Abstract
Diarrhoeal disease is a major cause of illness and death among infants and young children worldwide. Among the Escherichia coli (E. coli) causing intestinal diseases, there are six well-described categories: enteroaggregative E. coli (EAEC), diffusely adherent E. coli (DAEC), enteroinvasive E. coli (EIEC), entero-pathogenic E. coli (EPEC), enterohaemorrhagic E. coli (EHEC) and enterotoxigenic E. coli (ETEC).
The aim of the present study was to investigate the relative contribution of different groups of diarrhoe-agenic E. coli (DEC) in paediatric patients with diarrhoea. Clinical stool specimens from 380 children with diarrhoea, with ages ranging from birth to < 12 years, were selected for the study over a period of 17 months (August 2007 to December 2008). The study showed that 85/380 children (22%) had diarrhoea due to diarrhoeagenic E. coli. The most prevalent was enteropathogenic E. coli (EPEC) isolated from 46/85 paediatric patients (54%), followed by enterotoxigenic (ETEC) isolated from 19/85 (22.3%), en-terohaemorrhagic (EHEC) from 18/85 (21.1%) and enteroinvasive (EIEC) from 2/85 patients (2.3%). The most prevalent serotypes of EPEC were O86:K61 and O44:K74 isolated from 10/46 (21.7%), O128:K67 from 6/46 patients (13%), followed by O158:K- and O126:K71 isolated from 4/46 patients (8.6%). Among the ETEC the most prevalent serotypes were O78:K80 isolated from 10/19 (56.7%) and O25:K11 from 9/19 patients (47.3%), especially during the first twelve months: 9/19 patients (47.3%). The most prevalent EHEC strain found in this study was O145:K- and O103:K-: 5/18 patients (27.8%). Two isolated strains of EIEC belong to serotype O164:K-. The average age of the patients was 2 years. Two patients with bloody diarrhoea had EHEC serotype O157:H7 which progressed to haemolytic-uremic syndrome (HUS).
Our study shows that diarrhoeagenic E. coli is a significant causal agent of diarrhoeal diseases in paediatric patients in Bosnia and Herzegovina. This study is the first report about the frequency and most common serotypes of DEC in Bosnia and Herzegovina. Additionally, it is the first report of cases with an O157:K- infection which progressed to HUS, a serious and potentially fatal illness.
Keywords: diarrhoeagenic E. coli, enteropathogenic E. coli, enterotoxigenic E. coli, enterohaemorrhagic E. coli, enteroinvasive E. coli, haemolytic uremic syndrome
INTRODUCTION
Diarrhoeal diseases constitute a major public health problem, particularly in the developing world where the rate of mortality and morbidity is very high. E. coli is the type species of the genus Escherichia that contains mostly motile Gram-negative bacilli that fall within the family Enterobacteriaceae. It is the predominant non-pathogenic facultative flora of the human intestine. The organism typically colonizes the infant gastrointestinal tract within hours of life, and thereafter E. coli and the host derive mutual benefit (1). E. coli usually remains harmlessly confined to the intestinal lumen; however, in the debilitated or immunosuppressed host, or when gastrointestinal barriers are violated, even normal “non-pathogenic” strains ofE. coli can cause infection (2). E. coli that cause diarrhoea are extremely common worldwide. The classification of diarrhoeagenic E. coli strains is based on their virulence properties, and comprises six groups: entero-toxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enterohaemorragic E. coli (EHEC), enteroaggregative E. coli (EAEC) and diffuse adhering/diffusely adherent E. coli (DAEC). The associated clinical pictures comprise childhood and traveller’s diarrhoea (ETEC), bloody diarrhoea and haemolytic-uremic syndrome (EHEC), infantile diarrhoea (EPEC), and bacillary dysentery-like diarrhoea (EIEC) (1,2). ETEC are associated with two major clinical syndromes: weanling diarrhoea among children in the developing world, and traveller’s diarrhoea. Certain serotypes of ETEC occur worldwide; others have a limited recognized distribution. Epidemiologic investigations have implicated contaminated food and water as the most common vehicles for ETEC infection (3, 4, 5, and 6). ETEC diarrhoea may be mild, brief, and self-limiting or may result in severe purging similar to that seen in V. cholerae infection (7). EPEC is an important category of diarrhoeagenic E. coli which has been linked to infant diarrhoea in the developing world. As with other diarrhoeagen-ic E. coli strains, transmission of EPEC is faecal-oral, with contaminated hands, contaminated foods, or contaminated fomites serving as vehicles. EPEC adhere to the mucosal cells of the small bowel. The result of EPEC infection is watery diarrhoea, which is usually self-limited, but can be chronic (8). EHEC can be transmitted by food and water and from person to person. Most cases are caused by ingestion of contaminated foods, particularly foods of bovine origin (9). In most patients, the bloody diarrhoea will resolve without apparent sequelae, but in about 10% of patients younger than 10 years (and in many elderly patients), the illness will progress to HUS. HUS is defined by a trial of haemolytic anaemia, thrombocytopenia, and renal failure; initial clinical manifestations include oligouria or anuria, oedema, pallor, and, sometimes, seizures (10). There are some biochemical characteristics ofE. coli O157:H7 that have been exploited in the isolation and identification of this serotype. An important characteristic is that O157:H7 strains do not ferment D-sorbitol rapidly, in contrast to about 75 to 94% of other E. coli strains (11).
