGF2-alfa, when previously, beta-adrenergic receptors are blocked with propranolol.
MATERIALS AND METHODS
The study was conducted in vitro and in vivo conditions. In vitro, 39 examinations in tracheal isolated rings took place (24 piggies, 7 guinea pigs and persons that have passed away due to different reasons (8). Importance of the adrenergic receptors blockage in response of smooth musculature against Ach, histamines, 5-HT and PGF2-alfa in the presence and without the presence of propranolol was studied. Previously, animals were sacrified and then the isolated tracheal specimen of healthy piggy’s and guinea pigs was placed in Krebs solution. Investigation of the isolated lower tracheal rings response against mentioned substances was done within 3 hours, since the moment of taking of the sample from piggy’s and guinea pig. Human trachea were taken from autopsy whilst further experimental procedure is as same as above. During the experiment, preparation was placed in an bathroom with Krebs solution with pH = 7,4, whilst bathroom temperature during the development of the experiment was held constant in 37°C. Solution in the bathroom was aerosolized continuously with gas mixture (95% O2 and 5% O2), that has flown continuously in the bathroom solution. Rings were prepared and serially connected in between. Serial, composed of 2 up to 6 rings (depending from the age of experimental animal), was placed in bathroom for isolated organs (volume 50 ml), in a way that lower part of the rings is connected for retainer, whilst upper part of the ring is connected with elastic thread for transducer ("Force transducer”, Statham UC2). Response of TSM was registered in a multi-channel registration (Watanabe HSE 6600). After 30 minutes, first tonus of tracheal rings was registered (silence potential); afterwards preparation was exposed to different molar concentrations (10-1, 10-2, 10-3, and 10-4) of acetylcholine, histamine, serotonine and PGF2-alfa with propranolol and without propranolol. (See Scheme 1. of the experimental model in vitro). Doses have changed every 15 minutes, whereas effects of bronchi-constrictor agents were monitored for 3 minutes, after the application. Afterwards, preparation got rinsed couple of times with Krebs solution, prior application of the other substance, until the silence potential got reached (initial potential). Investigations in vivo have been done in 6 healthy persons. After taking the initial values for permeability of airways (Raw, ITGV, SRt), aerosolized histamine was given to the subjects (10 mg, 1%, 2 min). After 5 minutes of histamine application values for airways permeability were measured. Two hours after the histamine application the subjects were given aerosolized propranolol (20 mg, 2% min), and after three minutes values of Raw, ITGV respectively those of SRt were determined again. After 5 minutes, histamine aerosol (10 mg, 1%, 2 min) applied again; 10 minutes after inhalation of histamine values of Raw, ITGV were measured and also values of SRt were calculated. In the end, subjects were applied hexoprenaline as aerosol (2 inhalations by 0,2 mg) and 5 minutes after hexoprenaline values of Raw, ITGV were determined and also values of SRt were calculated.
SCHEME 1.
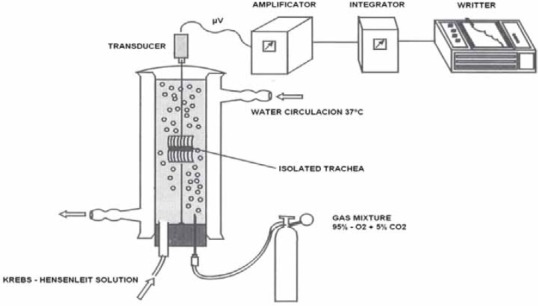
Experimental model for the measurement of the active tension of tracheal rings smooth musculature.
SRt = Raw x ITGV
SRt - Specific Resistance toracal,
Raw - Resistance airway,
ITGV - Intra-Toracal-Gas-Volume.
All experiments on human were approved by local ethic committee and are according to the Declaration of Helsinki.
Statistical analysis
Results were elaborated with statistical computer program GraphPad InStat III with T-test of comparison of two working groups.
