Abstract
Background
To determine the functional integrity of the neural systems involved in emotional responding, regulation and response control/inhibition in youths (age 10–18) with Disruptive Behavioral Disorder (DBD: Conduct Disorder and/or Oppositional Defiant Disorder) as a function of callous-unemotional (CU) traits.
Method
28 healthy youths and 35 youths with DBD (N=18 High CU, N=17 Low CU) performed the fMRI Affective Stroop task. Participants viewed positive, neutral, and negative images under varying levels of cognitive load. A 3-way ANOVA (group by emotion by task) was conducted on the BOLD response data.
Results
Youths with DBD-HCU showed significantly less activation of ventromedial prefrontal cortex (vmPFC) and amygdala in response to negative stimuli, compared to healthy youths and youths with DBD-LCU. VMPFC responsiveness was inversely related to CU symptoms in DBD. Youths with DBD-LCU showed decreased functional connectivity between amygdala and regions including inferior frontal gyrus in response to emotional stimuli. Youths with DBD (LCU and HCU) additionally showed decreased insula responsiveness to high load (incongruent trials) compared to healthy youths. Insula responsiveness was inversely related to ADHD symptoms in DBD.
Conclusion
These data reveal two forms of pathophysiology in DBD. One associated with reduced amygdala and vmPFC responses to negative stimuli and related to increased CU traits. Another associated with reduced insula responses during high load task trails and related to ADHD symptoms. Appropriate treatment will need to be individualized according to the patient’s specific pathophysiology.
Keywords: Disruptive Behavior Disorder, Callous-Unemotional trait, Emotional responding, Response inhibition, amygdala
Introduction
The disruptive behavioral disorders (DBD) of Conduct Disorder (CD) and Oppositional Defiant Disorder (ODD) are characterized by aggressive behavior, poor emotional regulation, and relationship difficulties (American Psychiatric Association, 2013). There have been recent claims that the impairments shown by these patients might relate to forms of dysfunction in several different neuro-cognitive mechanisms that manifest as specific forms of behavioral disturbance (Blair, 2013). Thus, considerable data indicate that a group of youths with DBDs show reduced amygdala responses to distress cues, the degree of which is positively associated with callous-unemotional (CU) traits (i.e. reduced guilt and empathy) and instrumental aggression (White et al., 2012, Lozier et al., 2014). These youths also showed atypical responses to reward and punishment within ventromedial prefrontal cortex (vmPFC) and caudate compared to typically developed youth (Finger et al., 2008, Finger et al., 2011) and youth with Attention-Deficity/Hyperactivity Disorder (ADHD) (Finger et al., 2008). A recent study suggested that these functional difference reflect compromised representation of reinforcement expectancies within the vmPFC and aberrant prediction error signaling within the caudate (White et al., 2013). There are also some data indicating a second group of youths show increased amygdala responses to threat and low callous-unemotional traits (Viding et al., 2012, Blair, 2013, Sebastian et al., 2014). Indeed, the importance of CU traits is recognized in the DSM-5 with the inclusion of the limited pro-social emotions specifier for CD (American Psychiatric Association, 2013).
Two additional neuro-cognitive mechanisms have been hypothesized, when dysfunctional, to increase the risk of antisocial behavior/ relate to the behavior problems of youth with CD (Patrick et al., 2009, Young et al., 2009, Miyake & Friedman, 2012, Blair et al., 2013). The first of these is top down attention (related to prefrontal (dorsomedial and lateral) regions; Buhle et al., 2014). Top down attention is implicated in emotional regulation in explicit cognitive reappraisal paradigms where subjects alter stimulus representations by priming non-emotional features (Buhle et al., 2014) and implicit attention distraction paradigms (e.g., the Affective Stroop task; aST) where subjects prime task features at the expense of the representation of emotional distracters (Pessoa et al., 2005, Blair et al., 2007). As such dysfunction in top down attention might lead to emotional dysregulation and an increased risk for reactive aggression. Indeed, studies have reported increased amygdala responses to negative stimuli in youth with conduct problems and low CU traits (Viding et al., 2012, Sebastian et al., 2014). It has been suggested that this might reflect deficient top down attention-based emotion regulation (Blair et al., 2013, Blair, 2013). However, no previous fMRI work has investigated this possibility.
The second neuro-cognitive mechanism hypothesized, when dysfunctional, to increase the risk of antisocial behavior/ relate to the behavior problems of youth with CD is response control/response inhibition (Patrick et al., 2009, Young et al., 2009, Miyake & Friedman, 2012). Response control/response inhibition is thought to be mediated by dorsomedial and inferior frontal/anterior insula cortices (Criaud & Boulinguez, 2013). Response control/inhibition is important for avoiding sub-optimal choices and can be indexed by the Stop, Go/No-Go and Stroop tasks (Criaud & Boulinguez, 2013). Impairment in response control/inhibition should result in an individual who will “impulsively” express behaviors (including antisocial behaviors) that are non-optimal for the situation. Such impairment has also been associated with an increased risk for antisocial behavior (Patrick et al., 2009, Young et al., 2009, Miyake & Friedman, 2012).
The current study uses the aST to investigate emotional responding, automatic emotion regulation and response control/inhibition in youths with DBD and high and low callous-unemotional traits (HCU/LCU). Considerable data, including from the aST and related tasks, demonstrate that the performance of a cognitive task reduces the response within the amygdala to an emotional stimulus (Critchley et al., 2000, Pessoa et al., 2002, Erthal et al., 2005, Blair et al., 2007, Mitchell et al., 2007); i.e., participants undertaking paradigms such as the aST demonstrate automatic emotion regulation. Our goal in using the aST is to elucidate the neuro-circuitry dysfunction related to symptom manifestation across disorders (in this case CD and ODD), thus departing from diagnosis-based approach to a mechanism-based approach towards the understanding of pathophysiology in DBD (Insel et al., 2010, Cuthbert & Insel, 2013).