Enteroaggregative E. coli (EAEC) is a subgroup of diar-rhoeagenic E. coli (DEC) that during the past decade has received increasing attention as a cause of watery diarrhoea, which is often persistent. EAEC have been isolated from children and adults worldwide. As well as sporadic cases, outbreaks of EAEC-caused diarrhoea have been described. The definition of EAEC is the ability of the micro-organism to adhere to epithelial cells such as HEp-2 in a very characteristic ’stacked-brick’ pattern. Typical illness is characterized by watery, mu- coid, secretory diarrhoea with low-grade fever and little to no vomiting. However, up to one third of patients with EAEC diarrhoea had grossly bloody stools (12,13). EIEC strains are biochemically, genetically, and pathoge-netically related closely to Shigella spp. Both organisms have been shown to invade the colonic epithelium, a phenotype mediated by both plasmid and chromosomal loci. In addition, both EIEC and Shigella spp. elaborate one or more secretory enterotoxins that may play roles in diarrhoeal pathogenesis (1). EIEC infection presents most commonly as watery diarrhoea, which can be indistinguishable from the secretory diarrhoea seen with ETEC. Only a minority of patients experience the dysentery syndrome, manifested as blood, mucus, and leukocytes in the stool, tenesmus, and fever. In two documented EIEC outbreaks, gross blood was observed in 0 and 7% of persons infected (14,15). Asymptomatic infections due to EIEC are probably unusual.
The term “diffusely adherent E. coli” was initially used to refer to any HEp-2-adherent E. coli strain that did not form EPEC-like microcolonies. With the discovery of EAEC, most authors now recognize DAEC as an independent category of potentially diarrhoeagenic E. coli. Several recent studies have implicated DAEC strains as agents of diarrhoea, while other studies have not recovered DAEC strains more frequently from diarrhoeal patients than from asymptomatic controls. An age-dependent susceptibility may explain this observation, because when populations are stratified by age, the association of DAEC with diarrhoea is found only in children older than infants. The reason for such an age-related phenomenon is as yet unknown. Other epidemiologic features, such as the mode of acquisition of DAEC infection, are also as yet undetermined (16). In 1944, Kauffman proposed a scheme for the sero-logic classification of E. coli which is still used in modified form today. According to the modified Kauffman scheme, E. coli is serotyped on the basis of their O (somatic), H (flagellar), and K (capsular) surface antigen profiles. A total of 170 different O antigens, each defining a serogroup, are recognized currently. A specific combination of O and H antigens defines the serotype of an isolate. E. coli of specific serogroups can be associated reproducibly with certain clinical syndromes, but it is not in general the serologic antigens themselves that confer virulence. Rather, the serotypes and sero-groups serve as readily identifiable chromosomal markers that correlate with specific virulent clones (1,3).
MATER1AL AND METHODS
Patients and stool samples
380 children with acute diarrhoeal diseases, admitted to the Paediatric clinic, Clinical Centre of the University of Sarajevo, were included. The age distribution ranged from birth to < 12 years with a mean age of 2 years. A total of 1140 stool samples were examined for the presence of diarrhoeagenic E. coli. Stools were collected in plastic containers.
