Abstract
Irritable bowel syndrome (IBS) is the most common gastrointestinal (GI) disorder worldwide, however treatment options for diarrhea-predominant IBS (IBS-D) remain limited. Eluxadoline, a µ- and κ-opioid receptor agonist and δ-opioid receptor antagonist, was recently approved for the treatment of IBS-D. A novel compound first described in 2008, eluxadoline was shown to normalize GI transit, with a subsequent phase I demonstrating its safety and tolerability in healthy adults. In 2016, two randomized, double-blind, placebo-controlled phase III trials studying eluxadoline use at 75 mg and 100 mg twice daily over 26 weeks demonstrated a significant improvement in stool consistency and many global symptoms of IBS. However, the data did not demonstrate a significant advantage over placebo using the United States Food and Drug Administration (US FDA) and European Medicines Agency (EMA) endpoints for abdominal pain. Safety and tolerability data, pooled from both phase II and III studies, suggest that eluxadoline is generally well tolerated with the most common adverse events (AEs) occurring in approximately 3–8% of patients and included nausea, constipation, and abdominal pain. The most common serious adverse event (SAE) is pancreatitis, which had a 0.4% incidence. Recent US FDA reports reporting severe pancreatitis and sphincter of Oddi dysfunction after short-term use of eluxadoline in patients without a gallbladder has added a history of cholecystectomy as an important contraindication. Eluxadoline is also contraindicated in patients with a history of biliary duct obstruction, sphincter of Oddi dysfunction, active alcohol abuse, history of pancreatitis or known pancreatic duct obstruction, severe hepatic impairment, severe or chronic constipation, or known mechanical gastrointestinal obstruction. As a new drug to enter the IBS-D market, the place of eluxadoline in the hierarchy of IBS treatments is still to be determined. In this article, we review the development and clinical trial data behind the approval of eluxadoline with a focus on safety data and its use in clinical practice.
Keywords: abdominal pain, diarrhea, eluxadoline, functional GI disease, irritable bowel syndrome, pancreatitis
Introduction
Irritable bowel syndrome (IBS), the most common gastrointestinal (GI) disorder worldwide, is a lifelong condition that leads to reduced quality of life and a high burden of healthcare utilization. Eluxadoline is an oral agent with mixed opioid effects that was approved for the treatment of IBS with diarrhea (IBS-D) in May 2015 by the United States Food and Drug Administration (US FDA) and in July 2016 by European Medicines Agency (EMA). It has undergone phase III trials demonstrating benefit over placebo, has a favorable AE profile, and is a promising addition to existing therapies. This review discusses the development of eluxadoline, safety and tolerability data, and its current place in clinical use. Eluxadoline appears to be a promising addition to the limited pharmaceutical options approved to treat IBS-D.
Background
Irritable bowel syndrome
IBS encompasses many pathophysiological processes, including disordered GI motility, visceral hypersensitivity, and psychosocial distress. Mechanistically, IBS may be mediated by alterations in multiple domains with psychological, genetic, dietary, brain–gut axis, and altered intestinal flora all playing a role.1 IBS is characterized by abdominal pain associated with altered bowel habits and can be broadly categorized into three subtypes: IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), and IBS with a mixed bowel pattern (IBS-M).2 This spectrum of presentation often complicates treatment options as patients are thought to move between subtypes over time regardless of therapy status. Additionally, changes in stool form often occur as a result of therapy, which largely are nonspecific to IBS but instead designed to treat general constipation or diarrhea.3
Existing therapies
Despite the prevalence of IBS, however, only a few agents have been approved for IBS treatment, with the mainstay of treatment involving pharmacologic agents in conjunction with lifestyle and dietary modifications. While there are a variety of over-the-counter antidiarrheal therapies, loperamide is the only such agent assessed by randomized studies in patients with IBS-D. The studies, though small and generally only evaluated short-term efficacy, demonstrated that while loperamide reduces stool frequency and improves stool consistency, these effects are equivalent to placebo with long-term treatment and do not address global symptoms such as abdominal pain.4–6 Alosetron is a 5-hydroxytryptamine-3 receptor (5HT-3) antagonist approved for the treatment of IBS-D in female patients, which was shown to improve global IBS symptoms and abdominal pain in women with major studies underpowered to demonstrate benefit in men. However, its use has been limited due to associations with ischemic colitis and severe constipation, and it remains an agent used primarily for refractory symptoms.7,8 Bile acid sequestrants such as cholestyramine are used empirically for a proportion of IBS-D patients who may have a component of bile acid maldigestion driving their symptoms, but these agents are generally poorly tolerated and limited data exist specific to IBS-D.1