We predicted: (i) Consistent with previous work (White et al., 2012, Lozier et al., 2014, Baker et al., 2015), DBD-HCU youths would show reduced amygdala responsiveness to threatening stimuli relative to healthy youths; (ii) Consistent with previous work (Viding et al., 2012, Sebastian et al., 2014, Baker et al., 2015), DBD-LCU youths would show increased amygdala responsiveness to threatening stimuli relative to healthy youths; (iii) and that amygdala responsiveness would be inversely associated with CU traits in youths with DBD; (iv) On the basis of previous hypotheses (Blair et al., 2013), we predicted DBD-LCU youths would show reduced recruitment of attention-based emotion regulation regions (dorsomedial and lateral frontal cortex; Blair et al., 2007) relative to healthy youths and DBD-HCU youths; (v) and that DBD youths would show reduced recruitment of regions implicated in response control (anterior insula/inferior frontal and dorsomedial frontal cortex) relative to healthy youths with responsiveness being inversely associated with ADHD symptoms in DBD youths; and (vi) Consistent with previous functional connectivity studies (Marsh et al., 2008, Herpers et al., 2014), we predicted DBD-HCU youths would show reduced connectivity between the amygdala and cortical regions to threatening stimuli relative to healthy youths and DBD-LCU youths and that level of connectivity would be inversely associated with CU traits in youths with DBD.
Methods
Participants
Sixty seven youths participated: 29 healthy and 38 with DBD (CD/ODD). Participants were recruited from the community through newspaper ads, fliers, and referrals from area mental health practitioners. Four participants (1 healthy and 3 with DBD) were excluded (due to, for example, excessive movement). Thus, data from 28 healthy (average age=13.88, 13 females) and 35 DBD (average age=14.81, 13 females) were analyzed; see Table 1. Statements of informed assent/consent were obtained from participating children/parents. This study was approved by the NIMH IRB.
Table 1.
Characteristics of healthy youths, youths with DBD, and youths with ADHD
| Healthy Youths (N=28) | Youths with DBD and LCU (N=17) | Youths with DBD and HCU (N=18) | P value | |
|---|---|---|---|---|
| Age | 13.88 (2.03) | 14.78 (2.39) | 14.56 (1.84) | 0.317 |
| IQ | 101.18 (10.70) | 96.65 (11.46) | 95.72 (9.69) | 0.182 |
| Gender | 15 male, 13 female | 12 male, 5 female | 10 male, 8 female | 0.504 |
| Handedness | 6 left, 22 right | 3 left, 14 right | 3 left, 15 right | 0.909 |
|
| ||||
| CD | 0 | 10 | 16 | 0.145 a |
| ODD | 0 | 7 | 2 | 0.06 a |
| ADHD | 0 | 12 | 7 | 0.125 a |
| SA f | 0 | 1 | 6 | 0.002 a |
|
| ||||
| Mean ICU score | 15.58 (6.76) | 33.12 (5.68) | 48.11 (5.67) | 0.000b |
| Conner Score | 2.31 (3.27) | 25.85 (13.45) | 28.67 (14.06) | 0.000 c |
| On Medication | 0 | 4d | 5e | 0.540 a |
CD: Conduct Disorder, ODD: Oppositional Defiant Disorder, ADHD: Attention-Deficit/Hyperactivity Disorder, SA: Substance Abuse
: between DBD-LCU and DBD-HCU
: DBD-LCU<DBD+HCU: t(33)=7.817, p=0.000.
: Healthy youths< DBD-LCU: t(43)=9.379, p=0.000; DBD-LCU=DBD+HCU: t(26)=0.540, p=0.594.
: 1 – Atomoxetine; 1 – Lamotrigine; 1 – Lamotrigine + Aripiprazole; 1 – Amphetamine, Intuitiv, Risperidone
: 4 – Amphetamine; 1 – Atomoxetine + Intuitiv;
: all cannabinoid abuse except one in HCU group (alcohol abuse)
Participants’ parents completed the Inventory of Callous-Unemotional Traits-Parent Version (ICU-P). The ICU-P is a 24-item scale assessing CU traits in youth with good construct validity (Frick, 2004, Kimonis et al., 2008) and reliability (Cronbach’s alpha=0.81). Following previous studies (Viding et al., 2012, Lozier et al., 2014), we divided the patients with DBD into two groups on the basis of a median split of the ICU-P scores (median score = 42; LCU/HCU: N=17/18); see Table 1. Previous community sample studies reveal average ICU scores between, for example, 22 and 31 (standard deviation: 7.88–10.98; Roose et al., 2010, Byrd et al., 2013). In contrast, clinical samples of patients with DBD and forensic samples reveal average ICU scores of 41 (White et al., 2009). As such, all HCU group showed a level of CU that was above average for patients with DBD and notably greater than that shown by healthy populations.
All youths and their parents completed the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 1997). Assessments were conducted by a doctoral-level clinician and supervised by expert child/adolescent psychiatrists. The K-SADS has demonstrated good validity and inter-rater reliability (Kaufman et al., 1997). The parents of 26/28 healthy youths and 28/35 youths with DBD completed the Connors Parent Rating Scale for ADHD, version 2 (Conners et al., 1998). IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (2-subtest form; Wechsler, 1999). Exclusion criteria are listed in Supplemental Material Section 1. The groups did not differ significantly in terms of age, sex, handedness or IQ; see Table 1.
Experimental Task
We used an adapted version of the aST described previously (Blair et al., 2007, Hwang et al., 2014) see Figure 1. On each trial, participants saw a central fixation point (400ms), a positive, neutral, or negative image (400ms), either a numerical array on task trials, or a blank screen on view trials (400ms), the same image previously displayed (400ms), and a second blank screen (1300ms). For task trials, participants pressed a button corresponding to how many numbers were displayed (numerosity: 3 to 6). On congruent trials, numerosity matched the actual number values displayed (e.g. three 3s). On incongruent trials, numerosity did not match the number values displayed (e.g. four 3s or six 3s). The numerical gap between numerosity and the number values ranged between 1 (e.g., four 3s) and 3 (e.g., six 3s). Participants were free to respond at any time between the initial numerical presentation and the end of the blank screen display (response window: 1700ms). Participants made no response for view trials.