Stool culture
Stool culture was done at the Institute of Microbiology, Clinical Centre of the University of Sarajevo. E. coli was recovered from clinical stool specimens on MacConkey agar (Becton Dickinson). The plates were incubated overnight at 37°C (±1.5°C) in bacteriological incubators under aerobic conditions. This media is specially designed to distinguish lactose-fermenting (pink to red) from non-lactose-fermenting colonies (colourless or slightly beige).
Biochemical identification
Enterobacteriaceae are usually identified via biochemical reactions. These tests were performed in individual culture tubes, while the indole test, positive in 99% ofE. coli strains, was used for the differentiation from other members of the Enterobacteriaceae. Fermentation of D-sorb- itol was used for the differentiation of EHEC O157:K-.
Serotyping
Bacterial growth on a primary-culture plate was agglutinated by slide agglutination with polyspecific O-antisera, Anti coli I (O26, O44, O114, O125, O142, and O158), Anti coli II (O55, O86, O111, O119, O126, O127, and O128) and Anti coli III (O157, O164, O145, O124, O118, O103, O78, and O25) (Sifin). A small amount of bacterial mass from 5-10 suspicious colonies was transferred onto a slide and mixed each with one drop of test serum (ca. 25 μ!), so that a homogenous, slightly milky suspension developed. The results were read with the naked eye by holding the slide in front of a light source against a black background while swaying the slide (tilting it back and forth). To control for spontaneous agglutination, a negative control must be performed in parallel using physiological saline (NaCl) in place of the test serum. From the colony which showed a positive reaction with polyspecific sera a subculture was prepared and strains were retested by using monospecific O:K antisera (Si- fin): O111:K58, O55:K59, O26:K60, O86:K61, O127:K63, O124:K72, O142:K-86, O145:K-, O126:K71, O128:K- 67, O158:K-, O164:K-, O103:K-, O157:K-, O44:K74, O78:K80, O114:K90, O118:K-, O25:K11, O119:K69, O125:K72. A positive result had to be checked in the confirmation test to exclude the effects of any parallel unspecific agglutination. Bacteria from the subculture was rinsed with NaCl and boiled for 1 hour to destroy the thermo-labile K-antigens. Then the bacteria suspension was adjusted with NaCl to a germ density of Mc-Farland Standard 3 and used as an antigen suspension.
RESULTS
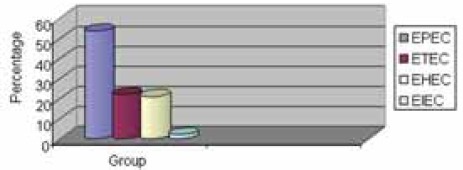
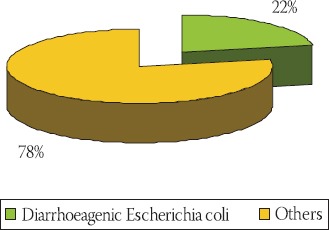
Clinical stool specimens from 380 children with diarrhoea, their ages ranging from birth to < 12 years, were selected for the study over a period of 17 months (August 2007 to December 2008). A study showed that 85/380 paediatric patients (22%) had diarrhoea due to diarrhoeagenic E. coli (Graph 1). The most prevalent was enteropathogenic E. coli (EPEC) isolated from 46/85 patients (54%), followed by enterotoxigenic (ETEC) found in 19/85 (22.3%), en-terohaemorrhagic (EHEC) in 18/85 (21.1%) and en-teroinvasive (EIEC) in 2/85 patients (2.3%) (Graph 2).
GRAPH 1.
Diarrhoea due to diarrhoeagenic£ coli
GRAPH 2.
