Abstract
In the present study, we investigated the fumigant potential of five edible essential oils (EOs) against Sitophilus oryzae and their phytochemical residues in treated grains. Among the tested EOs, peppermint oil proved significantly effective (P ≤ 0.05) on S.oryzae at 400 μl/L air concentration, inducing 83 and 100% mortalities in with-food and without-food conditions respectively over 72 h exposure. In addition, it was also observed that the binary mixtures of peppermint + lemon oil (1:1 ratio) produced an equivalent effect to that of peppermint oil alone treatments. The phytochemical residue analysis by GC-MS revealed the presence of six compounds upon 72 h exposure to EOs. Further, the analysis of physico-chemical properties of the compounds indicated a positive correlation between polar surface area (PSA) and its residual nature. The residue levels of eugenol were significantly elevated corresponding to its high PSA value (29) in clove and cinnamon oils. On the other hand, the compounds with zero PSA value imparted very less or no (D-Limonene, caryophyllene, pinene and terpinolene) residues in treated grains. With respect to the most active peppermint oil, L-menthone, menthyl acetate and eucalyptol residues were at 67, 41 and 23% levels respectively. The outcome of the present study indicate the peppermint oil as a potent fumigant against S. oryzae, and although the residues of phytochemicals in treated grains is higher; they belong to the generally recognised as safe (GRAS) status leaving no harmful effect.
Introduction
Plant essential oils (EOs) are considered as alternatives to conventional pesticides and have been well documented for their toxicity on stored product insect pests. Nearly, 3000 EOs has been reported from plants and about 10% of oils are commercially used as flavour and fragrants [1]. Naturally, EOs are derived from different parts of plant tissues, which play a vital role in plant defense system [2–3]. The EOs comprises of complex mixture of phytochemicals belonging to multiple groups and is responsible for various bioactivities in target insect pests. Among the phytochemicals in EOs, terpenes are known to have high vapour pressure and can easily diffuse in air at normal atmosphere [4]. Monoterpenes (C10H16) are volatile in nature and are responsible for fumigant actions in EOs [5]. Many studies have demonstrated the fumigant action of monoterpenes (e.g., limonene, menthone, eugenol, eucalyptol and menthol) against a range of insect pests [6–9].
Most recently, Isman and Grieneisen [10] stated that insecticide research on essential oils has increased nearly 15% during the past decade. However, no information is available in the literature regarding the commercialization of EO based fumigants for stored product insect control. In India, phytochemical based pesticides for commercial use are very less and EO based fumigants are nil as per Central Insecticides Board and Registration Committee [11]. Thus, alternative strategies and adequate data are required to formulate EO based fumigants. Rajendran and Sriranjini [12] had suggested that, further studies are required to understand the phytochemical residue/sorption levels on different food commodities and in EOs mixtures to validate the potentiality of EO based fumigants for stored product insect pest control. However, very less reports are available currently on the phytochemical residual analysis in EO fumigated commodities considering their high molecular weight and low vapour pressure. Similarly, the data on commercial use of EOs as food preservative agents remains unclear in the context of phytochemical residues and their toxicities [13].
The rice weevil, Sitophilus oryzae (L.) is one of the most destructive and cosmopolitan insect pest of stored cereals worldwide. Presently, phosphine is predominantly used across the globe to eradicate this pest species effectively from the infested commodities [14]. Due to resistance development in insects, the target concentration of phosphine required to achieve 100% insect control has increased from 80 ppm to 1000 ppm during the years 1992 to 2001 [15]. Reddy et al. [16] stated that, complete mortality of Sitophilus spp. population can be achieved at a dosage of 1 g/m3 phosphine held for seven days. Consequently, it is also known that, S. oryzae is one of the most tolerant species and had rapidly developed strong resistance to phosphine [17]. A wide range of plant based EOs have been screened for insecticidal activity targeting different modes of action on different insect species. However, the reports pertaining to phytochemical residue analysis on fumigated commodities still remains less. Therefore, the present investigation was undertaken to validate the fumigant potential of five edible EOs viz., Cinnamon (Cinnamomum zeylanicum), Clove (Syzygium aromaticum), Lemon (Citrus limon L.), Orange (Citrus aurantium) and Peppermint (Mentha piperita) against S. oryzae and to determine their phytochemical residues in treated rice grains. The fumigant toxicity of EOs on S. oryzae was compared in with and without-food conditions and the residual levels of active ingredients were also analysed. In addition, composition and physico-chemical properties of phytochemicals have been correlated with their residual property and accordingly discussed in this article.
Materials and methods
Solvents, chemicals, essential oils and other raw materials
HPLC grade solvents, EOs of five selected plants viz., cinnamon, clove, lemon, orange and peppermint and phytochemicals (eucalyptol, neoisocarvomenthol, menthone and menthyl acetate) were procured from Sigma-Aldrich Chemicals Ltd, India. Aluminium phosphide tablets ‘Quickphos’ (United Phosphorus Ltd., Mumbai, India) was obtained from commercial pesticide supplier. Rice grains (Raw rice, MR-gold variety) used for the experiment was procured from Local Super Market, Mysore, Karnataka, India. The obtained rice grains were held at -20°C in deep freezer (Blue Star, HF 300 model) for a week to disinfest any field carried infestation. The typical moisture content of the rice grains used for the experiment was about 12%.
Insect culture
Insect cultures of rice weevil, S. oryzae was regularly maintained in the insectary unit (with controlled condition 30±2°C, 75±5% humidity and 13:11—light:dark photoperiod) in Food Protectants and Infestation Control department at CSIR-Central Food Technological Research Institute, Mysore. A group (approximately 1000 individuals) of S. oryzae adults obtained from the stock culture were introduced into 2 L capacity individual glass jars containing 1 Kg rice grains. The insect culture was under regular observation until enough adults were developed. From this sub culture, adults of similar age groups were obtained and used for fumigant toxicity studies.
Fumigant bioassays
Individual EOs, phytochemicals and binary mixtures of EOs were performed in fumigant bioassays. Glass tubes (28 x 114 mm; 50 ml capacity) fitted with glass stoppers were used for performing the fumigation bioassays. Whatmann No. 1 filter paper strips (20 mm square) were prepared and pasted to the lower side of stopper. The top layer of each tube (about one inch length from the filter paper) was lined with Insect-A-slip to avoid insects coming in direct contact with the filter paper strips. All EOs, phytochemicals and binary mixtures were evaluated for fumigant toxicity both in with and without-food conditions. For fumigant bioassays under with-food conditions, 5 gm of rice was added to each tube. Ten individual adults of 15 days old were released into each tube. Among them, three highly active EOs were chosen to study the effect of binary mixtures of EO combinations in 1:1 ratio. Based on the individual EO fumigant bioassay results, major phytocompounds from the most active EO was selected for further studies. Different concentrations viz., 20, 100, 200, 400 μl/L air of EOs (including binary mixtures i.e., peppermint+lemon, peppermint+orange and lemon+orange) and phytocompound samples were loaded onto the filter paper. After loading each test concentrations, the open end of the individual tube was held with stoppers and further sealed with thin polythene films to make it air tight. Phosphine gas was used as a positive control for the phytocompound fumigant bioassay. Phosphine gas generation and fumigant dosing at various concentrations (20, 100, 200, 400 μl/L air) was carried out by following Manivannan et al., method [14]. Three sets were maintained for each of the treatment to observe mortalities at 24, 48 and 72 h exposures respectively. Likewise, the entire experiment was repeated in five replications.