There are relatively few agents that tackle the multidimensional symptom of IBS-D symptoms beyond diarrhea. Many patients are equally or more bothered by abdominal pain and discomfort, bloating, or gas that associate with their diarrhea. There are some data to support the use of tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) in reducing global symptom severity in patients with IBS, with the latter particularly useful when considering patients with concomitant affective disorders (anxiety, depression) contributing to their symptom severity; however data specific to IBS-D are limited.9 The anticholinergic side effect profile of TCAs make them a particularly attractive option in the treatment of IBS-D, however relative efficacy compared with agents other than placebo has not been studied. More recently, the antibiotic rifaximin has gained approval for IBS-D and demonstrated efficacy with repeat treatment (up to three 2-week courses) with significant improvements in daily ratings of IBS symptoms, bloating, abdominal pain, and stool consistency.10
Mechanism of action
Eluxadoline was approved for the treatment of IBS-D in May 2015 by the US FDA and in July 2016, by EMA.11 Eluxadoline influences bidirectional brain–gut signaling through the endogenous opioid system. Enteric opioid receptors µ, κ, and δ are involved in the regulation of gut motility, secretion, and sensation. The µ-opioid activation has an inhibitory effect on motility and secretion12 and is the primary mechanism through which loperamide exerts its effects. Expression of the κ-opioid receptor is increased in states of chronic visceral hypersensitivity, thought to play an important role in the abdominal pain component of IBS.13 Antagonism of the δ receptor counteracts the constipating effects of μ-opioid activation resulting from increased sphincter tone and inhibition of colonic peristalsis while enhancing the μ- and κ-opioid receptor-mediated effects on visceral sensation.14 Eluxadoline, a µ- and κ- opioid receptor agonist and δ- opioid receptor antagonist, was developed with the intention of utilizing this mixed opioid profile to treat both the diarrhea and abdominal pain associated with IBS-D.
Development
Early data
A novel compound, eluxadoline was first described in a 2008 study in which it was shown to normalize gastrointestinal (GI) transit over a wide dose-range in mice.15 This was in contrast to loperamide, which, when tested in the same murine models, led to the complete inhibition of intestinal contractility and secretion as opposed to slowing GI transit to a physiologic baseline. A subsequent phase I study demonstrated the safety and tolerability of eluxadoline in healthy adults.
Phase II studies
The first phase II study was a 12-week, placebo-controlled trial, conducted from May 2010 until April 2011. A total of 807 patients with IBS-D were assigned to a twice-daily dosing of oral placebo and variable dosing of eluxadoline.16 In accordance with 2012 US FDA guidelines for IBS clinical trials, the primary endpoints of the study were clinical response after 4 weeks of treatment as assessed by the validated Worst Abdominal Pain (WAP) and Bristol Stool Scale (BSS) questionnaires to assess changes in abdominal pain and stool consistency, respectively. Of note, an improvement in either score, not both, was sufficient to define clinical response. Secondary endpoints were response at week 12, changes in bowel movement frequency, urgency, incontinence, and other validated measures of IBS symptom severity with quality of life assessments. The study showed that at 4 weeks of treatment, significantly more patients in the 25 mg (12.0%; p = .041) and 200 mg eluxadoline (13.8%; p = .015) treatment arms showed clinical response compared with placebo (5.7%).16 Response was limited to improvements in BSS as the WAP scores were not significantly different from placebo for any eluxadoline treatment group. While no treatment arm showed significant clinical response by week 12, there were statistically significant clinical responses to eluxadoline in post-hoc analysis of the US FDA end point in which patients must have met both WAP and stool consistency response criteria on a given day. Patients in the 100 and 200 mg treatment arms were both significantly more likely to reach response than those receiving placebo (28% for 100 mg, 28.5% for 200 mg, 13.8% for placebo; p < 0.005) as well as show greater improvement in global symptoms and quality of life measures (p < 0.05).