The most common diarrhoeagenic E. coli strains
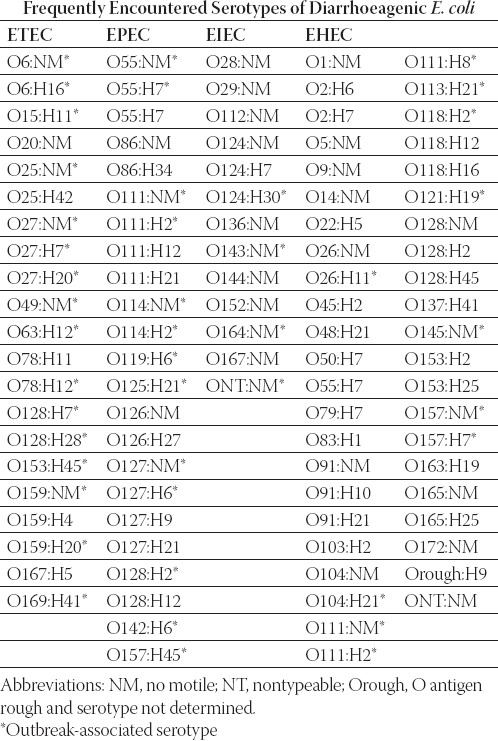
A variety of different serotypes of E. coli have been shown to cause diarrhoeal illness. The most common serotypes identified are shown in Table 1.
TABLE 1.
The most common serotypes identified. Adapted from: Bopp et al., 2003: Escherichia, Shigella, and Salmonella (34).
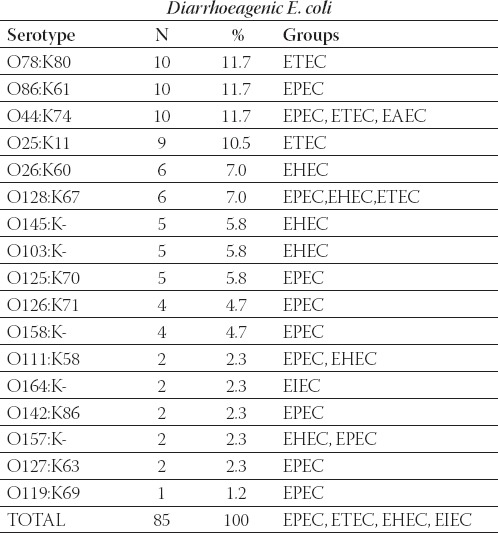
Among all serotypes of diarrhoeagenic E. coli the most prevalent in our study were O78:K80, O86:K61 and O44:K74 isolated from 10/85 patients (11.7%). The most prevalent EHEC strains found in this study were O145:K- and O103:K-. Two isolated strains of EIEC belong to serotype O164:K-. Table 2. shows all isolated serotypes and their frequency.
TABLE 2.
Frequency of diarrhoeagenic E. coli serotypes and groups
The most prevalent serotypes of EPEC were O86:K61 and O44:K74 isolated from 10/46 patients (21.7%), O128:K67 from 6/46 patients (13%), followed by O158:K- and O126:K71 isolated from 4/46 patients (8.6%). Among ETEC, the most prevalent se-rotypes were O78:K80 found in 10/19 (56.7%) and O25:K11 in 9/19 patients (47.3%) (Graphs 3. and 4.).
GRAPH 3.
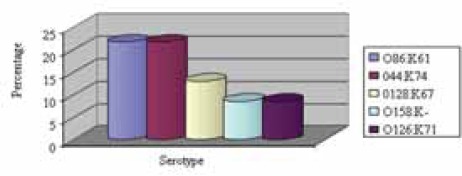
The most prevalent serotype of EPEC
GRAPH 4.
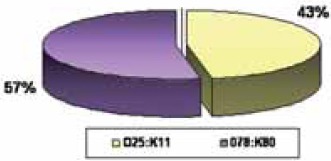
The most prevalent serotypes of ETEC
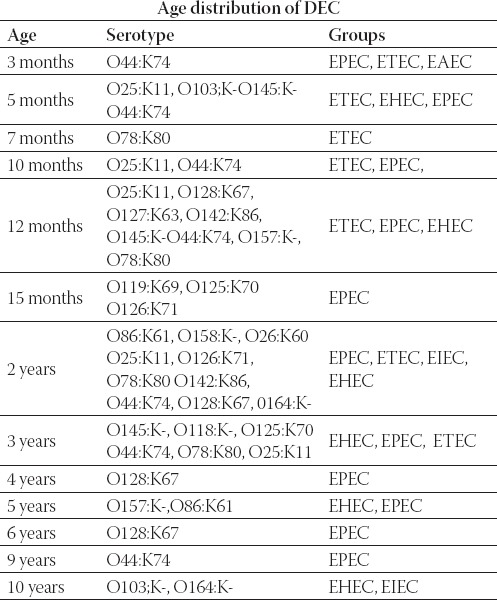
During the first twelve months, serotypes O78:K80 and O25:K11 of ETEC were the most common: 9/19 patients (473%). Table 3. shows the age distribution of diarrhoe-agenic E. coli. The average age of the patients was 2 years.