Phytochemical residue extraction
After 72 h exposure to respective EOs, the treated rice samples were extracted from the tubes and were subjected to phytochemical residue analysis. The fumigated rice grains obtained from all the five replicates were pooled together for phytochemical residue analysis. Accordingly, twenty-five grams of rice in each concentration was soaked in methanol at 1:3 (rice: methanol; w/v) ratio. The residual extract of each sample was collected and filtered through Whatmann No. 1 filter paper. Likewise, the extraction procedure was repeated thrice for each test sample. Obtained residual extracts were concentrated using a rotary vacuum evaporator. The final concentrates of the phytochemical extracts were stored in deep freezer (-20°C) for further GC-MS analysis.
GC-MS analysis
The GC-MS analysis was performed on a Perkinelmer system equipped with Turbo mass Gold mass spectrometer (Norwalk, CT. USA). Elite-5 capillary column (30 m x 0.25 mm, 0.25 μm film thickness) was used for analysis. Helium served as the carrier gas and all the samples were analyzed by the following specifications; Initial temp 40°C for 2 min, ramp 5°C/min to 290°C, Inj = 250°C, Volume = 1 μL, Split = 10:1, Delay: 5.00 min, Transfer Temp = 200°C, Source Temp = 180°C, Scan: 29 to 400Da. Compounds were identified by comparison of their respective mass spectra, retention indices (Kovats index) and above 40% of relative abundance of acceptance match criteria with those of standards and by comparing with the NIST mass spectral data system/library.
Data processing and statistical analysis
After the fumigant bioassays were complete, the per cent corrected mortalities were estimated following Abbot’s [18] formula for each of the treatment and represented along with their mean and standard error; percentage corrected mortality = (percentage mortality in treatment—percentage mortality in control/100—percentage mortality in control) x 100. One-way analysis of variance (ANOVA) with Tukey’s multiple range tests were performed for the mortality data to determine the significant difference between treatments and to identify the effective treatment at P ≤ 0.05 for further binary mixture studies. Lethal concentration for 50% mortality (LC50) was estimated for 72 h exposure for all the EOs and binary mixtures by probit analysis [19]. ANOVA and probit analysis were performed using SPSS statistical software (version 16.0). From the GC-MS data, relative concentration (RLC) and residue concentration (RSC) of each component in percentage was calculated by following formulas: RLC = (Component area / Total area) x 100; RSC = [(Sample area x Volume of sample) / (Reference area x Volume of reference)] x 100.
Results
Response of S. oryzae to EO treatments in without-food conditions
The results indicated that all the tested EOs in without-food condition exhibited concentration vs time dependent activities on S. oryzae (Table 1). After 24 h exposure, no or remarkable mortality was observed in any of the treatments, except for orange and peppermint oils which recorded 40 and 34 per cent mortalities at 400 μl/L air concentration. A minimum test concentration of 100 μl/L air was required to achieve more than 10% of mortality in S. oryzae for all the EO treatments exposed for 48 h. Among the five tested EOs, peppermint, lemon and orange oils displayed significant potential activities (>95 per cent mortality) at 400 μl/L air concentration exposed to S. oryzae over 72 h exposure (P ≤ 0.05). Complete adult mortality was recorded in peppermint and lemon oils treatments at maximum test concentration and long exposures.
Table 1. Mortality of S. oryzae adults exposed to five edible essential oils at different concentrations and times under without-food conditions.
| Essential oils | Concentration (μl/L air) | |||
|---|---|---|---|---|
| 20 | 100 | 200 | 400 | |
| 24 h treatment | ||||
| Cinnamon | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Clove | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Lemon | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 6.00 ± 5.98a |
| Orange | 0.00 ± 0.00a | 0.00 ± 0.00a | 6.00 ± 3.99a | 40.00 ± 5.47b |
| Peppermint | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 34.00 ± 6.77b |
| 48 h treatment | ||||
| Cinnamon | 4.08 ± 2.49a | 8.16 ± 5.58a | 10.20 ± 4.99a | 12.24 ± 6.91a |
| Clove | 0.00 ± 0.00a | 10.20 ± 3.81a | 14.29 ± 5.19ab | 24.49 ± 6.11a |
| Lemon | 0.00 ± 0.00a | 20.41 ± 3.81ab | 57.14 ± 3.81c | 89.79 ± 6.44c |
| Orange | 2.04 ± 2.49a | 10.20 ± 4.99a | 28.57 ± 4.56b | 65.31 ± 5.19b |
| Peppermint | 0.00 ± 0.00a | 36.73 ± 4.99b | 100.00 ± 0.00d | 100.00 ± 0.00c |
| 72 h treatment | ||||
| Cinnamon | 16.33 ± 4.99ab | 22.45 ± 2.49a | 24.49 ± 2.49a | 36.73 ± 3.81a |
| Clove | 0.00 ± 0.00a | 18.37 ± 3.22a | 22.45 ± 2.49a | 44.90 ± 6.91a |
| Lemon | 32.65 ± 2.49b | 46.94 ± 6.76ab | 61.22 ± 5.94b | 100.00 ± 0.00b |
| Orange | 51.02 ± 3.41c | 59.18 ± 3.22b | 71.43 ± 3.81b | 95.92 ± 4.07b |
| Peppermint | 12.24 ± 6.11a | 77.55 ± 14.55b | 100.00 ± 0.00c | 100.00 ± 0.00b |
Each value is a mean of five replicates with standard error (Mean ± SE). Means within a column and within exposure period (h) followed by the different letters are significantly different (P≤0.05) of mortality of S. oryzae adults as determined by Tukey’s test.
Response of S. oryzae to EOs treatments in with-food conditions
Mortality of the test insects decreased in fumigant bioassays carried out under with-food conditions (Table 2) than without-food (Table 1). Similar to without-food assay, adult mortality was also dependent on the concentration and exposure. However, lemon and orange oils exhibited moderate activities (55 and 46 respectively) at higher concentration and longer exposure. Maximum mortality (83%) was observed in peppermint oil treatment at 400 μl/L air concentration over 72 h exposure. Among the tested EOs, peppermint oil showed significant activities at P ≤ 0.05 at the test concentrations from 100 to 400 μl/L air over 72 h exposure.