Phase III studies
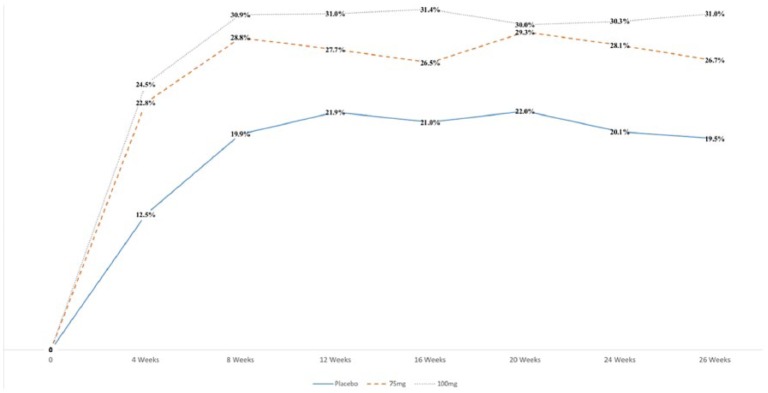
The two randomized, double-blind, placebo-controlled, parallel-group phase III trials were conducted between May 2012 and July 2014 in a total of 2427 patients with IBS-D (IBS-3001, IBS-3002 trials). The studies examined twice-daily dosing of eluxadoline 75 and 100 mg over 26 (IBS-3002) and 52 weeks (IBS-3001) of treatment, with the same methods of assessing clinical response as the prior phase II study as well as monitoring of symptoms using a daily diary.17 Of note, response was defined as a simultaneous daily improvement in both WAP and BSS stool consistency (i.e. patients who recorded a reduction of ⩾30% from their average baseline score for their WAP and, on the same days, a stool consistency score of <5 on ⩾50% of days during the study). There was significant composite symptom improvement in the 100-mg group over placebo in the monthly, 3-month (US FDA endpoint), and 6-month (EMA endpoint) intervals assessed over the 26-week course of treatment (Figure 1, p < 0.01). The eluxadoline 75 mg group showed significant composite symptom improvement at both endpoints in IBS-3002 (p < 0.001), however in IBS-3001 only reached significance at the US FDA (p = 0.001) and not EMA (p = 0.11) endpoint (Table 1). At both endpoints, there was no significant difference compared with placebo with respect to improvement in abdominal pain for either dose of eluxadoline. When using more stringent measures of reduction in these scores (i.e. ⩾40% and ⩾50%), significance was reached for the eluxadoline 100-mg group at both intervals assessed. Additionally, eluxadoline was superior to placebo with respect to assessments of global symptoms and quality of life measurements. Both dosages resulted in a significant composite response in the first 4 weeks of treatment, congruent with earlier findings in the phase II study.17,18 Eluxadoline at the 100-mg dose was significantly superior to placebo in all subpopulations explored, including age (stratified ⩾ or < 65 years) or sex, however there was no published subpopulation analysis for the 75-mg dosing group.
Figure 1.
Percent of patients with clinical response to eluxadoline in a pooled phase III trial. Adapted from Supplementary Table 1 of Lembo and colleagues.17
Table 1.
Efficacy results in two phase III clinical trials assessing clinical response to eluxadoline. Adapted from United States Food and Drug Administration 2015,21 original data from Lembo and colleagues.17
| IBS-3001 Trial |
IBS-3002 Trial |
|||||
|---|---|---|---|---|---|---|
| Eluxadoline 100 mg |
Eluxadoline 75 mg |
Placebo |
Eluxadoline 100 mg |
Eluxadoline 75 mg |
Placebo |
|
| n = 426 | n = 427 | n = 427 | n = 382 | n = 381 | n = 382 | |
| Compositea response over 12 weeks | ||||||
| Responder rates | 25% | 24% | 17% | 30% | 29% | 16% |
| Treatment difference | 8%b | 7%c | 13%d | 13%d | ||
| 95% CI (%) | (2.6,13.5) | (1.4,12.2) | (7.5,19.2) | (6.8,18.5) | ||
| Composite response over 26 weeks | ||||||
| Responder rates | 29% | 23% | 19% | 33% | 30% | 20% |
| Treatment difference | 10% | 4% | 13% | 10% | ||
| 95% CI (%) | (4.7, 16.1) | (−1.0, 9.9) | (6.4, 18.8) | (4.2, 16.4) | ||
| Abdominal pain response improved by ⩾30% | ||||||
| Responder rates | 43% | 42% | 40% | 51% | 48% | 45% |
| Treatment difference | 4% | 3% | 6% | 3% | ||
| 95% CI (%) | (−3.0, 10.2) | (−3.8, 9.4) | (−1.3,12.8) | (−4.3,9.8) | ||
| BSS <5 Response over 12 weeks | ||||||
| Responder rates | 34% | 30% | 22% | 36% | 37% | 21% |
| Treatment difference | 12% | 8% | 16% | 16% | ||
| 95% CI (%) | (6.3, 18.2) | (2.1, 13.8) | (8.4, 21.0) | (9.7, 22.4) | ||
Composite = Simultaneous improvement of WAP by ⩾ 30% and BSS <5 on the same day for ⩾50% of days over the interval.