Figure 1.
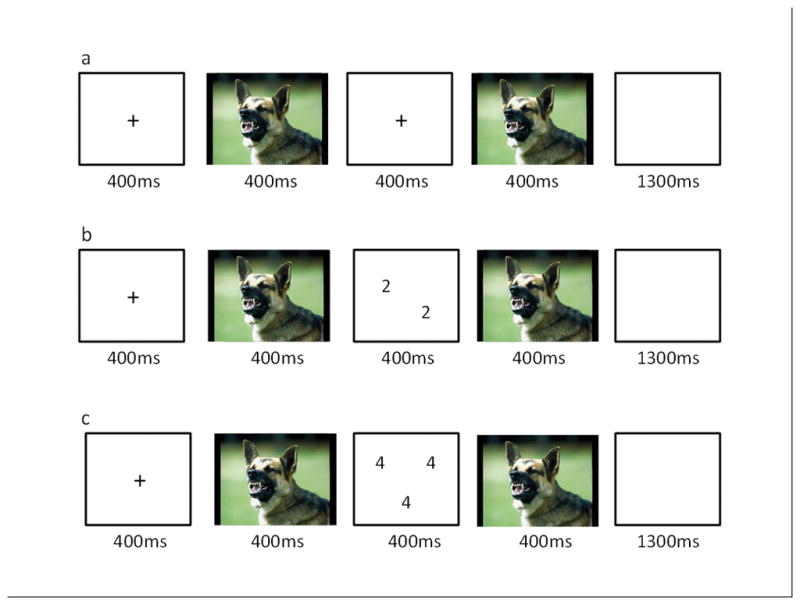
Example trial sequences. (a) negative view trial; (b) negative congruent trial; (c) negative incongruent trial.
The images consisted of 48 positive, 48 negative, and 48 neutral pictures selected from the International Affective Picture System (Lang PJ, 2005); see Supplemental Material Section 2 for mean valence and arousal values by stimulus class. Participants completed two runs. Each involved 288 trials (32 in each 9 categories [3 image type × 3 task type]) and 96 fixation trials (each of 2500ms length to generate a baseline). Trial order was randomized across participants.
Image Acquisition and Analysis
Whole-brain blood oxygen level-dependent (BOLD) fMRI data were acquired using a 3-T GE MRI scanner. Following sagittal localization, functional T2*-weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence with a matrix of 64×64 mm, repetition time (TR) of 3000ms, echo time (TE) of 30ms, field of view (FOV) of 240 mm, and voxels of 3.75×3.75×4 mm. Images were acquired in 30 continuous 4mm axial slices per brain volume across two runs. The duration of each run was 8min 13s. In the same session, a high-resolution T1-weighed anatomical image was acquired to aid with spatial normalization (three-dimensional Spoiled GRASS; TR=8.1ms; TE=3.2ms, flip angle 20˚; field of view=240mm, 128 axial slices, thickness=1.0mm; 256×256 acquisition matrix).
Functional MRI Analysis
Data were analyzed within the framework of a random effects general linear model using Analysis of Functional Neuroimages (AFNI). Both individual and group-level analyses were conducted. The first 5 volumes in each scan series, collected before equilibrium magnetization was reached, were discarded. Motion correction was performed by registering all volumes in the EPI dataset to a volume that was collected shortly before acquisition of the high-resolution anatomical dataset.
The EPI datasets for each participant were spatially smoothed (using an isotropic 6mm Gaussian kernel) to reduce the influence of anatomical variability among the individual maps in generating group maps. Next, the time series data were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients represented a percent signal change from the mean. The model involved six motion regressors and the following 9 task regressors: negative congruent, negative incongruent, negative view, neutral congruent, neutral incongruent, neutral view, positive congruent, positive incongruent and positive view. A regressor modeling incorrect responses was also included. All regressors were convolved with a canonical hemodynamic response function (HRF) to account for the slow hemodynamic response (with time point commencing at time of first image onset). There was no significant regressor collinearity.
The participants’ anatomical scans were individually registered to the Talairach and Tournoux atlas (Talairach & Tournoux, 1988). The individuals’ functional EPI data were then registered to their Talairach anatomical scan within AFNI. Linear regression modeling was performed using the 10 regressors (9 task plus incorrect responses) described earlier, plus regressors to model a first-order baseline drift function. This produced β coefficients and associated t statistics for each voxel and regressor.
The BOLD data were analyzed via a 3 (group: healthy youths, youths with DBD-LCU, youths with DBD-HCU) by 3 (emotion: negative, positive, neutral) by 3 (task: congruent, incongruent, view) ANOVA. Statistical maps were created for each main effect and interaction by thresh-holding at a single-voxel p value of p<0.005. ClustSim was then applied to these results yielding a minimum cluster size (22 voxels) with a map-wise false-positive probability of p<0.05, corrected for multiple comparisons.
Given our a priori hypotheses, regions of interest (ROIs), taken from the AFNI software’s anatomical maps (TT_Daemon atlas) were obtained from the amygdala (Talairach & Tournoux, 1988). A small volume-corrected ROI analysis via ClustSim was used on these regions (initial threshold: p<0.02, k=13, corrected p<.05).
Follow-up analyses were performed to facilitate interpretations. For these analyses, average percent signal change was measured across all voxels within each ROI generated from the functional masks, and data were analyzed using appropriate follow-up independent t-tests within SPSS.