Table 2. Mortality of S. oryzae adults exposed to five edible essential oils at different concentrations and times under with-food conditions.
| Essential oils | Concentration (μl/L air) | |||
|---|---|---|---|---|
| 20 | 100 | 200 | 400 | |
| 24 h treatment | ||||
| Cinnamon | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 4.00 ± 2.45a |
| Clove | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Lemon | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 2.00 ± 1.99a |
| Orange | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| Peppermint | 0.00 ± 0.00a | 0.00 ± 0.00a | 2.00 ± 1.99a | 6.00 ± 2.45a |
| 48 h treatment | ||||
| Cinnamon | 2.04 ± 2.49a | 4.08 ± 2.49a | 10.20 ± 3.81a | 16.33 ± 3.81a |
| Clove | 0.00 ± 0.00a | 8.16 ± 4.56ab | 12.24 ± 4.07a | 16.33 ± 2.04a |
| Lemon | 0.00 ± 0.00a | 10.20 ± 3.81ab | 20.41 ± 5.94a | 30.61 ± 3.81ab |
| Orange | 0.00 ± 0.00a | 4.08 ± 2.49a | 18.37 ± 3.22a | 20.41 ± 3.81a |
| Peppermint | 16.33 ± 3.81b | 20.41 ± 3.81b | 26.53 ± 2.04a | 36.73 ± 4.99b |
| 72 h treatment | ||||
| Cinnamon | 10.20 ± 3.81a | 18.37 ± 3.22a | 20.41 ± 5.94a | 32.65 ± 4.07ab |
| Clove | 8.16 ± 6.44a | 20.41 ± 3.81a | 24.49 ± 2.49ab | 26.53 ± 2.04a |
| Lemon | 28.57 ± 3.22bc | 42.86 ± 4.07b | 44.89 ± 2.49c | 55.10 ± 5.19c |
| Orange | 16.33 ± 3.81ab | 24.49 ± 2.49a | 38.78 ± 3.22bc | 46.94 ± 3.81bc |
| Peppermint | 38.78 ± 3.22c | 59.18 ± 4.56c | 65.31 ± 4.07d | 83.67 ± 2.49d |
Each value is a mean of five replicates with standard error (Mean ± SE). Means within a column and within exposure period (h) followed by the different letters are significantly different (P≤0.05) of mortality of S. oryzae adults as determined by Tukey’s test.
Fumigant toxicities of phytocompounds of most active peppermint oil on S. oryzae
The fumigant toxicities of selected four phytocompounds on S. oryzae are depicted in Table 3. Complete mortality (100%) of S. oryzae was observed in eucalyptol and menthone treatments at 200 and 400 μl/L air concentrations over 72 h exposure under without-food conditions. In with-food condition, more than 80% mortality was recorded for eucalyptol and menthone treatments at the highest test concentrations and maximum exposure. When compared to phosphine treatment (positive control), eucalyptol and menthone showed significant activities at P ≤ 0.05 in without and with-food conditions for 400 μl/L air concentration at 72 h exposure. Followed by eucalyptol and menthone, menthyl acetate also exhibited fumigant activities. No significant mortality was noticed for neoisocarvomenthol treatments over 24 h exposure, either in without-food or with-food condition.
Table 3. Mortality of S. oryzae adults exposed to selected four phytocompounds at different concentrations and times under with and without-food conditions.
| Condition | Compounds | Concentration (μl/L air) | |||
|---|---|---|---|---|---|
| 20 | 100 | 200 | 400 | ||
| Without-food | 24 h treatment | ||||
| Eucalyptol | 0.00 ± 0.00a | 9.09 ± 6.38a | 87.88 ± 5.88c | 95.96 ± 4.03d | |
| N. Menthol* | 0.00 ± 0.00a | 5.05 ± 2.47a | 9.09 ± 4.51a | 11.11 ± 5.88ab | |
| Menthone | 0.00 ± 0.00a | 1.01 ± 2.02a | 3.03 ± 4.03a | 7.07 ± 3.77a | |
| M. Acetate# | 15.15 ± 6.04b | 21.21 ± 8.67a | 39.39 ± 5.52b | 47.47 ± 4.94c | |
| Phosphine | 0.00 ± 0.00a | 6.00 ± 2.45a | 12.00 ± 3.74a | 32.00 ± 3.16bc | |
| 48 h treatment | |||||
| Eucalyptol | 0.00 ± 0.00a | 53.54 ± 5.14d | 100.0 ± 0.00c | 100.0 ± 0.00c | |
| N. Menthol* | 7.07 ± 5.88ab | 13.13 ± 2.47a | 23.23 ± 5.14a | 27.27 ± 3.77a | |
| Menthone | 0.00 ± 0.00a | 37.37 ± 5.88bc | 71.72 ± 7.41b | 95.96 ± 2.47c | |
| M. Acetate# | 19.19 ± 4.51b | 23.23 ± 4.03ab | 59.60 ± 3.19b | 61.62 ± 11.67b | |
| Phosphine | 8.00 ± 5.82ab | 32.00 ± 3.74ab | 52.00 ± 5.82b | 66.00 ± 3.99b | |
| 72 h treatment | |||||
| Eucalyptol | 10.20 ± 9.88a | 69.39 ± 3.22b | 100.0 ± 0.00c | 100.0 ± 0.00b | |
| N. Menthol* | 14.29 ± 8.28a | 32.65 ± 6.91a | 42.86 ± 2.49a | 51.02 ± 4.99a | |
| Menthone | 28.57 ± 7.20a | 65.31 ± 5.19b | 100.0 ± 0.00c | 100.0 ± 0.00b | |
| M. Acetate# | 36.73 ± 7.48a | 51.02 ± 5.94ab | 69.39 ± 4.56b | 87.76 ± 4.99b | |
| Phosphine | 13.13 ± 2.47a | 65.66 ± 5.14b | 73.74 ± 2.47b | 85.86 ± 4.03b | |
| With-food | 24 h treatment | ||||
| Eucalyptol | 0.00 ± 0.00a | 0.00 ± 0.00a | 9.09 ± 5.52a | 89.89 ± 7.81c | |
| N. Menthol* | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Menthone | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| M. Acetate# | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Phosphine | 2.00 ± 2.00a | 8.00 ± 3.74a | 34.00 ± 5.09a | 42.00 ± 5.82b | |
| 48 h treatment | |||||
| Eucalyptol | 0.00 ± 0.00a | 5.05 ± 4.03a | 31.31 ± 5.88c | 97.98 ± 2.02d | |
| N. Menthol* | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Menthone | 0.00 ± 0.00a | 11.11 ± 3.77a | 55.56 ± 2.47d | 73.74 ± 4.03c | |
| M. Acetate# | 3.30 ± 2.49a | 7.07 ± 2.02a | 15.15 ± 4.03b | 21.21 ± 3.77b | |
| Phosphine | 6.00 ± 2.45a | 40.00 ± 4.46b | 48.00 ± 3.74d | 62.00 ± 5.82c | |
| 72 h treatment | |||||
| Eucalyptol | 6.12 ± 3.81a | 24.49 ± 9.45b | 87.75±4.99cd | 100.0 ± 0.00c | |
| N. Menthol* | 0.00 ± 0.00a | 0.00 ± 0.00 a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Menthone | 14.29 ± 5.19a | 42.86 ± 6.91bc | 100.0 ± 0.00d | 100.0 ± 0.00c | |
| M. Acetate# | 8.16 ± 3.22a | 28.57 ± 1.77b | 38.78 ± 4.56b | 42.86 ± 8.28b | |
| Phosphine | 16.00 ± 5.09a | 68.00 ± 6.62c | 80.00 ± 4.46c | 88.00 ± 2.00c | |
*Neoisocarvomenthol;
#Menthyl acetate;
Each value is a mean of five replicates with standard error (Mean ± SE). Means within a column and within exposure period (h) followed by the different letters are significantly different (P≤0.05) of mortality of S. oryzae adults as determined by Tukey’s test.
