Abstract
Robot-assisted surgery has been developed to overcome limitations of conventional laparoscopy aiming to further optimize minimally invasive surgery. Despite the fact that robotics already have been widely adopted in urology, gynecology, and several gastro-intestinal procedures, like colorectal surgery, pancreatic surgery lags behind. Due to the complex nature of the procedure, surgeons probably have been hesitant to apply minimally invasive techniques in pancreatic surgery. Nevertheless, the past few years pancreatic surgery has been catching up. An increasing number of procedures are being performed laparoscopically and robotically, despite it being a highly complex procedure with high morbidity and mortality rates. Since the complex nature and extensiveness of the procedure, the start of a robotic pancreatic program should be properly prepared and should comply with several conditions within high-volume centers. Robotic training plays a significant role in the preparation. In this review we discuss the different aspects of preparation when working towards the start of a robotic pancreas program against the background of our nationwide experience in the Netherlands.
Keywords: Pancreatic surgery, robotic surgery, training in robotic surgery
Introduction
Minimally invasive pancreatic surgery is gaining popularity worldwide. Although less overwhelming compared with other sub disciplines of gastrointestinal surgery, the portion of pancreatic resections performed minimally invasive is clearly increasing (1). To date only non-randomized studies are available comparing open resection with minimally invasive techniques in pancreatic surgery. These studies suggest several benefits of minimally invasive surgery including less blood loss and shorter hospital stay (2-5). Currently, multicenter randomized controlled trials are being carried out in the Netherlands comparing open resection with a minimally invasive approach, for both distal pancreatectomy and pancreatoduodenectomy (6,7).
Despite its potential benefits, conventional laparoscopy has several technical drawbacks and is, independent of the outcomes of trials, technically more demanding than open surgery. Rigid (i.e., non-articulating) instruments and uncomfortable ergonomics may hinder the broader implementation of minimally invasive pancreatic surgery.
In 2000, the first commercially available robotic system was introduced to overcome these limitations. This robotic system aims to combine the benefits of open and conventional minimally invasive surgery by providing a 3D, magnified view of the operative field with intra-abdominal articulating instruments, thereby increasing surgical dexterity (8). Potentially, the use of the robotic system enables a larger proportion of pancreatic surgeries to be performed minimally invasively, since the technical benefits of the robot may especially be advantageous in reconstructing anastomoses during a Whipple procedure. Moreover, ergonomics are improved and the use of robotics in minimally invasive surgery potentially shortens the learning curve compared to conventional laparoscopy, as previously shown in different procedures (9,10).
Still, pancreatic surgery remains highly complex and is associated with significant morbidity and mortality rates (11-13). Therefore, when starting a robotic program for pancreatic surgery, it should be well prepared and several conditions must be met prior to performing the first procedures. Training of a dedicated multidisciplinary team should play a key-role in the setup. However, specific training programs for teams performing robotic pancreatic surgery are still scarce.
In the Netherlands, surgeons have been performing laparoscopic pancreatic surgery sporadically for over ten years (1). In 2012, the first robot-assisted distal pancreatectomies were performed and last year the first robot-assisted pancreatoduodenectomies were performed in the University Medical Center Utrecht (UMC Utrecht) after following the University of Pittsburgh Medical Center (UPMC) training program. Next, this program made available nationwide by the Dutch Pancreatic Cancer Group, similar as was done previously for laparoscopic pancreatic surgery (1). Other centers, including the Erasmus Medical Center Rotterdam, recently followed the program. In this review we discuss the steps we took on our road to our first successful robot-assisted pancreatoduodenectomy.
The start of the program
With support of the department and hospital leadership, programs should be started only in high-volume centers. A recent study demonstrated that centers with an annual volume less than 22 minimally invasive pancreatoduodenectomies have inferior outcomes (14). A team of dedicated members from several departments should be composed at the start of the project. A complete team should include experienced pancreatic surgeons, operating room nurses, anesthesiologists, and anesthesiology nurses.