Context-dependent psychophysiological interaction (gPPI) Analysis
A context-dependent psychophysiological interaction (gPPI) analysis was conducted to examine group differences in functional connectivity following the method described by McLaren and colleagues (McLaren et al., 2012). Our main goal was to examine group differences in functional connectivity between the amygdala and cortical regions. We took as a seed the region of right amygdala (coordinates: 25.5,−1.5, −12.5) showing a main effect of emotion from the main ANOVA conducted on the BOLD data (see Table S2). This seed can be considered relatively unbiased by group membership as it was identified by main effect of emotion (i.e., significant activity to emotion was seen within all groups). The average activation from this seed region was extracted across the time series. Interaction regressors were created by multiplying each of these average time series with nine task time course vectors (one for each task and emotion condition) which were coded 1 or 0 for task and emotion condition present or absent. The average activation for the seeds was entered into a linear regression model along with the nine interaction regressors and 6 motion regressors. A 3 (group)-by-3 (task)-by-3 (emotion) whole-brain repeated measures ANOVA was then applied to the data, and the regions showing significant group-by-emotion interaction were reported.
Results
Behavioral Data
Two 3(group: DBD-HCU, DBD-LCU, Healthy)-by-3(emotion: positive, neutral, negative)-by-2(task: congruent, incongruent) ANOVAs were applied to the reaction time (RT) and accuracy data; see Table S1. With respect to RT, there was a significant main effect of task [incongruent>congruent; F(1,60)=169.349, p<0.001] and a trend for emotion [F(2, 59)=2.898, p=0.059; negative & positive>neutral; t(62)=1.746 & 1.924, p=0.086 & 0.059, respectively]. With respect to accuracy, there was a significant main effect of task [incongruent < congruent; F(1,60)=22.565, p<0.001] and group [F(2,60)=4.578, p=0.014; DBD-LCU<DBD-HCU & healthy youths; t(43, 33)=2.352 & 2.486, p=0.023 & 0.018, respectively]. The performance of youth with DBD-HCU and healthy youths did not significantly differ [t(44)=1.056, p=0.297]. No other main effects or interactions for either ANOVA were significant.
Movement Data
There were no significant group differences in movement parameters; [F(1,60)=1.484–2.981, p>0.1].
MRI Data: Main analysis
A whole-brain 3(group)-by-3(emotion)-by-3(task) ANOVA was applied to the BOLD data. This revealed regions showing significant group-by-emotion, group-by-task and group-by-task-by-emotion interactions. Regions showing main effects of task and emotion and task-by-emotion interactions are presented in the Supplemental Material Section 3.
Group-by-Emotion Interaction
There was a group-by-emotion interaction within left vmPFC and right (but not left) amygdala ROI; see Table 2, Figure 2(A) and (D). Within both regions, youths with DBD-HCU showed significantly decreased activation to negative relative to neutral stimuli, compared to healthy youths and youths with DBD-LCU who did not significantly differ [vmPFC: t=3.573 & 3.891; p<0.001; right amygdala t=2.491 & 2.312; p=0.017 & 0.027]; see Figure 2(B) and (E). There were no group differences in either region’s response to positive relative to neutral stimuli [F=0.827 & 1.771, p>0.05].
Table 2.
(A) Brain Regions showing a significant interaction in comparison between healthy youths, youths with DBD-LCU and youths with DBD-HCU; (B) Brain regions showing a significant interaction of connectivity with right amygdala seed in comparison between healthy youths, youths with DBD-LCU, and youths with DBD-HCU
| Regiona | Coordinates of Peak Activation | F | Voxels | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Left/Right | BA | x | y | z | |||
| (A) | |||||||
| Group-by-Emotion | |||||||
| Ventro-medial prefrontal cortex | Left | 11 | −4.5 | 43.5 | −12.5 | 6.515 | 30 |
| Amygdala ROI | Right | 25.5 | −1.5 | −21.5 | 3.306 | 13 | |
| Group-by-Task | |||||||
| Insula | Left | 13 | −37.5 | 7.5 | −0.5 | 5.685 | 12* |
| Group-by-Task-by-Emotion | |||||||
| Superior frontal gyrus | Right | 9 | 22.5 | 37.5 | 29.5 | 3.976 | 24 |
| Caudate | Bilateral | 10.5 | 1.5 | 20.5 | 4.682 | 50 | |
|
| |||||||
| (B) | |||||||
| Rt. amygdala seed | |||||||
| Group-by-Emotion | |||||||
| Inferior frontal gyrus | Left | 10 | −40.5 | 40.5 | −0.5 | 6.241 | 38 |
| Caudate | Left | −4.5 | 13.5 | 8.5 | 6.314 | 46 | |
| Insula | Left | 13 | −34.5 | −22.5 | 14.5 | 6.130 | 24 |
| Posterior cingulate gyrus | Left | 24 | −4.5 | −13.5 | 38.5 | 4.696 | 22 |
According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon).
below the ClusterSim cluster size (22 voxels)
Figure 2.