p < 0.01.
p < 0.05.
p < 0.001.
BSS, Bristol Stool Score; CI, confidence interval; IBS, irritable bowel syndrome; WAP, Worst Abdominal Pain.
Safety and tolerability
Overview
Safety data from the phase III clinical trials was analyzed in up to 26 weeks (IBS-3002 trial) and 52 weeks (IBS-3001 trial) of treatment. There were no significant trends observed in hematologic, metabolic, or renal laboratory findings in eluxadoline treatment groups. Common adverse events (AEs) included nausea, abdominal pain, and constipation, which occurred in approximately 6–8% of patients. There was no significant difference in the discontinuation rate of therapy between eluxadoline and placebo, with no withdrawal period or worsening of symptoms following completion of treatment. SAEs included pancreatitis in patients with biliary sludge or excessive alcohol use defined as greater than three drinks per day, and sphincter of Oddi dysfunction, the latter of which exclusively occurred in patients without a gallbladder.17
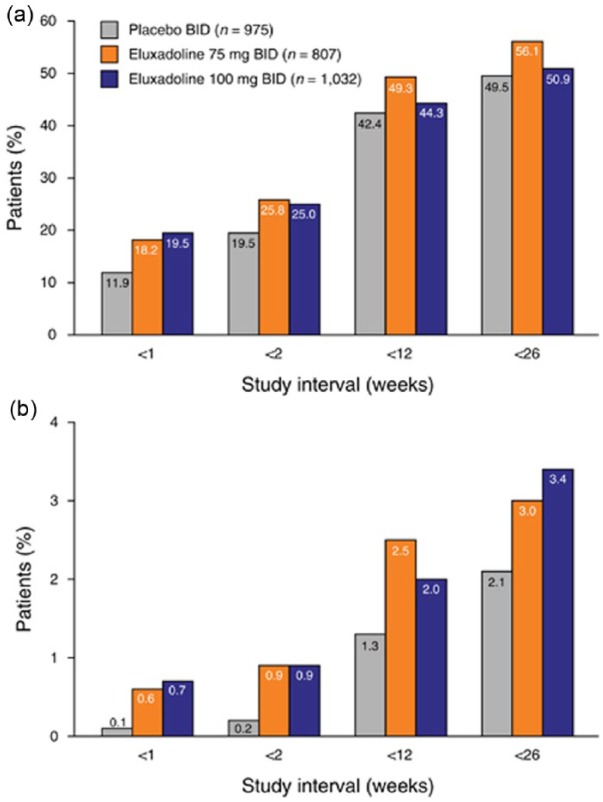
In 2016, Cash and colleagues pooled the data from the phase II and III clinical trials at the approved eluxadoline twice-daily doses of 75 and 100 mg to include a total of 2814 patients.19 Across the placebo (n = 974), 75 mg (n = 807), and 100 mg (n = 1032) groups the most commonly reported AEs included nausea (n = 49, 5%; n = 65, 8.1%; n = 73, 7.1%), constipation (n = 24, 2.5%; n = 60, 7.4%; n = 84, 8.1%), upper respiratory tract infection (n = 38, 3.9%; n = 27, 3.3%; n = 53, 5.1%), and abdominal pain (n = 25, 2.6%; n = 33, 4.1%; n = 47, 4.6%), which occurred more frequently than patients taking placebo (Figure 2). Constipation and abdominal pain were the most common AEs leading to discontinuation of treatment and were relatively infrequent (constipation 1.1% and 1.5% for 75 mg and 100 mg, respectively; abdominal pain: 1.1% at both dosages). There was a higher incidence of transaminase elevations in 2.1% and 2.5% of patients for the eluxadoline 75 mg and 100 mg groups, a rate higher than that seen in the placebo group (1.4%). When comparing older (age > 65) with younger (age ⩽ 65) patients, there was a slightly higher incidence of AEs (66% versus 59%) and SAEs (9% versus 4%) that did not reach statistical significance.19
Figure 2.