Response of S. oryzae to binary mixtures of EOs
The results of the binary mixtures of EOs from lemon, orange and peppermint oils tested against the adults of S. oryzae are presented in Table 4. All the tested binary mixtures of EOs expressed mortalities following 24 h exposure. Insect mortalities decreased in with-food assay compared to the without-food assay. Peppermint+lemon oil mixture displayed promising activities compared to other binary mixtures exposed for 72 h. The obtained mortalities over exposure to the test concentration of 400 μl/L air for 48 and 72 h exposure was significantly superior (P ≤ 0.05) over the other binary mixtures, resulting in 95 to 100% mortality.
Table 4. Mortality of S. oryzae adults exposed to binary mixtures of edible essential oils at different concentrations and times.
| Condition | Essential oils*(1:1) | Concentration (μl/L air) | |||
|---|---|---|---|---|---|
| 20 | 100 | 200 | 400 | ||
| Without-food | 24 h treatment | ||||
| Oe+Ln | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 2.00 ± 1.99a | |
| Pt+Oe | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Pt+Ln | 0.00 ± 0.00a | 0.00 ± 0.00a | 12.00 ± 5.82a | 30.00 ± 4.46b | |
| 48 h treatment | |||||
| Oe+Ln | 0.00 ± 0.00a | 12.24 ± 2.49a | 28.57 ± 3.22a | 38.78 ± 3.22a | |
| Pt+Oe | 2.04 ± 2.49ab | 12.24 ± 4.07a | 38.78 ± 3.22a | 51.02 ± 3.81a | |
| Pt+Ln | 14.29 ± 5.19b | 32.65 ± 2.49b | 36.73 ± 5.94a | 95.92 ± 4.07b | |
| 72 h treatment | |||||
| Oe+Ln | 6.12 ± 3.81a | 20.41 ± 2.04a | 30.61 ± 2.04a | 53.06 ± 2.49a | |
| Pt+Oe | 10.20 ± 3.81a | 24.49 ± 2.49a | 40.82 ± 3.81a | 61.22 ± 5.94a | |
| Pt+Ln | 18.37 ± 3.22a | 36.73 ± 3.81b | 69.39 ± 4.56b | 100.00 ± 0.00b | |
| With-food | 24 h treatment | ||||
| Oe+Ln | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Pt+Oe | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| Pt+Ln | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | |
| 48 h treatment | |||||
| Oe+Ln | 2.04 ± 2.49a | 12.24 ± 2.49a | 20.41 ± 3.81a | 26.53 ± 3.81a | |
| Pt+Oe | 10.20 ± 3.81a | 28.57 ± 3.22b | 40.82 ± 3.81b | 51.02 ± 3.81b | |
| Pt+Ln | 26.53 ± 2.04b | 30.61 ± 2.04b | 36.73 ± 3.81b | 67.35 ± 2.04c | |
| 72 h treatment | |||||
| Oe+Ln | 10.20 ± 2.04a | 22.45 ± 2.49a | 28.57 ± 3.22a | 53.06 ± 5.19a | |
| Pt+Oe | 22.45 ± 2.49b | 34.69 ± 2.49a | 55.10 ± 2.49b | 61.22 ± 3.81a | |
| Pt+Ln | 48.98 ± 3.22c | 63.27 ± 5.19b | 69.39 ± 3.22c | 85.71 ± 5.19b | |
*Oe—Orange oil; Ln—Lemon oil; Pt—Peppermint oil.
Each value is a mean of five replicates with standard error (Mean ± SE). Means within a column and within exposure period (h) followed by the different letters are significantly different (P≤0.05) of mortality of S. oryzae adults as determined by Tukey’s test.
The estimated probit analysis data for S. oryzae exposed to individual EOs, phytocompounds and binary mixtures of EOs for 72 h exposure are shown in Table 5. The LC50 concentrations recorded for orange and lemon oils were less than 65 and 550 μl/L air concentration for without-food and with-food exposures respectively. Peppermint oil proved to be the potent EO fumigant, recording the least LC50 (47.8 μl/L air) and LC90 (124.4 μl/L air) values for without-food exposure compared to the other EOs. Similarly, the peppermint oil treatment also exhibited promising toxicity on S. oryzae under with-food conditions, recording least LC50 (45.2 μl/L air) and LC90 (1372.8 μl/L air) values. Moreover, the estimated fiducial limits for LC50 and LC90 concentrations, cinnamon and clove oils showed wide ranges with maximum lethal concentration values. On the other hand, the most active peppermint oil showed narrow range of fiducial limits; 32.0–66.4 and 87.2–219.6 μl/L air ranges obtained for without-food exposure and 28.0–63.4 and 732.0–3993.4 μl/L air ranges obtained for with-food exposure (LC50 and LC90 respectively). Compared to the positive control (phosphine), the phytocompounds eucalyptol and menthone showed notable LC50 and LC90 values than neoisocarvomenthol and menthyl acetate in fumigation bioassays. The LC50 and LC90 values were <55 and <150 μl/L air in without-food condition and <110 and <300 μl/L air in without-food condition for eucalyptol and menthone respectively. In the EO binary mixture bioassays, the minimal LC50 and LC90 values were noticed in peppermint+lemon oil mixture. Less than 100 μl/L air values of LC50 and LC90 values of 427.8 and 1425.4 μl/L air were obtained for peppermint+lemon oil combination in without-food and with-food exposures respectively. Narrow range of fiducial limits was obtained for peppermint+lemon oil combination than the other treatments.