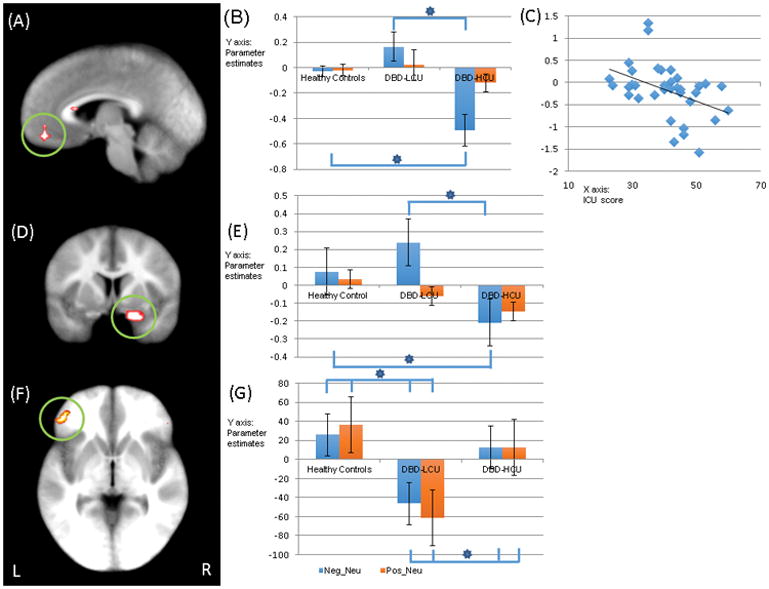
Regions showing a significant group-by-emotion interaction: (A) lBOLD response data: eft ventromedial prefrontal cortex (coordinates: −4.5, 43.5, −12.5, at =0.005); (B) Parameter estimates for left vmPFC; (C) Negative correlation between symptom severity of callous-unemotional trait measured by the ICU (x-axis) and BOLD response parameter estimates of negative relative to neutral trials (y-axis) in left vmPFC; (D) right amygdala ROI (at p=0.05); (E) Parameter estimates for this region; (gPPI data with right amygdala seed: ); (E) left inferior frontal gyrus (coordinates: −40.5, 40.5, −0.5 at p=0.005) and; (G) Parameter estimates for this region;.
Key to Figure 1: Neg: Negative; Neu: Neutral; Pos: Positive; Healthy: healthy youths; LCU: youths with DBD-LCU; HCU: youths with DBD-HCU. * = significant contrasts for interaction variables (p<0.05).
The results are showing on the Talairach space.
Group-by-Task Interaction
There was a group-by-task interaction within left insula; see Table 2. Within this region, youths with DBD-LCU and youths with DBD-HCU did not differ [t=1.542, p=0.133]. However, both showed a significantly decreased differential response to incongruent task trials relative to view trials [t=3.471 & 2.579; p=0.001 & 0.013] and to incongruent relative to congruent task trials [t=3.517 & 3.406, p=0.001] compared to healthy youths; see Figure 3(D) and (E). There were no group differences in differential response to congruent relative to view trials [t=0.621 & 0.118, p=0.538 & 0.907]. No other regions showed a significant group-by-task interaction. While left insula did not survive multiple comparison correction (k=22), this likely reflects a Type II error; the reduced insula activity (as well as left inferior parietal lobule) was seen in both groups of youths with DBD and an exploratory ANOVA contrasting healthy youth with a combined DBD group revealed a highly significant group-by-task interaction within this region (k=57); see Figure 3(A) and (B). The only other region showing a significant group-by-task interaction for this second analysis was left inferior parietal lobule (k=28; for analysis details, see Supplemental Material Section 4).
Figure 3.
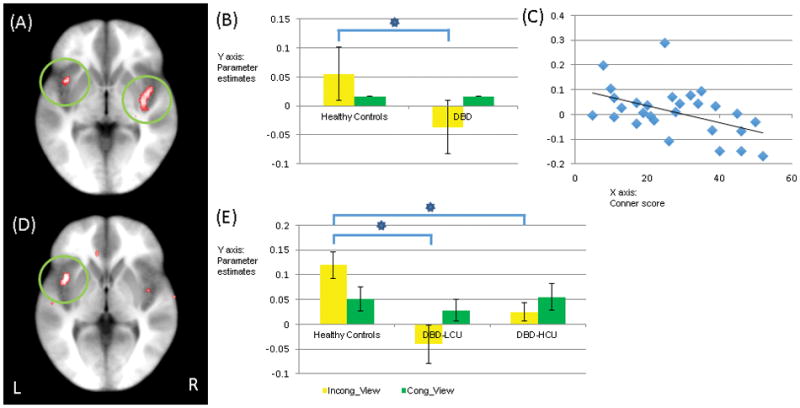
Regions showing a significant group-by-task interaction: (A) bilateral insula (coordinates: 37.5, −13.5, −6.5; −37.5, 7.5, −0.5 at p=0.005) via Healthy versus DBD ANOVA; (B) Parameter estimates for this region; (C) Negative correlation between ADHD symptom severity measured by Connor parent report scale (x-axis) and BOLD response parameter estimates of congruent relative to view trials (y-axis); (D) left insula (coordinates: −37.5, 7.5, −0.5 at p=0.005) via Healthy vs. DBD-LCU vs. DBD-HCU ANOVA; (E) Parameter estimates for this region.
Key to Figure 3: Incong: Incongruent trial; Cong: Congruent trial; View: View trial; Healthy: healthy youths; DBD: youths with DBD; LCU: youths with DBD-LCU; HCU: youths with DBD-HCU. * = significant contracts for interaction variables (p<0.05).
The results are showing on the Talairach space.
Group-by-Task-by-Emotion Interaction
There was a significant group-by-emotion-by-task interaction within right superior frontal gyrus and bilateral caudate; see Table 2. Within both regions, youths with DBD-HCU showed greater activity on negative incongruent trials relative to comparison groups [t=2.013–3.319, p=0.002–0.005], and youths with DBD-LCU showed greater activity on positive incongruent trials than both other comparison groups [t=2.438–2.668, p=0.011–0.020]. All other contrasts were not significant except within bilateral caudate where healthy youths showed less activity than DBD-LCU for negative congruent trials [t=2.146, p=0.038].
MRI results: gPPI results
A 3(group)-by-3(emotion)-by-3(task) ANOVA was conducted on the gPPI data using the right amygdala seed. Regions displaying a significant group-by-emotion interaction included left inferior frontal gyrus, left posterior cingulate gyrus, left caudate, and left insula; see Table 2. Youths with DBD-LCU compared to healthy youths and youths with DBD-HCU showed significantly reduced connectivity between the right amygdala seed and these regions in response to emotional (negative and positive) relative to neutral stimuli [t=2.452–5.355, p=0.000–0.018; though for left posterior cingulate gyrus in response to negative relative to neutral stimuli t=1.916, p=0.062; see Figure 2(F) and (G) for left inferior frontal gyrus. Healthy youths and youths with DBD-HCU showed no significant differences in gPPI connectivity [t=0.067–1.947, p=0.058–0.947], except that youths with DBD-HCU showed significantly increased connectivity between right amygdala and caudate in response to positive relative to neutral stimuli relative to healthy youth [t=2.152, p=0.037].