TABLE 3.
Age distribution of diarrhoeagenic E. coli serotypes and groups
DISCUSSION
Diarrhoea remains one of the main sources of morbidity and mortality in today’s world and a large proportion is caused by diarrhoeagenic E. coli (17). The frequency was investigated in many studies and ranges from 1050 % (16, 18, 19, 20) with EPEC and ETEC as the most common (11, 17, 21, 22, 23, 24 and 25). In our study, 22% of the patients had diarrhoea due to diarrhoeagenic E. coli. EPEC and ETEC were the most prevalent groups (54% and 22.3% respectively). The most common sero-types were O78:K80, O86:K61, O44:K74 (10/85 patients, i.e. 11.7% each) and O25:K11 (9/85 patients, i.e. 10.5%). The percentage of cases of sporadic endemic infant diarrhoea which are due to ETEC usually varies from 10 to 30% (17,21). The study of diarrhoeagenic E. coli in four different age groups among 905 children in Thailand (26) showed that the isolation rate of diarrhoe-agenic E. coli was significantly higher in children less than 1 year of age, compared with children older than 1 year. ETEC were more common in children older than 2 years, compared with the younger children. In our study, the average age of patients was 2 years, and ETEC was more common during the first twelve months. The reservoir of EPEC infection is thought to be symptomatic or asymptomatic children and asymptomatic adult carriers, including mothers and persons who handle infants (8). Numerous studies have documented the spread of infection through hospitals, nurseries, and day care centres from an index case. In symptomatic patients, EPEC can be isolated from stools up to 2 weeks after cessation of symptoms In addition to profuse watery diarrhoea, vomiting and low-grade fever are common symptoms of EPEC infection. Faecal leukocytes are seen only occasionally (27).
The most notable feature of the epidemiology of the disease due to EPEC is the striking age distribution seen in persons infected with this pathogen. EPEC infection is primarily a disease of infants younger than 2 years. As reviewed by Levine and Edelman numerous case-control studies in many countries have shown a strong correlation of isolation of EPEC from infants with diarrhoea compared to healthy infants (8). The correlation is strongest with infants younger than 6 months. In children older than 2 years, EPEC can be isolated from healthy and sick individuals, but a statistically significant correlation with disease is usually not found. In our study, EPEC has been isolated in all age groups (from 3 months to 9 years).
We have isolated EIEC from stool samples of two children aged 2 and 12 years. Epidemiologic studies of EIEC mostly describe outbreaks. In sporadic cases, many EIEC strains are probably misidentified as Shigella spp. because EIEC strains are biochemically, genetically, and pathoge-netically closely related to Shigella spp. (1). Documented EIEC outbreaks are usually foodborne or waterborne, although person-to-person transmission does occur (14).
We report two cases of an E. coli O157:K- infection in patients aged 5 years and 1 year with severe bloody diarrhoea and abdominal cramps. It progressed to hae-molytic uremic syndrome (HUS) approximately 7 to 10 days after the onset of diarrhoea. Treatment was limited largely to supportive care. This syndrome is the most worrisome complication of EHEC infection because it is a serious and potentially fatal illness. “Haemolytic” refers to the break-up of red blood cells which leads to anaemia. There is also destruction of platelets (thrombo- cytopenia) which, in turn, promotes abnormal bleeding. “Uremic” refers to the failure of the kidneys. In addition, problems in the brain with seizures and coma may occur. HUS most commonly affects children under the age of 10 years and is the most common cause of acute renal failure in infants and young children. A very low infectious dose for EHEC infection has been estimated from outbreak investigations. This number, on the order of 100 to 200 organisms for infection, is similar to the number required for Shigella infection and is consistent with the numerous reports of person-to-person transmission in outbreaks and in the institutional setting (28). The incubation period of EHEC diarrhoea is usually 3 to 4 days, although incubation times as long as 5 to 8 days or as short as 1 to 2 days have been described in some outbreaks. The initial complaint is usually non-bloody diarrhoea, although this is preceded by abdominal cramps and pain, as well as a short-lived fever in many patients. Vomiting occurs in about half of the patients during the period of non-bloody diarrhoea and/or at other times during the illness. Within 1 or 2 days, the diarrhoea becomes bloody and the patient experiences increased abdominal pain. This stage usually lasts between 4 and 10 days. In severe cases, faecal specimens are described as “all blood and no stool” (29).