Table 5. Probit analysis of mortality for S. oryzae adults exposed to individual essential oils, phytocompounds and binary mixtures of essential oils for 72 h exposure.
| Essential oils* | LC50 (μl/L) |
95% Confidence Limit (LL–UL) |
LC90 (μl/L) |
95% Confidence Limit (LL–UL) |
Slope ± SE | X2 (df) |
|---|---|---|---|---|---|---|
| Oils—Without-food | ||||||
| Cn | 3356.2 | 878.6–554252.0 | 2950159.6 | 64497.8–1.391E13 | 0.44 ± 0.07 | 66.43 (18) |
| Ce | 504.0 | 353.0–930.2 | 4169.2 | 183480.0–20693.4 | 1.39 ± 0.09 | 101.87 (18) |
| Ln | 63.0 | 34.8–96.4 | 598.6 | 318.6–2219.8 | 1.31 ± 0.07 | 255.48 (18) |
| Oe | 26.8 | 8.8–46.6 | 655.4 | 322.8–3163.6 | 0.92 ± 0.06 | 153.64 (18) |
| Pt | 47.8 | 32.0–66.4 | 124.4 | 87.2–219.6 | 3.08 ± 0.11 | 398.59 (18) |
| Oils—With-food | ||||||
| Cn | 3066.2 | 843.2–469073.8 | 609992.8 | 24003.4–3.670E11 | 0.56 ± 0.07 | 96.87 (18) |
| Ce | 3707.4 | 856.8–7012794.0 | 895645.8 | 23497.0–3.527E14 | 0.54 ± 0.07 | 112.17 (18) |
| Ln | 233.4 | 145.0–511.8 | 92794.8 | 12083.8–14224132.8 | 0.49 ± 0.06 | 50.52 (18) |
| Oe | 534.6 | 330.8–1249.8 | 38814.8 | 8851.2–750989.2 | 0.69 ± 0.07 | 53.31 (18) |
| Pt | 45.2 | 28.0–63.4 | 1372.8 | 732.0–3993.4 | 0.86 ± 0.06 | 62.95 (18) |
| Phytocompounds—Without-food | ||||||
| El | 54.2 | 39.2–71.2 | 143.0 | 106.0–222.6 | 3.04 ± 0.11 | 285.82 (18) |
| Nm | 327.8 | 187.2–1084.8 | 12522.4 | 2541.0–1437557.0 | 0.81 ± 0.07 | 162.52 (18) |
| Me | 40.6 | 28.4–54.0 | 154.6 | 112.2–246.4 | 2.21 ± 0.09 | 203.38 (18) |
| Ma | 52.8 | 27.0–81.6 | 928.2 | 451.0–4238.2 | 1.03 ± 0.06 | 167.26 (18) |
| Pe | 75.6 | 59.5–93.1 | 449.9 | 333.2–681.6 | 1.66 ± 0.07 | 87.82 (18) |
| Phytocompounds—With-food | ||||||
| El | 101.0 | 68.4–139.6 | 283.0 | 195.8–556.8 | 2.87 ± 0.11 | 460.47 (18) |
| Nm | - | - | - | - | - | - |
| Me | 65.8 | 45.6–89.4 | 207.2 | 145.6–362.4 | 2.57 ± 0.09 | 336.27 (18) |
| Ma | 472.8 | 292.4–1167.2 | 13782.8 | 3692.8–252515.0 | 0.88 ± 0.07 | 96.19 (18) |
| Pe | 66.2 | 49.5–84.3 | 370.4 | 268.7–586.1 | 1.71 ± 0.07 | 121.17 (18) |
| Oil Mixtures—Without-food | ||||||
| Oe+Ln | 406.8 | 297.4–648.2 | 5211.0 | 2385.0–19707.4 | 1.16 ± 0.08 | 69.04 (18) |
| Pt+Oe | 270.6 | 199.8–412.6 | 3533.8 | 1647.8–13543.0 | 1.15 ± 0.07 | 90.24 (18) |
| Pt+Ln | 85.6 | 61.8–114.2 | 427.8 | 286.2–819.0 | 1.84 ± 0.07 | 204.59 (18) |
| Oil Mixtures—With-food | ||||||
| Oe+Ln | 463.6 | 315.8–857.4 | 10448.2 | 3760.4–67018.4 | 0.95 ± 0.07 | 66.23 (18) |
| Pt+Oe | 173.4 | 133.8–234.4 | 6396.2 | 2867.4–23189.2 | 0.82 ± 0.06 | 40.72 (18) |
| Pt+Ln | 24.6 | 8.0–43.2 | 1425.4 | 604.8–9509.8 | 0.73 ± 0.06 | 93.03 (18) |
*Cn—Cinnamon; Ce—Clove; Oe—Orange oil; Ln—Lemon oil; Pt—Peppermint oil; El—Eucalyptol; Nm—Neoisocarvomenthol; Me—Menthone; Ma—Menthyl acetate; Pe—Phosphine
Phytochemical residue of EO compounds correlating with the polar surface area
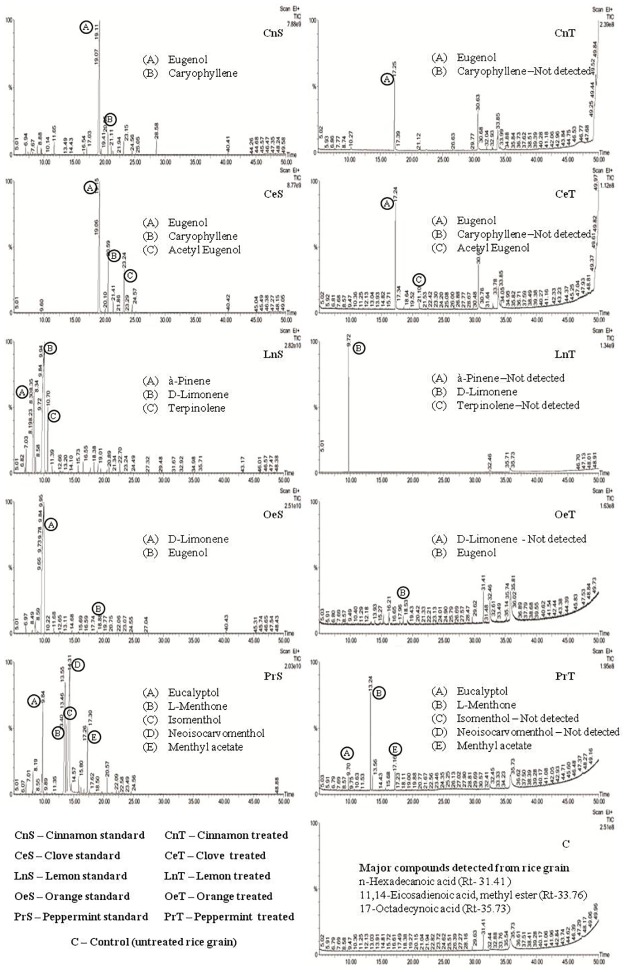
Phytochemical composition and RLC of compounds obtained from the tested EOs are presented in Table 6. GC-MS analysis revealed that, cinnamon, clove, orange, lemon and peppermint oils contained a total of 27, 17, 25, 23 and 25 compounds respectively. The following are the major compounds identified in the selected EOs; eugenol detected in cinnamon (76.76%) and clove (74.55%) oils, D-limonene in orange (93.48%) oil, D-limonene and α-pinene in lemon (66.60 and 17.30% respectively) oil, and neoisocarvomenthol, 1-menthone and eucalyptol in peppermint (40.55, 27.62 and 10.61% respectively) oil. The phytochemicals were classified into five categories based on the RLC levels (I = 0–5%, II = 6–20%, III = 21–45%, IV = 46–70%, V = 71–100%) in EOs for comparative analysis and presented in Table 7. No RLC value is depicted in the Table for the EO with level I, except for eugenol in orange oil which showed reasonable traces of RSC in the treated samples. The results suggest that the RSC values did not correspond to their respective RLC levels recorded in the study. Over all, about six compounds were only detected as residues in the fumigated rice grains. Menthone, menthyl acetate and eucalyptol residues were detected in peppermint oil treatments. While, eugenol residue was detected in cinnamon, clove and orange oil treatments and D-limonene and acetyl eugenol in lemon and clove oil treatments respectively. Menthone showed maximum RSC value (67.28%) followed by menthyl acetate, eucalyptol, eugenol, acetyl eugenol and D-limonene with 41.77, 23.50, 18.86 and 1.57% RSC’s respectively. While comparing the RSC of a compound with its total composition in the EO, eugenol showed maximum RSC (about 15% in clove and 20% in cinnamon oils). Fig 1 shows GC MS chromatogram of residual compounds detected in rice grains fumigated with peppermint oil. Physico-chemical properties of phytochemicals of EOs were compared with two conventional fumigants, phosphine and methyl bromide (S1 Table). RSC level was the least or nil for the compounds having zero polar surface area (PSA) and no remarkable variation was observed between the RSC and physico-chemical properties of the compounds except PSA.