Correlations with symptom severity
Ten correlations were conducted examining the relationship between BOLD response parameters and symptom severity in the patients with DBD. CU symptom severity was negatively correlated with differential (negative-neutral) BOLD response for the vmPFC [r = −0.370, p=0.026] but not the amygdala [r=−0.238, p>0.05]; see Figure 2(C). However, it was positively correlated with amygdala-caudate connectivity in response to the negative relative to neutral stimuli [r = 0.420, p=0.012]. ADHD symptom severity (as indexed by the Connor Parent Report Scale) was negatively correlated with differential (congruent-view, but not incongruent-view or incongruent-congruent) BOLD response within left insula [r=−0.477, p=0.010]; see Figure 3(C). Following a reviewer’s suggestion and for completion, we also examined the relationship between CU symptom severity and differential (incongruent-view, congruent-view, and incongruent-congruent) BOLD response within left insula. However, these were non-significant [r=0.188, −0.084, and 0.105, p=0.280, 0.630, and 0.459, respectively]. In addition, we examined the relationship between ADHD symptom severity and differential (negative-neutral) BOLD response for the vmPFC and amygdala. However, these were also non-significant [vmPFC: r=−0.028, p=0.889; amygdala: r=−0.174, p=0.377].
Potential confounds
We conducted analyses excluding youths on psychotropic medications and substance abusers. These analyses revealed similar results to the main analysis reported above (See Supplemental Material Section 5 & 6).
Discussion
We investigated emotional responding, automatic emotion regulation and response control/inhibition in youths with DBD and HCU/LCU. There were two main results: First, youth with DBD-HCU showed significantly decreased left vmPFC and right amygdala activation to negative relative to neutral stimuli, compared to healthy youths and youths with DBD-LCU. Moreover, the vmPFC response to negative versus neutral stimuli was inversely related to level of CU traits in the patients with DBD. Second, youths with DBD (LCU and HCU) showed decreased activation of bilateral insula on task trials relative to healthy youth. Insula responsiveness was inversely related to ADHD symptomatology in the youths with DBD.
In line with our first prediction and previous work (Viding et al., 2012, White et al., 2012, Lozier et al., 2014), youth with DBD-HCU showed reduced amygdala responses to threat stimuli relative to comparison youth. In addition, the current study extended the literature in two ways. First, it indicated that reduced amygdala recruitment is specific for negative relative to positive emotional stimuli (though it remains possible that amygdala responding to happy expressions may be disrupted in youths with DBD-HCU; cf. Fusar-Poli et al., 2009). Second, it indicated dysfunction in emotional responding in both the amygdala and vmPFC. VMPFC and amygdala responsiveness to negative relative to neutral stimuli were correlated in all three groups and level of vmPFC response was inversely related to CU traits level in patients with DBD. The relationship between the amygdala and vmPFC is complex. VMPFC may regulate amygdala activity (Milad & Quirk, 2012). However, vmPFC lesions suppress amygdala activity and “protect” the individual from the development of PTSD/depression (Koenigs & Grafman, 2009). These latter results are consistent with a more interactive role where valence information is provided by the amygdala to vmPFC for representation (Schoenbaum et al., 2006). We assume that the current data of decreased activation in vmPFC and amygdala for youths with HCU reflects a failure in this interaction (cf. Marsh et al., 2008, Motzkin et al., 2011).
In contrast to our second prediction, youth with DBD-LCU did not show significantly increased amygdala responses to threat stimuli relative to healthy youth (only a non-significant trend) though their amygdala responses to threat stimuli were significantly greater than those of youth with HCU. It should be noted that while some previous studies have reported increased amygdala responses to negative stimuli in youth with conduct problems and LCU (Viding et al., 2012, Sebastian et al., 2014), not all studies have (Lozier et al., 2014). However, previous work has consistently shown, as was seen here, that youth with DBD-LCU show increased amygdala responses relative to youth with DBD-HCU (Viding et al., 2012, White et al., 2012; cf. prediction (iii), Sebastian et al., 2014). Moreover, it is worth noting that while the group with DBD-HCU was selected for showing elevated CU traits, the group with DBD-LCU was selected for not showing CU traits. Selecting a second group of youth with DBD for impairment potentially associated with heightened threat sensitivity (possibly irritability; Thomas et al., 2011) might prove beneficial in future research.
Our fourth prediction, that DBD-LCU patients would show reduced recruitment of attention-based emotion regulation regions (dorsomedial and lateral frontal cortex; Blair et al., 2007) relative to healthy youths and DBD-HCU patients was not supported. The suggestion had been that increased emotional responsiveness in youth with DBD-LCU might reflect a failure on top down attention driven emotion regulation (cf. Blair et al., 2007). However, no regions showed significant group-by-task or group-by-task-by-emotion interactions that were consistent with reduced recruitment of systems implicated in top down attention in patients with DBD-LCU. It should be noted though that youths with DBD-LCU showed decreased connectivity between the amygdala and both (right/left) insula and (right/left) inferior frontal cortex. These regions have been implicated in some accounts of emotion regulation in previous work (Davidson et al., 2000, Gold et al., 2015). The youth with DBD-LCU thus show some pathology consistent with impaired emotional regulation. However, it is important to note that though they showed significantly increased amygdala and vmPFC responsiveness to negative stimuli relative only to youth with DBD-HCU (there was increased responsiveness relative to healthy youth but this was not statistically significant). As such data from this study did not indicate heightened responsiveness to aversive stimuli (i.e., emotional dysregulation) in youth with DBD-LCU relative to healthy youth though this has been reported in other studies (Viding et al., 2012, Sebastian et al., 2014).