Team: experienced HPB surgeons/pancreatic surgeons
Pancreatic resections are complex procedures, with considerable morbidity and mortality. Performing these procedures in a minimally invasive manner makes it even more complex. We are convinced that extensive experience in open hepato-pancreato-biliary (HPB) surgery is essential when setting up a robotic program. All surgeons involved in our project had extensive experience in open pancreatic surgery. Besides that, the surgeons enrolled in the robotic pancreas program had prior experience with conventional laparoscopic pancreatic surgery or had experience with other robotic procedures, like liver resection. The robotic pancreatoduodenectomy is mostly performed by two surgeons. Thus, preferably, the same surgeons should be involved in the setup.
Team: dedicated scrub nurses
All participating scrub nurses were dedicated HPB scrub nurses with extensive experience in open HPB surgery. Besides this, they had extensive experience in high complex robotic surgery (esophagectomies, liver resections, and/or donor nephrectomies). Especially the combination of these two ensures a short learning curve and a rapid buildup of experience.
Team: anesthesiology
Dedicated HPB anesthesiologists and anesthesiology nurses are needed to ensure fast standardization of the procedure. Performing a pancreatoduodenectomy robotically requires several adjustments, also from the anesthesia team. Airway access can be suboptimal with a docked robot (not with the da Vinci Xi system), sequential compression devices are necessary since the patient will be lying in anti-Trendelenburg for a significant period of time and extra long IV lines may be necessary to obtain enough space for the robotic system.
Equipment
Alongside the dedicated team, the right equipment should be available. In the Netherlands, most centers started with robotic pancreatic surgery relatively late compared to other robotic procedures; therefore most of the needed equipment and instruments were already available. Intuitive Surgical’s da Vinci S system, as well as the da Vinci Si system and da Vinci Xi system are suited for the robotic pancreatoduodenectomy (Intuitive Surgical, Sunnyvale, California, USA). In our experience most of the needed instruments were already available in the hospital. Although not used in open pancreatic resections, instruments like laparoscopic liver retractors, silk sutures, v-loc sutures and beanbags were already available.
Training
Training in minimally invasive surgery has been shown beneficial (1,15,16). However, specific training programs for robotic pancreatic surgery are not widely available yet. When starting up a robotic program for a complex procedure like a pancreatic resection, surgical training should have a significant share in the preparation. Especially reconstructions following a pancreatoduodenectomy require advanced suture skills and therefore should be trained extensively.
In the Netherlands, the nationwide LAELAPS training program for laparoscopic pancreatic surgery was initiated in 2013 (1). In this program, surgeons were trained for laparoscopic distal pancreatectomy. Training consisted of video training, detailed description of the technique/procedure and on-side proctoring by an experienced laparoscopic pancreatic surgeon. In procedures performed after the training program, a significant lower conversion rate (38% to 8%), less blood loss and a shorter hospital stay were observed compared to procedures performed before the training program. This program showed that training is feasible, beneficial and was followed by a 7-fold increase the proportion of distal pancreatectomies performed laparoscopically in the Netherlands (1). In 2016, the LAELAPS-2 program for laparoscopic pancreatoduodenectomy was started.
As a continuation of the successful LAELAPS-1 and -2 programs and after the success of the transatlantic implementation of the UPMC training program, a nationwide program for the safe introduction of the robot-assisted pancreatoduodenectomy in the rest of the Netherlands was developed in 2016: LAELAPS-3. The aim of this program was to introduce robotic pancreatoduodenectomy without a learning curve in complications, but only a learning curve in operating time. This program was set-up in close collaboration with Dr. Herbert Zeh and Dr. Melissa Hogg, initiators of the UPMC robotic pancreas program and the specific training program on robot pancreatic surgery, respectively. Their program was the basis of the LAELAPS-3 program.
Nationwide training program: LAELAPS-3
Training in LAELAPS-3 consists of simulation exercises, suture exercises, practicing anastomoses on artificial organs, watching multiple video recordings of all phases of the procedure and on-site proctoring of the first procedures by a UPMC surgeon. Currently, surgeons in four hospitals have performed their first robotic pancreatoduodenectomy.