Table 6. Relative concentrations of phytochemicals estimated from five edible essential oils.
| RT | Compound name | Relative concentration (%)* | ||||
|---|---|---|---|---|---|---|
| Cn | Ce | Ln | Oe | Pr | ||
| 5.01 | 1,2-Propanediamine | - | 0.03 | - | - | - |
| 5.06 | Hydrazinecarboxamide | 0.28 | 0.41 | - | 0.11 | - |
| 6.75 | β-Thujene | 0.10 | - | - | 0.38 | - |
| 6.77 | Amphetamine-3-methyl | - | 0.05 | - | - | - |
| 6.93 | à-Pinene | 1.53 | - | 17.30 | 0.82 | 1.75 |
| 6.99 | Acetohydrazide | - | 0.04 | - | - | - |
| 7.33 | Camphene | 0.38 | - | 0.06 | - | - |
| 7.46 | Borane carbonyl | - | 0.03 | - | - | - |
| 7.67 | Benzaldehyde | 0.20 | - | - | - | - |
| 8.01 | á-Phellandrene | 1.14 | - | - | 0.53 | 0.11 |
| 8.49 | á-Myrcene | 0.13 | - | 1.86 | 2.48 | 0.07 |
| 8.81 | Octanal | - | - | - | 0.27 | - |
| 8.93 | 2,6-Octadien-1-ol, 2,7-dimethyl- | - | - | 0.07 | - | - |
| 9.02 | 3-Carene | 0.08 | - | - | 0.20 | - |
| 9.22 | Terpinolene | 0.22 | - | 9.43 | - | - |
| 9.46 | o-Cymene | 0.98 | - | 0.12 | - | 0.12 |
| 9.58 | Cyclohexene,1-methyl-5-(1-methylethenyl)-,(R) | 0.79 | - | - | - | - |
| 9.65 | D-Limonene | - | 0.06 | 66.60 | 93.48 | 1.70 |
| 9.84 | Eucalyptol | 0.13 | - | - | - | 10.61 |
| 10.27 | á-Ocimene | - | - | 0.09 | - | - |
| 10.49 | ç-Terpinene | - | - | - | - | 0.07 |
| 11.64 | Linalool | 2.50 | - | - | 0.38 | - |
| 11.77 | Butanoic acid, 2-methyl-, 2-methylbutyl ester | - | - | - | - | 0.04 |
| 11.79 | Nonanal | - | - | 0.09 | 0.03 | - |
| 12.65 | Limonene oxide, cis- | - | - | 0.10 | 0.13 | - |
| 12.77 | Limonene oxide, trans- | - | - | 0.08 | 0.08 | - |
| 13.00 | 1-Methoxy-1,3-cyclohexadiene | - | - | 0.05 | - | |
| 13.19 | Citronellal | - | - | 0.04 | 0.05 | - |
| 13.46 | L-Menthone | - | - | - | - | 27.62 |
| 13.78 | Isomenthol | - | - | - | - | 6.31 |
| 14.30 | Neoisocarvomenthol | - | - | - | - | 40.55 |
| 14.45 | trans-p-Mentha-2,8-dienol | - | - | - | 0.02 | - |
| 14.57 | à-Terpineol | - | - | - | - | 0.53 |
| 14.67 | Decanal | - | - | - | 0.22 | - |
| 15.12 | Pentanoic acid, 4-methyl-, 1-buten-1-yl ester | - | - | - | - | 0.06 |
| 15.79 | Pulegone | - | - | - | - | 0.88 |
| 15.80 | Carvone | - | - | - | 0.09 | 0.08 |
| 15.82 | Cyclopentane,1-methyl-2-acetyl-3-(1-methylethenyl)- | - | - | 0.07 | - | - |
| 16.18 | Piperitone | - | - | - | - | 0.33 |
| 16.54 | Cinnamaldehyde, (E)- | 0.87 | - | - | - | - |
| 16.55 | Citral | - | - | 1.11 | 0.05 | - |
| 17.03 | trans-Isosafrole | 1.05 | - | - | - | - |
| 17.26 | Menthyl acetate | - | - | - | - | 7.45 |
| 17.75 | 3,7-Nonadien-2-one, 4,8-dimethyl- | - | - | 0.96 | - | - |
| 19.26 | 2-Ethylbutyric acid, 3-phenylpropyl ester | 0.08 | - | - | - | - |
| 18.34 | Geranyl methyl ether | - | - | - | 0.02 | - |
| 18.88 | Eugenol | 76.76 | 74.55 | 0.08 | 0.26 | 0.07 |
| 19.00 | Neryl Acetate | - | - | 0.49 | - | - |
| 19.41 | α-Copaene | 0.72 | - | - | - | - |
| 19.41 | à-Cubebene | - | 0.35 | - | - | - |
| 19.49 | Geranyl acetate | - | - | 0.22 | - | - |
| 19.64 | (-)-á-Bourbonene | - | - | - | - | 0.10 |
| 19.73 | Germacrene D | - | - | - | 0.04 | - |
| 20.10 | Methyleugenol | - | 0.54 | - | - | - |
| 20.12 | Dodecanal | - | - | - | 0.03 | - |
| 20.52 | Caryophyllene | 3.58 | 9.44 | 0.20 | 0.04 | 1.02 |
| 20.77 | 1,6-Cyclodecadiene,1-methyl-5-methylene-8- (1-methylethyl)-, [S-(E,E)]- | - | - | - | - | 0.05 |
| 20.89 | Alpha-Bergamotene | - | - | 0.36 | - | - |
| 21.11 | Acetic acid, cinnamyl ester | 1.40 | - | - | - | - |
| 21.34 | cis-á-Farnesene | - | 0.01 | 0.04 | - | 0.13 |
| 21.39 | Humulene | 0.59 | 1.56 | - | - | - |
| 21.86 | cis-muurola-3,5-diene | - | 0.01 | - | - | - |
| 22.36 | Valencen | - | - | - | 0.06 | - |
| 22.52 | (-)-Mintlactone | - | - | - | - | 0.22 |
| 22.69 | á-Bisabolene | - | - | 0.58 | - | - |
| 23.07 | d-Cadinene | 0.19 | - | - | - | 0.06 |
| 23.14 | Acetyl eugenol | 2.20 | 11.28 | - | 0.03 | - |
| 23.81 | Caryophyllene oxide | 0.62 | 1.23 | - | - | 0.07 |
| 25.16 | Epoxy (1,11) humulene | 0.08 | 0.12 | - | - | - |
| 28.57 | Benzyl Benzoate | 3.02 | - | - | - | - |
| 40.41 | Piperonyl butoxide | 0.38 | 0.29 | - | 0.20 | - |
RT-Retention time,
*Cn—Cinnamon oil; Ce—Clove oil; Oe—Orange oil; Ln—Lemon oil; Pt—Peppermint oil.