In line with predictions, youths with DBD (HCU and LCU) showed reduced task-related bilateral anterior insula cortex activity, a region implicated in response control (Chambers et al., 2009), during incongruent trials relative to healthy youths. This is consistent with previous reports of insula dysfunction in DBD (Crowley et al., 2010, Fairchild et al., 2014, White et al., 2014), and the relationship between dysfunctional response inhibition and externalizing behaviors (Young et al., 2009, Patrick et al., 2013) particularly impulsivity (Loeber et al., 2009). BOLD responses in anterior insula cortex correlated inversely with ADHD symptom severity in youth with DBD. This indicates a second form of pathophysiology in DBD related not to CU but rather impulsiveness and doubtless exacerbating antisocial and risky behavior (such as substance abuse) in these youth (Crowley et al., 2010, Blair et al., 2013).
In contrast to our final prediction, youth with DBD-HCU youths did not show reduced connectivity between the amygdala and cortical regions to threatening stimuli relative to comparison youths and DBD-LCU youth. Instead, youths with DBD-HCU showed increased connectivity between right amygdala and caudate in response to positive relative to neutral stimuli relative to healthy youth. This contrasts with previous functional connectivity findings indicating reduced connectivity between the amygdala and particularly vmPFC in patients with high psychopathic traits (Marsh et al., 2008, Marsh et al., 2011, Motzkin et al., 2011). However, it should be noted that these previous studies reflect connectivity during either resting state (Motzkin et al., 2011) or across all task conditions (Marsh et al., 2008, Marsh et al., 2011). The current study investigated group differences in differential connectivity across specific conditions. It is thus possible that while amygdala-vmPFC global connectivity is reduced in youth with elevated CU traits, any increase in connectivity for emotional relative to neutral stimuli is comparable for youth with DD-HCU and healthy youth.
Implications for treatment
Our results support suggestions that CU traits/emotional responsiveness should be considered when assessing patients with DBD. Youths with DBD-HCU showed decreased activation in the areas of emotional responsiveness including amygdala and vmPFC, which may lead to exercising proactive aggression, whereas youths with DBD-LCU showed decreased connectivity between amygdala and inferior frontal cortex which may lead to difficulty in emotion regulation and in turn exercising more of reactive aggression (Blair et al., 2013, Blair et al., 2014). Optimal treatment for patients with hypo-emotionality may differ from patients with hyper-emotionality. Indeed, youths with high CU traits benefit less from current interventions (Frick et al., 2014). The current data are not particularly supportive of interventions designed to augment emotional regulation in youth with DBD-LCU but this may reflect patient assessment and/or the form of emotion regulation assessed (Gyurak et al., 2011). The current data support suggestions that response control dependent on anterior insula cortex might be deficient in patients with DBD (Young et al., 2009, Patrick et al., 2013) and it may well be an integral part of assessment for youths with DBD.
Limitations and Conclusion
Two caveats should be considered: First, we included youths with substance abuse and those receiving medication treatment. However, subsequent analyses excluding these subjects yielded similar results to the main analysis (see Supplemental Material Section 5 and 6). Second, there were relatively few group differences in behavioral task performance. The patients with DBD-LCU were less accurate in their responding than both the healthy youth and youth with DBD-HCU who did not differ in performance. However, this was seen for both congruent and incongruent trials and there were no group differences in impact of emotional distracters. This likely reflects the relatively minor differential effect of emotional distracters in this task (there were only trends for trials involving positive or negative emotional distracters to be slower than trials involving neutral distracters). Given the relatively weak impact of these distracters here, it is less surprising that we did not observe, for example, an anticipated reduction in interference from emotional distracters in the youth with DBD-HCU (Mitchell et al., 2006). More arousing/negatively valenced distracters might have been more successful in producing group differences in behavior. However, such stimuli are unlikely to be considered ethical for research with adolescents.
In summary, we demonstrated two forms of pathophysiology in youths with DBD that related to different forms of behavioral impairment. One is associated with reduced amygdala and vmPFC responses to negative stimuli and related to increased CU traits. Another with reduced insula responses during response control and related to ADHD symptoms. Appropriate assessment/intervention will need to be individualized according to specific pathophysiology of youths with DBD.
Supplementary Material
Acknowledgments
Drs. Hwang, White, Sinclair, and Blair are with the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health. This work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health under grant number 1-ZIA-MH002860-08 to Dr. Blair. Ethics approval for this study was granted by the NIH Combined Neuroscience Institutional Review Board under protocol number 05-M-0105.