Basic robot training course
Prior to starting robotic surgery in general, there are several official courses available one can follow in order to get familiarized with the basic use of the robotic system. Although this is not part of the official LAELAPS-3 training program, every surgeon involved in this program is required to have basic knowledge on the use of the robot, preferably obtained after following one of the official courses, e.g., Intuitive Surgical’s the da Vinci® Technology Training Pathway (17).
Simulation training
The first steps of the program consist of simulation exercises. These exercises can be done on a training robot (e.g., Mimic®, Mimic technologies, Seattle, Washington, USA) or on a da Vinci robotic system with the use of a da Vinci Skills Simulator, or ‘backpack’ simulator (Intuitive Surgical, Sunnyvale, California, USA).
In the LAELAPS-3 program simulation is subdivided in three categories: pretest, curriculum and posttest. Pretest and posttest consist of the same exercises: several basic exercises on a Mimic or with help of the backpack simulator and three different box trainer exercises (Figure 1). The middle part of the simulation training is the ‘curriculum’ (18). These are 25 exercises on a Mimic or backpack simulator in which one must obtain a predetermined 90% level of proficiency before passing. Every exercise is taped and scored by the coordinators of the training program using a standardized scoring form.
Figure 1.
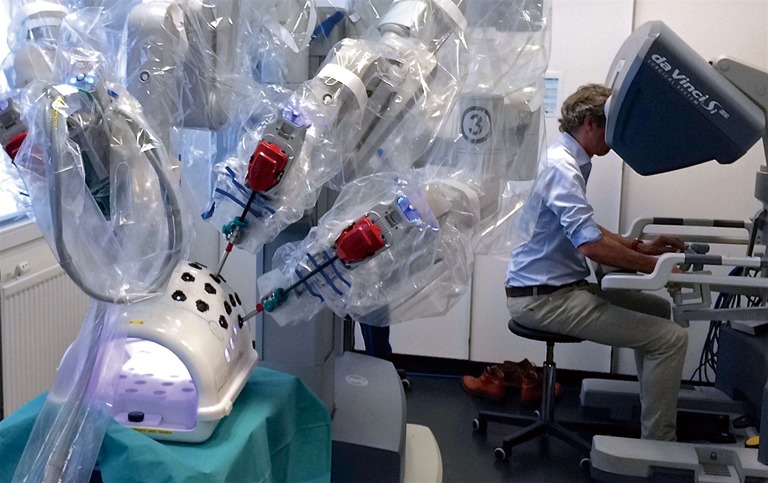
The box trainer.
Advanced suturing and anastomoses training on artificial tissue
In the reconstruction phase of a pancreatoduodenectomy, precise suturing is required for the pancreato-, hepatico- and gastrojejunostomy anastomoses. Fortunately, the suturing within these anastomoses can be practiced in a simulated situation (19). Hence, simulation plays an important role in this second step of the training program. One will start with basic suture exercises on a piece of artificial human skin. These exercises can be done on a training robot (if available) or in the OR. Next, the anastomoses of the Whipple procedure (e.g., pancreaticojejunostomy and hepaticojejunostomy) are performed on artificial tissue (Figure 2). All exercises are recorded and scored by the coordinators of the LAELAPS-3 program. Different aspects of a surgeon’s performance are scored using the objective structured assessment of technical skills (OSATS) method, e.g., gentleness, time, flow of the exercise, and instrument handling (20). Currently, these scores are collected in prospective databases for research purposes.
Figure 2.
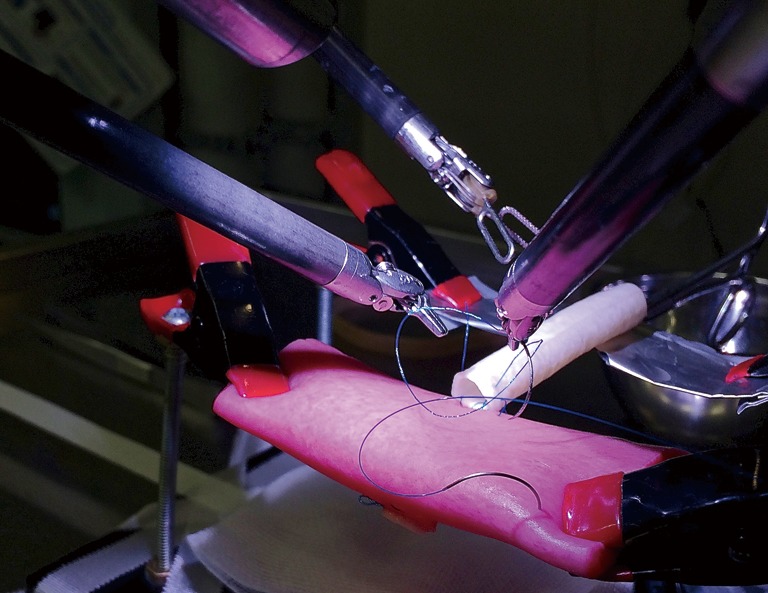
Construction of a hepaticojejunostomy on artificial organs.