RESULTS
Results gained in vitro in preparations of piggy’s isolated tracheal rings, guinea pigs and persons passed away due to different reasons, shows that Ach, histamine, 5-HT and PGF2-alfa without previous treatment with propranolol, induces constriction of TSM. Reactions on above mentioned substances remain unchanged in the presence of propranolol. This conclusion derives from data gained in experiment, as follows:
Ach in concentration 10-2 mol/dm3 has caused a constriction of 1,58±0,20 g/cm. (p<0,01). Ach with propranolol (P+Ach) (10-2 mol/dm3) has induced constriction of 1,72±0,73 g/cm., but not in a significant manner (p>0,1) (Figures 1 and 4).
Histamine in concentration 10-2 mol/dm3 has caused a constriction of TSM: 0,54±0,12 g/ cm. (p<0,01). Histamine with propranolol (P+hist.) (10-2 mol/dm3) has induced constriction of SM of tracheal rings 0,61±0,13 g/cm., but not in a significant manner (p>0,1) (Figures 2, 5).
5-HT in concentration 10-2 mol/dm3 has caused a constriction of TSM: 0,61±0,16 g/cm. (p<0,01). 5-HT (10-2 mol/dm3) with propranolol (P+5-HT) (10-2 mol/dm3) has induced constriction of SM of tracheal rings in experimental animals 0,46±0,11 g/cm., but not in a significant manner (p>0,1) (Figure 3).
PGF2-alfa in concentration (10-2 mol/dm3) has caused a constriction of TSM: 2,33±1,25 g/cm (p<0,01). PGF2-alfa with propranolol (P+PGF) (10-2 mol/dm3), do not cause constriction of SM in a significant manner (p>0,1) (Figure 6.).
FIGURE 1.
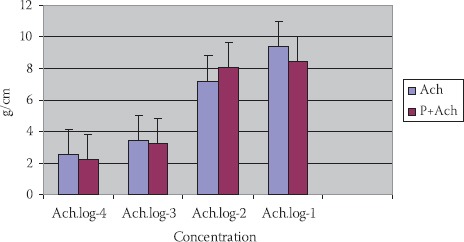
Cumulative reaction of Ach, propranololAch in TSM at piggy’s of different maturity ages (n=24; X±SEM).
FIGURE 2.
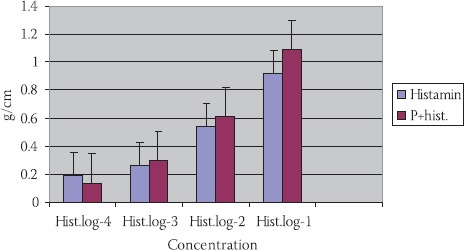
Reaction of TSM in different histamines concentrations, propranolol histamine in tracheas’ of guinea pigs of different maturity ages (n=7; X±SEM).
FIGURE 3.
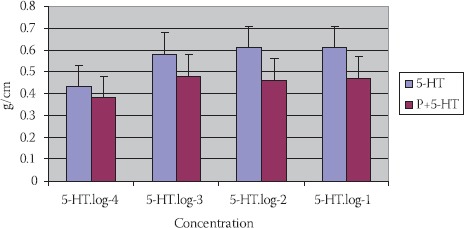
Reaction of TSM in different 5-HT concentrations, propranolol+5-HT in tracheas’ of guinea pigs of different maturity ages (n=7; X±SEM).
FIGURE 4.
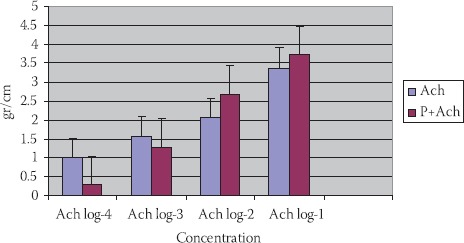
Reaction of TSM in different Ach concentrations, propranolol Ach in tracheal of people with different maturity ages (n=8; X±SEM).
FIGURE 5.