Table 7. Phytochemical residues detected in essential oils fumigated rice grains.
| Compound name | Name of essential oil | RLC level* | RSC obtained / compound | RSC obtained / oil |
|---|---|---|---|---|
| Acetyl eugenol | Clove | II | 12.16 ± 3.51 | 1.37 ± 0.39 |
| Caryophyllene | Clove | II | nd | nd |
| Eucalyptol | Peppermint | II | 23.50 ± 4.87 | 0.25 ± 0.05 |
| Eugenol | Cinnamon | V | 17.37 ± 5.67 | 20.08 ± 7.99 |
| Clove | V | 18.86 ± 4.04 | 14.06 ± 3.01 | |
| Orange | I | 13.43 ± 4.34 | 0.04 ± 0.01 | |
| Isomenthol | Peppermint | II | nd | nd |
| D-Limonene | Lemon | IV | 1.57 ± 0.19 | 0.10 ± 0.01 |
| Orange | V | nd | nd | |
| Neoisocarvomenthol | Peppermint | III | nd | nd |
| L-Menthone | Peppermint | III | 67.28 ± 8.79 | 1.86 ± 0.24 |
| Menthyl acetate | Peppermint | II | 41.77 ± 5.14 | 0.31 ± 0.04 |
| à-Pinene | Lemon | II | nd | nd |
| Terpinolene | Lemon | II | nd | nd |
*I = 0–5%, II = 6–20%, III = 21–45%, IV = 46–70%, V = 71–95%;
Each value is a mean of three replicates with standard error (Mean ± SE).
Fig 1. Comparison of GC-MS chromatogram of methanolic extract of fumigated rice grains with standard essential oils.
Discussion
In spite of many investigations to explore the potential of bio-fumigants for stored product insect control, the major disadvantage is the low vapor pressure and high boiling point leading to absorption/ settling of residues on the treated commodities imparting off odors. Hence, in the present study, we comparatively investigated the fumigant toxic potentialities and the residue profiles of cinnamon, clove, lemon, orange and peppermint EOs in fumigated rice grains. Insect mortality results revealed that, all the tested EOs exerted concentration dependent toxic effects on S. oryzae. Peppermint oil was regarded as highly toxic; while lemon and orange oils were reported to be moderately toxic to S. oryzae. Previously, Khani et al. [20] and Shaaya et al. [21] reported 85 and 7.5 μl/L air as the LC50 concentrations for peppermint oil treatments against S. oryzae. Whereas in the present study, LC50 values of 47.8 (LCL:UCL = 32.0–66.4) and 45.2 μl/L air (LCL:UCL = 28.0–63.4) were recorded for peppermint oil in both with and without-food conditions. The LC90 values for peppermint oil increased in with-food conditions (124.4 to 1372 μl/L air) compared to without-food conditions as similar to the other oils. The obtained slope values significantly decreased (P ≤ 0.05) in with-food conditions for all oils except for the less active cinnamon oil. Hence, it was predicted that fumigant toxicities of EOs decreased under with-food conditions indicating that a proportion of active ingredients/phytochemicals might have got absorbed/adhered onto the rice grains thereby reducing the lethality. Similarly, Rajendran and Sriranjini [22] stated that the toxicity of bio-fumigants against insect pests will be less in with-food conditions due to low vapour pressure (≤1 mmHg) and high sorption by the grains.
Based on the toxicity data of the individual oils, peppermint, orange and lemon oils were selected for the binary mixture fumigant bioassays on S. oryzae. Mortality results showed that, S. oryzae was more susceptible to peppermint with lemon oil mixture at P ≤ 0.05. This mixture expressed 100% mortality at 400 μl/L air concentration in without-food condition, which decreased to 85% mortality in with-food condition exposed for 72 h. It was observed that peppermint+ lemon oil mixture (1:1) had more fumigant action than the other two mixtures. Earlier bioactivity studies have indicated that the mixtures of essential oils are more effective than individual oils. For example, Krishnarajah et al. [23] reported that, binary mixtures of essential oils were more toxic to Sitotroga cerealella than the individual oils. This also holds good for conventional fumigants like phosphine, which showed synergistic effects on Rhyzopertha dominica when combined with CO2, and the report suggested that PH3+CO2 admixture increased the insect mortality than phosphine alone treatments [24]. While in the present study, fumigant toxicity of peppermint oil alone was relatively par to peppermint+lemon oil mixture. Similarly, Nattudurai et al. [25] observed that eucalyptus+camphor oil mixture had similar effects (LC50) on Tribolium castaneum as posed by the individual oils. In the present study, similar to the results of individual oils, the slope values for the binary mixtures greatly varied between with and without-food conditions. This strongly indicated that the toxicity of EOs is altered by the sorptive nature of the commodity.
Monoterpenoids in EOs are known to play a vital role in mitigating fumigant actions on stored product insect pests [12]. Consequently in the present study, peppermint oil showed promising activities due to the presence of major monoterpenoids such as eucalyptol and menthone. When compared to the commercial fumigant (phosphine), menthone and eucalyptol alone treatments showed significant activities at P ≤ 0.05 and were identified as active compounds in peppermint oil exhibiting fumigant activities. Likewise, orange and lemon oils offered moderate toxicities on S. oryzae attributed by the presence of D-limonene and α-pinene monoterpenoid compounds. Previously, Lee et al. [26] suggested that menthone had more fumigant potential followed by α-pinene and limonene. Later, eucalyptol was also recognised as potent fumigant against S. oryzae, T. castaneum and R. dominica as described by Lee et al. [6]. The present study also insists the fact that, not all monoterpenoids found in EOs possess fumigant actions. Though, eugenol belongs to monoterpenoid group, it displayed less activity against S. oryzae, despite having a RLC value around 75% in cinnamon and clove oils. Similarly, Liska et al. [7] also reported that eugenol was not effective as a fumigant against T. castaneum.