Video training
Although the reconstruction phase of the Whipple procedure can be practiced in a simulated setting easily, this differs for the resection phase of the procedure. The resection phase is trained in our program by a recommended six hours of video observing. These videos are provided on an online platform by UPMC. The platform includes full videos of resections for various pathologies, as well as multiple videos of each phase of the resection and reconstruction. Especially for the resection phase of the procedure, we are convinced that extensive experience in open pancreatic surgery will simplify this part of the operation.
Proctoring of the first procedures
Once the official LAELAPS-3 training program has been successfully completed, the first procedures can be planned. Despite extensive training, the robotic Whipple remains a technically challenging procedure. Hence a more experienced robotic pancreatic surgeon should proctor the first cases. A proctor is more experienced and better aware of the potential obstacles that can be encountered and the possible solutions. Moreover, the direct help of the proctor ensures that the procedure will be finished in a reasonable amount of time.
In our nationwide training program we aim to strategically plan the training sessions for the participating surgeons, so their first procedures can be preferably planned during a single week. In this week, a proctor from UPMC visits the Netherlands to attend the first procedures in different hospitals. The UMC Utrecht has performed over 15 robotic Whipple procedures at this moment and therefore will accompany the proctoring process once the initial learning curve of 20 procedures has been completed.
Patient selection
After finishing training, the most important next step is the initial patient selection. Currently, no guidelines exist for patient selection for minimally invasive pancreatoduodenectomy. In our nationwide experience, patients who underwent pancreatic radiotherapy, extensive upper abdominal surgery, have chronic pancreatitis, who have medical conditions that preclude them from lying in anti-Trendelenburg or who were expected to have problems tolerating pneumoperitoneum, were excluded for undergoing robotic pancreatic resection.
Besides these general exclusion criteria, there are a few other patient and tumor characteristics that should be taken into account. First, body mass index (BMI). There is no consensus currently on ideal BMI for robotic pancreatic surgery. In fact, gaining adequate working space can be difficult when an operating on a patient with a very low BMI. On the other hand, in patients with a significantly higher BMI, it can be troublesome to reach the pancreas with the robotic instruments. When starting up a program, a BMI between 20 and 35 kg/m2 should be considered for robotic pancreatic surgery. These guidelines can be extended after increased experience. In the ongoing Dutch trials on minimally invasive pancreatic surgery patients with a BMI over 35 are excluded (6,7).
Tumor characteristics should be considered as well, especially in the beginning of one’s learning curve. Patients with recurrent acute or chronic pancreatitis, tumors with abutment of the portal vein or SMV that may require vascular reconstruction and large (duodenal) tumors (>6 cm) should not be selected. Although vascular resections have been demonstrated to be safe and feasible in robotic pancreatoduodenectomy, this demands a certain level of expertise and experience (21,22). When selecting patients for a robotic pancreatoduodenectomy, benign lesions (e.g., IPMN or ampullary adenoma) or patients who have a dilated pancreatic duct and/or bile duct, are eminently suited for the first procedures.
Tips, tricks and pitfalls
The vital factor in making a success of your robotic program is team work. Dedication of surgeons, OR staff and the anesthesia team is key. The same team should be involved in, at least, the first ten procedures. Additionally, robotic experts from other departments should be consulted during your startup. Prior to the first procedure, we recommend doing a comprehensive run-through the protocol with the entire team. In this way, the availability of the right instruments is assured and everybody is well aware of one’s tasks and attuned to each other.