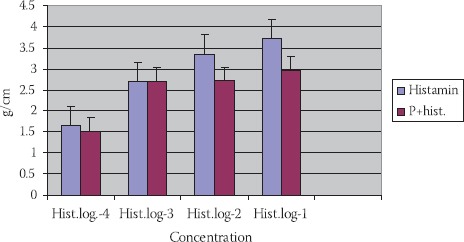
Reaction of TSM in different concentrations of histamine, propranolol+histamine in trachea’s of people with different maturity ages (n=8; X±SEM).
FIGURE 6.
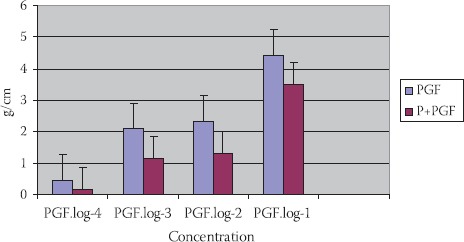
Reaction of TSM in different concentrations of PGF2alfa, propranoIol+PGF2alfa in tracheal of people with different maturity ages (n=8; X±SEM).
Results gained in vivo in healthy individuals show that propranolol in the reaction of tracheal rings against histamines, also do not have any significant influence. This conclusion derives from data gained as follows:
Initial values (i.v.) of SRt = 0,7310,07 kPax sec.
SRt after administration of the histamines-aerosol (hist.aer.) (10 mg, 1%, 2 min) =1,0310,17 kPa × sec. (p>0,1).
SRt 2 hours after histamines = 0,89 ± 0,11 kPa × sec. (p>0,1).
SRt after administration of the propranolol-aerosol (prop. aer.) (20 mg, 2%, 2 min) = 0,89 ± 0,16 kPa × sec. (p>0,1)
SRt after histamines at persons with previous administration of the propranolol = 0,93 ± 0,17 kPa × sec. (p>0,1).
SRt after hexoprenaline-aerosol (hex.aer.) is 0,510,05 kPa × sec. (p>0,1) (Figure 7).
FIGURE 7.
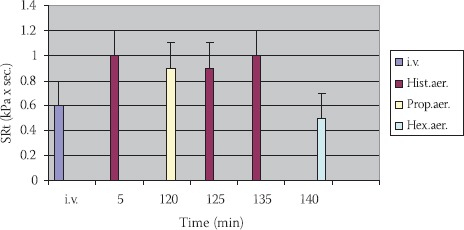
Action of propranolol in reaction to histamine in healthy persons (n=6; X±SEM).
DISCUSSION
Blockage of the beta-adrenergic receptors at the asthmatics cause constriction of the airways, whilst at healthy persons there was no certain activity of these receptors in silence condition noticed. Whilst some of the authors claim that application of the propranolol (blocker of the beta-adrenergic receptors) causes increase of the airways resistance (4), other authors were not able to prove these results. Blockage of the beta-adrenergic receptors in sick people with increased resistance of the airways leads to bronchi-constriction. This proves the importance of the increased beta-adrenergic activity in order to oppose cholinergic bronchi-constrictor affection (5). Results of this in vitro research, without prior treatment with propranolol, shows that ACh, histamine, 5-HT and PGF2-alfa induces the contraction of the tracheal smooth musculature, whilst this constriction, in the presence of the propranolol, remains unchanged. It has to be stressed out that applied propranolol with intravenous way in healthy goat also had no important reaction in the regulation of the basic lung function (9). Concentration-reaction curb, gained from the different aged tissue, suggest that response which is mediated by the muscarinic receptors do not depend on age of the tracheal smooth musculature cells. Contraction caused by potassium ions correlates with age and can be inhibited by the blockage of the calcium channels with calcium blocker D-600. Response on applying prostaglandin PGE2 is associated with the increase of the calcium ions influx through the “receptor-activating” channels, by causing depolarization of smooth musculature cells (10). This mechanism, which is described for the PGE, can also be activated even for PGF2-alfa and to cause a contraction. Despite of this, “voltage operating” calcium channels are open during this depolarization, by