References
- American Psychiatric Association. Diagnostic and Statistical Manual. Vol. 5. American Psychiatric Association; Washington D.C: 2013. [Google Scholar]
- Baker RH, Clanton RL, Rogers JC, De Brito SA. Neuroimaging findings in disruptive behavior disorders. CNS Spectrums. 2015;20:369–81. doi: 10.1017/S1092852914000789. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–40. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Vythilingam M, Crowe SL, McCaffrey DE, Ng P, Wu CC, Scaramozza M, Mondillo K, Pine DS, Charney DS, Blair RJ. Cognitive control of attention is differentially affected in trauma-exposed individuals with and without post-traumatic stress disorder. Psychological Medicine. 2013;43:85–95. doi: 10.1017/S0033291712000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience. 2013;14:786–99. doi: 10.1038/nrn3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Leibenluft E, Pine DS. Conduct disorder and callous-unemotional traits in youth. New England Journal of Medicine. 2014;371:2207–16. doi: 10.1056/NEJMra1315612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex. 2014;24:2981–90. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, Kahn RE, Pardini DA. A Validation of the Inventory of Callous-Unemotional Traits in a Community Sample of Young Adult Males. Journal of Psychopathology and Behavioral Assessment. 2013:35. doi: 10.1007/s10862-012-9315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience & Biobehavioral Reviews. 2009;33:631–46. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26:257–68. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neuroscience & Biobehavioral Reviews. 2013;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, Van Amelsvoort T, Robertson D, David A, Murphy D. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, Banich MT. Risky decisions and their consequences: neural processing by boys with Antisocial Substance Disorder. PLoS One. 2010;5:e12835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Erthal FS, de Oliveira L, Mocaiber I, Pereira MG, Machado-Pinheiro W, Volchan E, Pessoa L. Load-dependent modulation of affective picture processing. Cognitive, Affective & Behavioral Neuroscience. 2005;5:388–95. doi: 10.3758/cabn.5.4.388. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Passamonti L, Walsh ND, Goodyer IM, Calder AJ. Atypical neural responses during face processing in female adolescents with conduct disorder. Journal of the American Acadedmy of Child & Adolescent Psychiatry. 2014;53:677–687. e5. doi: 10.1016/j.jaac.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, Pine DS, Blair RJ. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Joujrnal of Psychiatry. 2011;168:152–62. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS, Chen G, Towbin KE, Leibenluft E, Pine DS, Blair JR. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives of General Psychiatry. 2008;65:586–94. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick . The Inventory of Callous-Unemotional Traits. University of New Orleans; New Orleans: 2004. [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, Kahn RE. Annual research review: A developmental psychopathology approach to understanding callous-unemotional traits in children and adolescents with serious conduct problems. Journal of Child Psychology and Psychiatry. 2014;55:532–48. doi: 10.1111/jcpp.12152. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Morey RA, McCarthy G. Amygdala-prefrontal cortex functional connectivity during threat-induced anxiety and goal distraction. Biological Psychiatry. 2015;77:394–403. doi: 10.1016/j.biopsych.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cognition and Emotion. 2011;25:400–12. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpers PC, Scheepers FE, Bons DM, Buitelaar JK, Rommelse NN. The cognitive and neural correlates of psychopathy and especially callous-unemotional traits in youths: a systematic review of the evidence. Development and Psychopathology. 2014;26:245–73. doi: 10.1017/S0954579413000527. [DOI] [PubMed] [Google Scholar]
- Hwang S, White SF, Nolan ZT, Sinclair S, Blair RJ. Neurodevelopmental changes in the responsiveness of systems involved in top down attention and emotional responding. Neuropsychologia. 2014;62:277–85. doi: 10.1016/j.neuropsychologia.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Skeem JL, Marsee MA, Cruise K, Munoz LC, Aucoin KJ, Morris AS. Assessing callous-unemotional traits in adolescent offenders: Validation of the inventory of callous-unemotional traits. International Journal of Law and Psychiatry. 2008;31:241–252. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–8. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJBM, Cuthbert BN. International affective picture system (IAPS): Affetive ratings of pictures and instruction manual. University of Florida; Gainesville, FL: 2005. [Google Scholar]
- Loeber R, Burke J, Pardini DA. Perspectives on oppositional defiant disorder, conduct disorder, and psychopathic features. Journal of Child Psychology and Psychiatry. 2009;50:133–42. doi: 10.1111/j.1469-7610.2008.02011.x. [DOI] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, Marsh AA. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71:627–36. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Jurkowitz IT, Schechter JC, Yu HH, Pine DS, Blair RJ. Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research. 2011;194:279–86. doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology. 2012;63:129–51. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ. The impact of processing load on emotion. Neuroimage. 2007;34:1299–309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DG, Richell RA, Leonard A, Blair RJ. Emotion at the expense of cognition: psychopathic individuals outperform controls on an operant response task. Journal of Abnormal Psychology. 2006;115:559–66. doi: 10.1037/0021-843X.115.3.559. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. Journal of Neuroscience. 2011;31:17348–57. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: Developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology. 2009;21:913–938. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: application to assessment of externalizing psychopathology. Journal of Abnormal Psychology. 2013;122:902–16. doi: 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences U S A. 2002;99:11458–63. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28:249–55. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose A, Bijttebier P, Decoene S, Claes L, Frick PJ. Assessing the affective features of psychopathy in adolescence: a further validation of the inventory of callous and unemotional traits. Assessment. 2010;17:44–57. doi: 10.1177/1073191109344153. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends in Neuroscience. 2006;29:116–24. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJ, Dadds MR, Cecil CA, Lockwood PL, Hyde ZH, De Brito SA, Viding E. Neural responses to fearful eyes in children with conduct problems and varying levels of callous-unemotional traits. Psychological Medicine. 2014;44:99–109. doi: 10.1017/S0033291713000482. [DOI] [PubMed] [Google Scholar]
- Talairach, Tournoux . Co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Thomas LA, Hall JM, Skup M, Jenkins SE, Pine DS, Leibenluft E. A developmental neuroimaging investigation of the change paradigm. Developmental Science. 2011;14:148–61. doi: 10.1111/j.1467-7687.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, McCrory EJ. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. American Journal of Psychiatry. 2012;169:1109–16. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- White SF, Cruise KR, Frick PJ. Differential correlates to self-report and parent-report of callous-unemotional traits in a sample of juvenile sexual offenders. Behavioral Science & the Law. 2009;27:910–28. doi: 10.1002/bsl.911. [DOI] [PubMed] [Google Scholar]
- White SF, Fowler KA, Sinclair S, Schechter JC, Majestic CM, Pine DS, Blair RJ. Disrupted expected value signaling in youth with disruptive behavior disorders to environmental reinforcers. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:579–88. e9. doi: 10.1016/j.jaac.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, Sinclair S, Pine DS, Blair RJ. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. American Journal of Psychiatry. 2012;169:750–8. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Pope K, Sinclair S, Fowler KA, Brislin SJ, Williams WC, Pine DS, Blair RJ. Disrupted expected value and prediction error signaling in youths with disruptive behavior disorders during a passive avoidance task. American Journal of Psychiatry. 2013;170:315–23. doi: 10.1176/appi.ajp.2012.12060840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–30. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