This bacteria and the toxin produced (Shiga-like toxin) should be searched for in cultures of stools and colonic biopsies in the case of bloody diarrhoea, in particular when a haemolytic-uremic syndrome is associated. The isolation of E. coli O157:H7 or other Stx-producing E. coli strains from stool specimens depends upon culturing early in the course of the disease. Unfortunately, many patients are not extensively evaluated until they have HUS symptoms, which usually begin several days after the onset of diarrhoea (30,31).
The most common non-O157:H7 serotypes associated with human disease include O26:K60, O103:K, O111:K58, O113:H21. At least 10 outbreaks due to these organisms have been reported in Japan, Germany, Italy, Australia, the Czech Republic and the United States (32). In our study, O103:K- and O145:K- were the most common se-rotypes of EHEC (27.8% each). O26:K6O and O111:K58 constituted 7% and 2,3% of all isolated DEC serotypes. As with other diarrhoeal pathogens, the primary goal of treatment of diarrhoea due to DEC is to prevent dehydration by correcting fluid and electrolyte imbalances. Oral rehydration may be sufficient for milder cases, but more severe cases require parenteral rehydration. Correction of nutritional imbalance with lactose-free formula or breast milk may be insufficient for some severely ill patients, and total parenteral nutrition may be required (25). There are no vaccines currently available or in clinical trials to prevent disease due to EPEC.
Although EHEC strains are generally susceptible to a variety of antibiotics, there are no prospective studies showing conclusively that the use of antibiotics alters the outcome of the disease. There are, however, retrospective studies which suggest that patients who received antibiotics may be at greater risk of developing HUS. The use of antibiotics may be harmful for two potential reasons: first, lyses of bacteria by some antibiotics leads to increased release of toxin, at least in vitro; second, antibiotic therapy could kill other intracolonic bacteria, thereby increasing the systemic absorption of toxin (33).
CONCLUSION
This study confirms that diarrhoeagenic E. coli is an important causal agent of diarrhoeal diseases in paediatric patients in Bosnia and Herzegovina. Enteropathogenic E. coli (EPEC) and enterotoxigenic E. coli (ETEC) are the most common. This is the first report about the frequency and distribution of the most common serotypes of DEC in Bosnia and Herzegovina. Simultaneously, it is the first report ofE. coli O157:H7, an emergent pathogen causing the haemolytic-uremic syndrome, a serious and potentially fatal illness. Therefore, research in diarrhoeagenic E. coli remains an important task to pursue.
List of Abbreviations
DEC - diarrhoeagenic E. coli
EAEC - enteroaggregative E. coli
EIEC - enteroinvasive E. coli
EHEC - enterohaemorrhagic E. coli
EPEC - enteropathogenic E. coli
ETEC - enterotoxigenic E. coli
STEC - Shiga-toxin-producing E. coli
DAEC - diffusely adherent E. coli
HUS - haemolytic-uremic syndrome
ST - heat-stable enterotoxins
LT - heat-labile enterotoxin
HEp2 - alveolar epithelial carcinoma cell line
REFERENCES
- 1.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 2.Gloria LP, Eric M, Octavio G, Javier A, Erasmo N, Sergio V. Two or more enteropathogens are associated with diarrhoea in Mexican children. Ann. Clin. Microbiol Antimicrob. 2007;6:17. doi: 10.1186/1476-0711-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hukić M. Fakultativno anaerobni gram-negativni bacilli. Sarajevo: U: Bak-teriologija; Hukić i saradnici. Jež; 2005. pp. 205–207. [Google Scholar]