Incidence of (a) AEs and (b) SAEs by time interval: pooled analysis for eluxadoline phase II and III studies.
AE, adverse event; BID, twice daily; SAE, serious adverse event.
Reprinted with permission from Cash and colleagues.19
Sphincter of Oddi spasm and pancreatitis
Of note, patients with a known history of sphincter of Oddi spasm were excluded from all eluxadoline trials.16,17 This was based on prior data demonstrating that patients experiencing sphincter of Oddi spasm are sensitive to opioids and can have a higher incidence of abdominal pain and pancreatitis after exposure to opioid-containing medications.20 Among the patients receiving eluxadoline, 0.2% (2 of 807) of the 75 mg dosage group and 0.8% (5 of 1032) of the 100 mg group experienced sphincter of Oddi spasm compared with no reported cases (0 of 975) in the placebo group.19 All cases occurred in patients without a gallbladder and resolved with discontinuation of treatment. The EMA listed prior cholecystectomy as a contraindication to eluxadoline use after it was made commercially available in May 2015, however initially the US FDA recommended using the 75-mg dose when indicated in this patient population. In March 2017, the US FDA added prior cholecystectomy as a contraindication to eluxadoline use after receiving 120 reports of these patients developing serious pancreatitis resulting in hospitalization with two deaths.22 In both patients who died, their symptoms occurred shortly after receiving a single dose of eluxadoline. The current US FDA recommendation for patients with mild hepatic impairment (Child-Pugh Class A) is to treat with the 75-mg dose and monitor for signs of aminotransferase elevation and episodic abdominal pain, which were present in every reported case of sphincter of Oddi spasm in the pooled study.19,21 SAEs were relatively rare when pooling trial data, with only approximately 4% of patients on eluxadoline experiencing SAEs compared with 2.6% on placebo.19 There was no dose-dependent association between risk of SAE and eluxadoline. The most commonly reported SAE was pancreatitis, which had a 0.4% incidence (7 of 1839) with both eluxadoline dose groups and 0% incidence for placebo.19 There was no increased risk of cardiovascular complications or QTc-prolonging effect over placebo and no deaths were reported during the studies.21 Eluxadoline is contraindicated in patients with a history of biliary duct obstruction, sphincter of Oddi dysfunction, prior cholecystectomy, pancreatitis or known pancreatic duct obstruction, severe hepatic impairment (Child-Pugh Class C), severe or chronic constipation, active alcohol abuse (more than three drinks per day), or known mechanical gastrointestinal obstruction.21
Pregnancy
The US FDA has not currently assigned eluxadoline a pregnancy category as none of the clinical studies have included pregnant women. In animal reproduction studies, administration of eluxadoline at doses over 50 times the human exposure after a single oral dose demonstrated no teratogenic or postnatal developmental effects in offspring. Coadministration of eluxadoline with oral contraceptives (combined progesterone/estrogen) did not change the bioavailability or efficacy of either drug. There are no published data available regarding the presence of eluxadoline in breast milk, the effects of eluxadoline on the breastfed infant, or its effects on milk production.21
Drug interactions
Eluxadoline is metabolized by cytochrome P450 (CYP) proteins, however the exact CYP pathway has not been clearly established.21 Strong CYP inhibitors such as ciprofloxacin, gemfibrozil, fluconazole, clarithromycin, and paroxetine among other agents will therefore increase exposure to eluxadoline through inhibition of its metabolism. Coadministration with OATP1BI inhibitors (e.g. rifampin, antiretrovirals, cyclosporine) or substrates (e.g. rosuvastatin) will also increase eluxadoline exposure. For example, dosing eluxadoline with rosuvastatin 20 mg resulted in a 40% increase in concentration exposure of the statin compared to administration of rosuvastatin alone, increasing the risk of rhabdomyolysis.21 Patients using eluxadoline should be advised to limit use of other antidiarrheal agents such as loperamide to an as-needed basis for acute management of severe diarrhea as routine use of concomitant antidiarrheals increases the risk for constipation-related AEs.