allowing further more increase of the calcium ions influx. This suggests that calcium enters the cell contributing to the increase of the cytoplasmatic calcium. Reaction to prostaglan-din is inhibited by the blockage of the “voltage operating” channels with calcium blocker D-600. It seems that development of the “voltage operating” calcium channels in the guinea pigs tracheal smooth musculature cells depends on the age and reaches the stability only after 4 months of extra-uterine life (11,12,13). Thus, our results in vitro suggest that ACh derived from the epithelium can be seen as final mediator of the constriction in piggy’s, guinea pigs, people, and that it can play an important role in the changed response in airways diseases (14). in vitro and in vivo investigations show that propranolol also does not emphasize action of histamine. Calcium ions function as well as potassium channels in an age dependent manner (15). These observations suggests that blockage of the beta-adrenergic receptors do not play any role in the modulating process of the constrictory response of the airway smooth muscles. Responses that are induced by substances such as ACh, histamine, 5-HT and PGF2-alfa. A module of the provoked bronchi-constriction was demonstrated by Lammers et al. (2). These authors concluded that inhalation of the capsaicin (a stimulator of C fibres) induces interim bronchi-dilation in healthy persons, previously provoked with leukotriene D4. This bronchi-dilation effect of the capsaicin is blocked fully with preliminary inhalation of lidocaine (local anaesthetic) as aerosol, but it cannot be blocked neither with ipratropium-bromide (muscarinic antagonist) nor with propranolol (beta-blocker). This suggests that bronchi-dilation caused by inhaling of capsaicin is mediated neither by adrenergic nerve fibres nor with cholinergic ones, but it seems that with lidocaine afferent nerve fibers are blocked, that mediate bronchi-dilation caused by cap- saicin. Non-adrenergic-non-cholinergic nerve system (NANC) is demonstrated in people and some of the animals (2,16). It seems that this system is the only neurohumoral bronchi-dilator mechanism in the airways (17). Physiologic and physiopathologic role of this system remains as not clarified, as long as neurotoxin cannot be applied in people. We think that until the existence of the NANC system is not proved in people, balance between adrenergic system and cho-linergic system is irreplaceable for the normal maintaining of the bronchi-motor tonus of the airways
CONCLUSION
ACh induces constriction of the tracheal rings smooth muscles in vitro (p<0,01). This constriction is not modified by propranolol (p>0,1).
Histamine causes constriction of the TSM (p<0,01). Histamine combined with propranolol induces constriction of the TSM, but not in a significant manner (p>0,1).
5-HT and PGF2-alfa also causes constriction of the TSM MLT (p<0,01), but 5-HT with propranolol, and PGF2-alfa with propranolol do not induce constriction of the TSM (p>0,1).
Contraction of the airways is induced by histamine in vivo in healthy people, propranolol (p>0,1) does not intensify this mechanism.