Second, one should take their time for training and getting the team ready for the first procedure. Although it can be tempting to quickly go through training and start the program, one should not rush into it. This also applies to surgeons who are experienced in pancreatic surgery. Rushing into a procedure like a robotic pancreatic resection can potentially jeopardize patient safety.
Lastly, for the safe setup and expansion of the program an adequate learning curve is essential. Therefore, when starting your program, OR time and robotic availability should be assured for the upcoming months.
Evolution of robots, tools and education
As the Intuitive Robotic systems evolve, and new entries from other companies come into the market, it is likely that complex operations such as pancreatoduodenectomy will get easier, safer, and be accessible to a wider faction of surgeons. With the advent of the Xi robot for example, multi-quadrant surgery no longer requires moving the robot, but simply retargeting the instruments and redocking from the robot in the same location (23). With ever improving stapling and vessel sealing capabilities, the safety of the operation will undoubtedly improve. We will need to be sure educational materials, such as Atlases of robotic surgery are widely available for reference and for ongoing refresh for clinical practice (24). Some professional societies, such as the Society of American Gastrointestinal and Endoscopic Surgery (SAGES), in preparation for widespread adoption of robotic surgery and complex robotic surgery, have begun publication of such atlases.
Conclusions
In conclusion, if well prepared, robotic pancreatoduodenectomy can be safely implemented within high-volume centers. Studies have shown promising results (e.g., reductions in major complications, less blood loss) of the use of a robotic system in pancreatic surgery (2). In order to safely start a robotic program for pancreatic surgery, several components are necessary, including a dedicated team, prior experience with pancreatic surgery and minimally invasive surgery and first and foremost structured training. In our opinion, these factors are essential for the safe and successful implementation. Even though structured training programs for robotic pancreatic surgery are scarce nowadays, it is to be expected that training will be become broader implemented and more important in the future.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Dr. Yuman Fong is a scientific advisor to Intuitive Surgical, Medtronic, AVRA Medical Robotics, Inc., Perfint Healthcare Robotics and Johnson & Johnson. The other authors have no conflicts of interest to declare.
References
- 1.de Rooij T, van Hilst J, Boerma D, et al. Impact of a Nationwide Training Program in Minimally Invasive Distal Pancreatectomy (LAELAPS). Ann Surg 2016;264:754-62. 10.1097/SLA.0000000000001888 [DOI] [PubMed] [Google Scholar]
- 2.Zureikat AH, Postlewait LM, Liu Y, et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann Surg 2016;264:640-9. 10.1097/SLA.0000000000001869 [DOI] [PubMed] [Google Scholar]
- 3.de Rooij T, Lu MZ, Steen MW, et al. Minimally Invasive Versus Open Pancreatoduodenectomy: Systematic Review and Meta-analysis of Comparative Cohort and Registry Studies. Ann Surg 2016;264:257-67. 10.1097/SLA.0000000000001660 [DOI] [PubMed] [Google Scholar]
- 4.Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59. 10.1097/SLA.0b013e318251ee09 [DOI] [PubMed] [Google Scholar]
- 5.Magge D, Gooding W, Choudry H, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg 2013;148:525-31. 10.1001/jamasurg.2013.1673 [DOI] [PubMed] [Google Scholar]
- 6.Nederlands Trial Register. Laparoscopic versus open pancreatoduodenectomy: a multicenter randomized controlled phase 2 trial: LEOPARD-2. Available online: http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5689. Accessed April, 6, 2017.