Clinical use
As a new drug to enter the IBS-D market, the place of eluxadoline in the hierarchy of IBS treatments is still to be determined. Many patients self-treat their diarrheal symptoms with over-the-counter loperamide before even seeking primary or specialty care. While eluxadoline may be a reasonable first-line prescription agent, along with rifaximin, for patients seeking medical care for confirmed IBS-D, the current US FDA inquiry into the reported deaths associated with eluxadoline use in patients with prior cholecystectomy is still ongoing. Despite its demonstrated efficacy in addressing both diarrheal and global symptoms of IBS, the latter of which not addressed by loperamide in clinical trials of IBS-D patients, further studies are still needed to clarify its benefit relative to existing therapy modalities. Direct comparison of eluxadoline with other recently approved IBS-D agents such as alosetron and rifaximin is not possible due to differences in the clinical trial methodologies and study populations.23 Second-line prescription agents may include TCAs, which have not been as rigorously studied in IBS-D and have significantly more AEs than first-line agents. Potential advantages of eluxadoline include better quality evidence than loperamide and that its treatment not limited to use in only women as with alosetron; however, head-to-head studies directly comparing eluxadoline with other agents are currently lacking. An additional benefit of eluxadoline is its favorable side effect profile when excluding patients with contraindications such as prior cholecystectomy, alcohol abuse, and pancreatic or hepatobiliary dysfunction. Identifying which subpopulations of IBS-D patients are more likely to benefit from eluxadoline use will likely be an area of future study.
Approved as a schedule IV substance by the US Drug Enforcement Administration, eluxadoline has a theoretical abuse potential given its properties of µ- and κ- opioid agonism and δ-opioid antagonism.24 However, in cross-over studies examining the abuse potential of eluxadoline versus placebo and oxycodone as an active control, eluxadoline was not shown to have a significantly increased abuse potential over placebo when used intranasally. In comparing oral abuse potential through use of validated questionnaires, eluxadoline 300 mg and 1000 mg dosages had significantly higher abuse potential than placebo but significantly lower versus oxycodone.25 A larger study using data from the IBS-3001 and IBS-3002 trials demonstrated that the overall incidence of AEs potentially related to abuse did not differ significantly among the groups given placebo, eluxadoline 75 mg, or eluxadoline 100 mg (2.8%, 2.7%, and 4.3%, respectively). Opiate Withdrawal Scale scores did not differ significantly among the three groups (3.0, 2.0, and 3.0, respectively).26
Among patients trialed on eluxadoline, current data would suggest that if tolerated, the 100 mg dose is more efficacious than the 75 mg dose, but is also associated with a slightly higher incidence of adverse effects. Patients that respond to therapy may expect improvement within 1 month of starting treatment. There is relatively low oral bioavailability (1.02%) of eluxadoline, which was intended to reduce the possibility of central opioid effects. Intake of fatty meals (defined as >800 total kcal, with ⩾50% derived from fat) has been shown to decrease eluxadoline serum levels by 50% and therefore administration with a fatty meal is recommended, as reduced plasma levels are ultimately desirable as the drug is felt to optimally exert its effect intraluminally as opposed to systemically.21 Additionally, eluxadoline has approximately linear pharmacokinetics with no evidence of accumulation with repeated, twice-daily dosing.
Conclusion
IBS-D remains a challenging syndrome to address, however, early data with eluxadoline show it to be a promising addition to the limited existing treatment options available. The two large phase III studies have shown it to improve stool consistency, frequency, and quality of life metrics. While these studies of eluxadoline did not demonstrate a significant improvement in abdominal pain at US FDA and EMA endpoints, it has been shown to improve abdominal pain in subsequent analyses. Future studies comparing eluxadoline to alternative therapies used to treat IBS-D will hopefully further elucidate its role in treating a complex and common disorder.
Footnotes
Funding: Kyle Staller has received research funding from Astra-Zeneca, Pathway Genomics, and Gelesis.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Kenneth Barshop, Department of Internal Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Kyle Staller, Division of Gastroenterology, Center for Neurointestinal Health, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA.