List ofAbbreviations
5-HT - serotonine
PGF2-alfa - prostaglandine
Ach - acetylcoline
TSM - tracheal smooth musculature
Hist. - histamine
P - propranolol
SRt - specific resistance toracal
Raw - resistance airway
ITGV - intra-toracal-gas-volume
kPa - kilopascal
i.v. - initial value
REFERENCES
- 1.Shibata K, Taketani K. Excitatory effect of noradrenaline on rat airway parasympathetic ganglion neurons. Fukuoka Igaku Zasshi. 2001;92:377–383. [PubMed] [Google Scholar]
- 2.Lammers J.J, Minett P, McCusker M, Fan Chung K, Barnes P.J. Nonadrenergic bronchodilator mechanisms in normal subjects in vivo. J. Appl. Physiol. 1988;64:1817. doi: 10.1152/jappl.1988.64.5.1817. [DOI] [PubMed] [Google Scholar]
- 3.Martin J.G, Wang A, Zacour M, Biggs D.F. The effects of vaso-active intestinal polypeptide on cholinergic neurotransmission in an isolated innervated guinea pig tracheal preparation. Respir. Physiol. 1990;79:111–121. doi: 10.1016/0034-5687(90)90011-m. [DOI] [PubMed] [Google Scholar]
- 4.Food J.M, Farrell M.H, Krumholz H. Beta-blocker therapy in heart failure. JAMA. 2002;287:883. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 5.Cleland J.G. Beta-blockers for heart failure Why, Which, when and where? Med. North. Am. 2003;87:339. doi: 10.1016/s0025-7125(02)00173-6. [DOI] [PubMed] [Google Scholar]
- 6.Roffel A.F, Elzinga C.R.S, Zaagsma J. Muscarinic M3 receptors mediate contraction of human central and peripheral airway smooth muscle. Pulm. Pharm. 1990;3:47–51. doi: 10.1016/0952-0600(90)90009-8. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Ward J.K, Tadjkarimi S, Yacoub M.D, Barnes P.J, Belvisi M.G. 5-Hydroxytryptamine facilitates cholinergic bron-choconstriction in human and guinea-pig airways. Am. J. Respir. Crit. Care. Med. 1995;152:377–380. doi: 10.1164/ajrccm.152.1.7599849. [DOI] [PubMed] [Google Scholar]
- 8.Moffatt J, Cocks T, Page C. Role of the epithelium and acetylcho-line in mediating the contraction to 5-hydroxytryptamine in the mouse isolated trachea. B. J. Pharmacol. 2004;141:1159–1166. doi: 10.1038/sj.bjp.0705720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islami H.I, Haxhiu M.A. The role of beta-adrenergic receptors on regulation of depth and rate of breathing in conscious goats. Abstract, Skopje. 1985;15:25. [Google Scholar]
- 10.Den Hertog A, Van den Akker. The section of prostaglandine E2 on the smooth muscle cell of the guinea-pig taenia coli. Europ. J. Pharmacol. 1980;21:225. doi: 10.1016/0014-2999(79)90471-0. [DOI] [PubMed] [Google Scholar]
- 11.Vermue N.A, Den Hertog A. The prostaglandin response in guinea pig tania caeci and tracheal smooth muscle of different ages. Europ. J. Pharmacol. 1988;147:273. doi: 10.1016/0014-2999(88)90786-8. [DOI] [PubMed] [Google Scholar]
- 12.Blair L, Dionne V.E. Developmental acquisition of Ca2+ sensitivity by K+ channels in spinal neurons. Nature. 1885;315:6017. doi: 10.1038/315329a0. [DOI] [PubMed] [Google Scholar]
- 13.Waterman S.A. Voltage-gated calcium channels in autonomic neuroeffector transmission. Prog. Neurobiol. 2000;60:181–210. doi: 10.1016/s0301-0082(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 14.Southgate M, Bruce E, Pichoff M.D, Edward L. Ontogeny of epithelial modulation of airway smooth muscle function in the guinea pig. Peditr. Pulmonol. 2005;15:105–110. doi: 10.1002/ppul.1950150207. [DOI] [PubMed] [Google Scholar]
- 15.Ford L.E, Gilbert Sh. The importance of maturational studies in airway smooth muscle. Am. J. Physiol. Lung. Cell. Mol. Physol. 2005;289:L898–L901. doi: 10.1152/ajplung.00328.2005. [DOI] [PubMed] [Google Scholar]
- 16.Moffatt J.D, Dumsday B, McLean J.R. Non-adrenergic, non-cho-linergic neurons innervating the guinea-pig trachea are located in the oesophagus: evidence from retrograde neuronal tracing. Neurosci. Lett. 1998;248:37–40. doi: 10.1016/s0304-3940(98)00321-8. [DOI] [PubMed] [Google Scholar]
- 17.Barnes P.J. Neuronal control of human airways in health and disease. Am. Rev. Respir. Dis. 1986;134:958. doi: 10.1164/arrd.1986.134.5.1289. [DOI] [PubMed] [Google Scholar]