- 7.de Rooij T, van Hilst J, Vogel J, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): study protocol for a randomized controlled trial. Trials 2017;18:166. 10.1186/s13063-017-1892-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung U, Fong Y. Robotic liver surgery. Hepatobiliary Surg Nutr 2014;3:288-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim PC, Kang E, Park DH. A comparative detail analysis of the learning curve and surgical outcome for robotic hysterectomy with lymphadenectomy versus laparoscopic hysterectomy with lymphadenectomy in treatment of endometrial cancer: a case-matched controlled study of the first one hundred twenty two patients. Gynecol Oncol. 2011;120:413-8. 10.1016/j.ygyno.2010.11.034 [DOI] [PubMed] [Google Scholar]
- 10.Yohannes P, Rotariu P, Pinto P, et al. Comparison of robotic versus laparoscopic skills: is there a difference in the learning curve? Urology 2002;60:39-45; discussion 45. 10.1016/S0090-4295(02)01717-X [DOI] [PubMed] [Google Scholar]
- 11.Elberm H, Ravikumar R, Sabin C, et al. Outcome after pancreaticoduodenectomy for T3 adenocarcinoma: A multivariable analysis from the UK Vascular Resection for Pancreatic Cancer Study Group. Eur J Surg Oncol 2015;41:1500-7. 10.1016/j.ejso.2015.08.158 [DOI] [PubMed] [Google Scholar]
- 12.Menahem B, Guittet L, Mulliri A, et al. Pancreaticogastrostomy is superior to pancreaticojejunostomy for prevention of pancreatic fistula after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Ann Surg 2015;261:882-7. 10.1097/SLA.0000000000000806 [DOI] [PubMed] [Google Scholar]
- 13.Gooiker GA, Lemmens VE, Besselink MG, et al. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br J Surg 2014;101:1000-5. 10.1002/bjs.9468 [DOI] [PubMed] [Google Scholar]
- 14.Adam MA, Thomas S, Youngwirth L, et al. Defining a Hospital Volume Threshold for Minimally Invasive Pancreaticoduodenectomy in the United States. JAMA Surg 2017;152:336-42. 10.1001/jamasurg.2016.4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palter VN, Orzech N, Reznick RK, et al. Validation of a structured training and assessment curriculum for technical skill acquisition in minimally invasive surgery: a randomized controlled trial. Ann Surg 2013;257:224-30. 10.1097/SLA.0b013e31827051cd [DOI] [PubMed] [Google Scholar]
- 16.Walliczek-Dworschak U, Mandapathil M, Förtsch A, et al. Structured training on the da Vinci Skills Simulator leads to improvement in technical performance of robotic novices. Clin Otolaryngol 2017;42:71-80. 10.1111/coa.12666 [DOI] [PubMed] [Google Scholar]
- 17.Intuitive Surgical. da Vinci Training. Available online: https://www.intuitivesurgical.com/training/. Accessed April, 12, 2017.
- 18.Hogg ME, Tam V, Zenati M, et al. Mastery-Based Virtual Reality Robotic Simulation Curriculum: The First Step Toward Operative Robotic Proficiency. J Surg Educ 2017;74:477-85. 10.1016/j.jsurg.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 19.Tam V, Zenati M, Novak S, et al. Robotic Pancreatoduodenectomy Biotissue Curriculum has Validity and Improves Technical Performance for Surgical Oncology Fellows. J Surg Educ 2017. [Epub ahead of print]. 10.1016/j.jsurg.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 20.Martin JA, Regehr G, Reznick R, et al. Objective structured assessment of technical skill (OSATS) for surgical residents. Br J Surg 1997;84:273-8. 10.1002/bjs.1800840237 [DOI] [PubMed] [Google Scholar]
- 21.Giulianotti PC, Addeo P, Buchs NC, et al. Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas 2011;40:1264-70. 10.1097/MPA.0b013e318220e3a4 [DOI] [PubMed] [Google Scholar]
- 22.Kauffmann EF, Napoli N, Menonna F, et al. Robotic pancreatoduodenectomy with vascular resection. Langenbecks Arch Surg 2016;401:1111-22. 10.1007/s00423-016-1499-8 [DOI] [PubMed] [Google Scholar]
- 23.Yuh B, Yu X, Raytis J, et al. Use of a mobile tower-based robot--The initial Xi robot experience in surgical oncology. J Surg Oncol 2016;113:5-7. 10.1002/jso.24094 [DOI] [PubMed] [Google Scholar]
- 24.Ross SB, Downs DJ, Sucandy I, et al. Robotic pyloric preserving pancreaticoduodenectomy. In: Fong Y, Woo Y, Lau C, et al. editors. SAGES Atlas in Robotic Surgery. 2017. [Google Scholar]