- 4.Black RE, Brown KH, Becker S, Abdul Alim ARM, Merson M.H. Contamination of weaning foods and transmission of enterotoxigenic Escherichia coli diarrhoea in children in rural Bangladesh. Trans. R. Soc. Trop. Med. Hyg. 1982;76:259–264. doi: 10.1016/0035-9203(82)90292-9. [DOI] [PubMed] [Google Scholar]
- 5.Long K. Z, Wood J.W, Vasquez Gariby E, K, et al. Proportional hazards analysis of diarrhoea due to enterotoxigenic Escherichia coli and breast feeding in a cohort of urban Mexican children. Am J. Epidemiol. 1994;139:193–205. doi: 10.1093/oxfordjournals.aje.a116981. [DOI] [PubMed] [Google Scholar]
- 6.Wood L.V, Ferguson L.E, Hogan P, et al. Incidence of bacterial enteropathogens in foods from Mexico. Appl. Environ. Micro-biol. 1983;46:328–332. doi: 10.1128/aem.46.2.328-332.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattila L. Clinical features and duration of traveller’s diarrhoea in relation to its etiology. Clin. Infect. Dis. 1994;19:728–734. doi: 10.1093/clinids/19.4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine M.M, Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhoea: epidemiology and pathogenesis. Epidemiol. Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien A.D, Tesh V.L, Donohue-Rolfe A, et al. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 1992;180:65–94. doi: 10.1007/978-3-642-77238-2_4. [DOI] [PubMed] [Google Scholar]
- 10.Tesh V.L, O’Brien A.D. The pathogenic mechanisms of Shi-ga toxin and the Shiga-like toxins. Mol. Microbiol. 1991;5:18171822. doi: 10.1111/j.1365-2958.1991.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 11.Farmer J.J. Enterobacteriaceae: introduction and identification. In: Murray P.R, Baron E.J, Pfaller M.A, Tenover F.C, Yolken R.H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 438–449. [Google Scholar]
- 12.Andrej W. Enteroaggregative Escherichia coli: epidemiology, virulence and detection. J. Med. Microbiol. 2007;56:4–8. doi: 10.1099/jmm.0.46930-0. [DOI] [PubMed] [Google Scholar]
- 13.Scavia G, Staffolani M, Fisichella S, et al. Enteroaggregative Es-cherichia coli associated with a foodborne outbreak of gastroenteritis. J. Med. Microbiol. 2008;57:1141–1146. doi: 10.1099/jmm.0.2008/001362-0. [DOI] [PubMed] [Google Scholar]
- 14.Harris J.R, Mariano J, Wells J.G, Payne B.S, Donnel H.D, and Cohen M.L. Person-to-person transmission in an outbreak of enteroinvasive Escherichia coli. Am. J. Epidemiol. 1985;122:245252. doi: 10.1093/oxfordjournals.aje.a114095. [DOI] [PubMed] [Google Scholar]
- 15.Taylor D.N, Echeverría P, Sethabutr O, et al. Clinical and mi-crobiologic features of Shigella and enteroinvasive Escherichia coli infections detected by DNA hybridization. J. Clin. Microbiol. 1988;26:1362–1366. doi: 10.1128/jcm.26.7.1362-1366.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baqui A.H, Sack R.B, Black R.E. Enteropathogens associated with acute and persistent diarrhoea in Bangladesh children <5 years of age. J. Infect. Dis. 1992;166:792–796. doi: 10.1093/infdis/166.4.792. [DOI] [PubMed] [Google Scholar]
- 17.Clarke S.C, Haigh R.D, Freestone P.P, Williams P.H. Entero-pathogenic Escherichia coli infection: history and clinical aspect. Br J Biomed Sci. 2002;59(2):123–127. doi: 10.1080/09674845.2002.11783647. [DOI] [PubMed] [Google Scholar]
- 18.Regua-Mangia A.H, Gomes T.A, Vieira T.A, Andrade J.R, Irino K, Teixeira L.M. Frequency and characteristic of diarrhoeagenic Escherichia coli strains isolated from children with and without diarrhoea in Rio de Laneiro, Brazil. J. Infect. 2004;48(2):161–167. doi: 10.1016/s0163-4453(03)00138-5. [DOI] [PubMed] [Google Scholar]