References
- 1. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015; 313: 949–958. [DOI] [PubMed] [Google Scholar]
- 2. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 3. Camilleri M, Buéno L, Andresen V, et al. Pharmacologic, pharmacokinetic, and pharmacogenomic aspects of functional gastrointestinal disorders. Gastroenterology 2016; 150: 1319–1331.e20. [DOI] [PubMed] [Google Scholar]
- 4. Cann PA, Read NW, Holdsworth CD, et al. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci 1984; 29: 239–247. [DOI] [PubMed] [Google Scholar]
- 5. Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol 1996; 31: 463–468. [DOI] [PubMed] [Google Scholar]
- 6. Lavo B, et al. Loperamide in treatment of irritable bowel syndrome—a double-blind placebo controlled study. Scand J Gastroenterol Suppl 1987; 130: 77–80. [DOI] [PubMed] [Google Scholar]
- 7. Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 2014; 63: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andresen V, Montori VM, Keller J, et al. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol 2008; 6: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lacy B. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med 2016; 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lembo A, Pimentel M, Rao SS, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 2016; 151: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 11. Mullard A. FDA approves two IBS drugs. Nat Rev Drug Discov 2015; 14: 449–449. [DOI] [PubMed] [Google Scholar]
- 12. Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept 2009; 155: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hughes PA, Castro J, Harrington AM, et al. Increased κ-opioid receptor expression and function during chronic visceral hypersensitivity. Gut 2014; 63: 1199–1200. [DOI] [PubMed] [Google Scholar]
- 14. Ananthan S. Opioid ligands with mixed μ/δ opioid receptor interactions: an emerging approach to novel analgesics. AAPS J 2006; 8: E118–E125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wade P, Palmer J, McKenney S, et al. Modulation of gastrointestinal function by MuDelta, a mixed µ opioid receptor agonist/ µ opioid receptor antagonist. Br J Pharmacol 2012; 167: 1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dove LS, Lembo A, Randall CW, et al. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology 2013; 145: 329–338.e1. [DOI] [PubMed] [Google Scholar]
- 17. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med 2016; 374: 242–253. [DOI] [PubMed] [Google Scholar]
- 18. Fichna J, Sobolewska-Wlodarczyk A, Wlodarczyk M, et al. Clinical potential of eluxadoline in the treatment of diarrhea-predominant irritable bowel syndrome. Ther Clin Risk Manag 2016; 12: 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cash BD, Lacy BE, Schoenfeld PS, et al. Safety of eluxadoline in patients with irritable bowel syndrome with diarrhea. Am J Gastroenterol. Epub ahead of print 6 December 2016. DOI: 10.1038/ajg.2016.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Helm JF, Venu RP, Geenen JE, et al. Effects of morphine on the human sphincter of Oddi. Gut 1988; 29: 1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. US Food and Drug Administration. Highlights of prescribing information for eluxadoline. FDA Label. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206940s000lbl.pdf (2015, accessed 22 December 2016).
- 22. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about increased risk of serious pancreatitis with irritable bowel drug Viberzi (eluxadoline) in patients without a gallbladder. Center for Drug Evaluation and Research; Available at: https://www.fda.gov/Drugs/DrugSafety/ucm546154.htm (accessed 17 March 2017). [Google Scholar]
- 23. Cash BD. Eluxadoline: a promising therapy that raises many questions. Transl Gastroenterol Hepatol 2016; 1: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Drug Enforcement Administration. Placement of eluxadoline into schedule IV. 80. Available at: https://www.deadiversion.usdoj.gov/fed_regs/rules/2015/fr1112_3.htm (2015, accessed 8 January 2017). [PubMed]
- 25. Levy-Cooperman N, McIntyre G, Bonifacio L, et al. Abuse potential and pharmacodynamic characteristics of oral and intranasal eluxadoline, a mixed μ- and κ-opioid receptor agonist and δ-opioid receptor antagonist. J Pharmacol Exp Ther. 2016; 359(3): 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fant RV, Henningfield JE, Cash BD, et al. Eluxadoline demonstrates a lack of abuse potential in phase 2 and 3 studies of patients with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. Epub ahead of print 3 February 2017. DOI: 10.1016/j.cgh.2017.01.026. [DOI] [PubMed] [Google Scholar]