- 19.Akinyemi K.O, Oyefolu A.O, Opere B, Otunba-Payne V.A, Oworu A.O. Escherichia coli in patients with acute gastroenteritis in Lagos, Nigeria. East Afr. Med. J. 1998;75(9):512–525. [PubMed] [Google Scholar]
- 20.Bui Thi T.H, Trang D.T, Flemming S, Phung D.C, Kare M, Anders D. Diarrhoeagenic Escherichia coli and other causes of childhood diarrhoea: a case-control study in children living in a wastewater-use area in Hanoi, Vietnam. J. Med. Microbiol. 2007;56:1086–1096. doi: 10.1099/jmm.0.47093-0. [DOI] [PubMed] [Google Scholar]
- 21.Jan E. A, Kare B, Lars B. High prevalence of atypical entero-pathogenic Escherichia coli (EPEC) in Norwegian children with diarrhoea. J. Med. Microbiol. 2003;52:1015–1019. doi: 10.1099/jmm.0.05287-0. [DOI] [PubMed] [Google Scholar]
- 22.Flores-Abuxapqui J.J, Suarez-Itoil G.J, Heredia-Navarrete M.R, Puc-Franco M.A, Franco-Monsreal J. Frequency of entero-toxigenic Escherichia coli in infants during the first three months of life. Arch. Med. Res. 1994;25:303–307. [PubMed] [Google Scholar]
- 23.Fagundes-Neto U. Enteropathogenic Escherichia coli infection in infants: clinical aspects and small bowel morphological alterations. Rev. Microbiol. Sao Paulo. 1996;27(Suppl. 1):117–119. [Google Scholar]
- 24.Arduino R.C, DuPont H.L. Traveller’s diarrhoea. Baillieres Clin. Gastroenterol. 1993;7:365–385. doi: 10.1016/0950-3528(93)90046-u. [DOI] [PubMed] [Google Scholar]
- 25.Black R. E. Epidemiology of traveller’s diarrhoea and relative importance of various pathogens. Rev. Infect. Dis. 1990;12(Suppl. 1):73–79. doi: 10.1093/clinids/12.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 26.Orn-Anong R, Sarayoot S, Hideo H, William B.T. Prevalence of childhood diarrhoea-associated Escherichia coli in Thailand. J. Med. Microbiol. 2004;53:237–243. doi: 10.1099/jmm.0.05413-0. [DOI] [PubMed] [Google Scholar]
- 27.Wu S.X, Peng R.Q. Studies on an outbreak of neonatal diarrhoea caused by EPEC O127:H6 with plasmid analysis restriction analysis and outer membrane protein determination. Acta Paediatr. Scand. 1992;81:217–221. doi: 10.1111/j.1651-2227.1992.tb12207.x. [DOI] [PubMed] [Google Scholar]
- 28.Belongia E.A, Osterholm M.T, Soler J.T, Ammend D.A, Braun J.E, Macdonald K.L. Transmission of Escherichia coli O157:H7 infection in Minnesota child day-care facilities. JAMA. 1993;269:883–888. [PubMed] [Google Scholar]
- 29.Riley L.S, Remis W.R, Helgerson S.D, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 30.Elisabeth P, Ralph H.Z, Sonja R, Alexander A, Stefan W, Peter G.K, Wolfgang G. Frequency and virulence properties of diar-rheagenic Escherichia coli in children with diarrhoea in Gabon. Am. J. Trop. Med. Hyg. 2003;69(4):406–410. [PubMed] [Google Scholar]
- 31.Nguyen T.V, Le Van P, Le Huy C.H, Gia K.N, Wemtraub W. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J. Clin. Microbiol. 2005;43:755–760. doi: 10.1128/JCM.43.2.755-760.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson R.P, R.C, Clarke J.B, Wilson S.C, et al. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J. Food Prot. 1996;59:1112–1122. doi: 10.4315/0362-028X-59.10.1112. [DOI] [PubMed] [Google Scholar]
- 33.Cimolai N.S, Basalyga D.G, Mah B.J, Carter J.E. A continuing assessment of risk factors for the development of Escherichia coli O157:H7-associated haemolytic uremic syndrome. Clin. Nephrol. 1994;42:85–89. [PubMed] [Google Scholar]
- 34.Bopp C.A, Brenner F.W, Fields P.I, et al. Escherichia, Shigella, and Salmonella. In: Murray P.R, Baron E.J, Jorgensen J.H, editors. Manual of clinical microbiology. 8th edition. Vol. 1. Washington, DC: ASM Press; 2003. pp. 654–671. [Google Scholar]