It is imperative that, a thorough knowledge on residual properties of EOs is essential to formulate a bio-fumigant. The present phytochemical residual analysis revealed that, only six compounds settle/adhere on the rice grains out of the total detected compounds in individual EO. Among the tested five EOs, phytochemical residues of eugenol (14–20% of RSC per oil) was remarkably higher in clove and cinnamon oils than the other oils. Further, it was also observed that RSC of phytochemicals were associated with their respective PSAs. It is known that PSA is the electrostatic potential of a substance/compound to form physical interactions between surfaces. Palm et al. [27] stated that PSA has been used for prediction of absorption of drugs in pharmaceutical industry. Accordingly, in the present study, we propose a positive correlation between the PSA values of phytocompounds from EOs with their residual nature. For instance, the residues of major compounds like acetyl eugenol, eucalyptol, eugenol, L-menthone and menthyl acetate were more and correlated with their respective PSA values. The phytochemical analysis of eugenol showed notable RLC and corresponding RSC levels in cinnamon and clove oils. While for orange oil, though the RLC level was very low for eugenol, remarkable RSC was noticed due to its high PSA value (29). Similarly, the RLC values were less for acetyl eugenol and menthyl acetate compounds, whereas the RSC values were more, mainly attributed by its high PSA values (36 and 26 respectively). Contrastingly it was observed that, though D-limonene was a major compound in orange (93%) and lemon (66%) oils, the obtained RSC was very less (0.10% of RSC per oil) in lemon and was not detected in orange oil, due to its zero PSA value. Similarly, the PSA value for the other non-residual compounds such as caryophyllene, pinene and terpinolene is zero though their RLC values were about 6–20% in the respective oils. More interestingly, PSA value and RLC level of L-menthone was less in peppermint oil when compared to eugenol in cinnamon and clove oils though the detected residues were very high (40–70%). In contrast, though PSA and RLC values were at noticeable levels for isomenthol and neoisocarvomenthol compounds they did not impart any residue in the fumigated rice grains. These facts denote the instability of the compounds and probable chemical reactions taking place during fumigations. To support this view, Belitz et al. [28], stated that terpene hydrocarbons undergo rapid autooxidation at atmospheric air in many of the essential oils. Hence it was assumed/predicted that menthol isomers (isomenthol and neoisocarvomenthol) might have autooxidized to L-menthone leading to more residue formation. In addition, it is known that isomenthol and neoisocarvomenthol were not stable when compared to menthol because of an axial methyl group and may rapidly get oxidized to menthone. Interestingly, in the present study it was noticed that insect mortality was nil for neoisocarvomenthol in with-food condition and about 50% mortality (at 400 μl/L air concentration and 72 h exposure) was observed in without-food condition. Probably, it was assumed that, in without-food condition, direct exposure/contact of oxidized neoisocarvomenthol (i.e., menthone) on insects might have induced mortality. On the other hand, in with-food condition, a proportion of oxidized neoisocarvomenthol might have settled on rice grains and the actual concentration required to induce insect mortality may be lacking. Krishnaswamy [29] described the oxidation mechanism of menthol and their isomers into menthone isomers. Furthermore, Pecar and Gorsek [30] had described the reaction kinetics of menthol getting oxidized to menthone under laboratory conditions. According to Lee et al. [6], residual level of phytocompound on the commodity is dependent on the quantity of compound being absorbed. In addition, Cartalade and Vernhet [31] stated that, maximum adsorption of phytochemical was associated to the PSA of the material. Similarly, in the present study, the compounds such as acetyl eugenol, eugenol, menthone and menthyl acetate have more PSA values subsequently imparting higher levels of residues in treated grains. Hence, it is proposed that the PSA value may determine the residual property of compounds getting adhered to the surface of commodity.
Furthermore, Reddy et al. [16] stated that sorption/residue of fumigants on commodities is mainly dependent on the moisture content of the grain, size of particle, composition of fumigant agent, exposure phase and dose. Likewise, the conventional fumigants, phosphine and methyl bromide have zero PSA value thereby the residue formation in fumigated commodities will be very less [22, 32].
Conclusions
Our study signifies that peppermint oil is a potent bio/phyto-fumigant and could be used for the control of S. oryzae under storage conditions. In addition, the study implies that peppermint oil can also be used in mixed form with lemon oil for effective fumigations. With respect to the phytochemical residues, the identified major residual compounds such as 1-menthone, menthyl acteate, eucalyptal and eugenol, and their respective oils are generally recognised as safe (GRAS) [33–36]. Accordingly, we study suggests that, peppermint and lemon oils can be considered as safer alternatives for commodity treatments. Also, the phytochemical residues could be probably removed by 24 hours aeration, as suggested by Lee et al. [6] for eucalyptol compound. Likely, the residual compounds in peppermint oil fumigated grains may also be removed upon aeration. In addition, Isman [37] reviewed that residues from edible EOs are beneficial to human health by pass through diet. Furthermore, peppermint oil is exempted from Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) for pesticide formulation in alone or in combination with other ingredients [38]. Hence, our study suggests peppermint oil as a potent bio-fumigant and could be applied with the reduced residual risk under direct exposure to commodities in alone or in mixture with lemon oil for the control of S. oryzae in stored product commodities.
Supporting information
(DOC)
Acknowledgments
We thank Prof. Ram Rajasekharan, Director, CSIR-Central Food Technological Research Institute, Mysore for the support and facilities provided for this study. This research is financially supported by Council of Scientific and Industrial Research (CSIR), New Delhi, Government of India, under the MLP-196 project. We also thank Mr. Bhavani Eswaran, CIFS, CSIR-CFTRI for his assistance in GC-MS analysis. The authors sincerely thank the anonymous reviewers for their valuable comments/suggestions on the earlier version of the manuscript and Dr. P. Prabashankar, Senior Principal Scientist, CSIR-CFTRI, Mysore and Prof. James Premdoss Clement, Jawaharlal Nehru Centre for Advanced Scientific Research, India for editing the previous version of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by Council of Scientific and Industrial Research, New Delhi, India MLP 196 (In-House) to EV.
References
- 1.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004; 94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 2.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012; 3:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Contrl. 2010; 21:1199–1218. [Google Scholar]
- 4.Dudareva N, Pichersky E, Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004; 135:1893–1902. doi: 10.1104/pp.104.049981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koul O, Walia S, Dhaliwal GS. Essential oils as green pesticides: potential and constraints. Biopestic Int. 2008; 4:63–84. [Google Scholar]
- 6.Lee BH, Annis PC, Tumaalii F. The potential of 1,8-cineole as a fumigant for stored wheat. In: Wright EJ, Webb MC, Highley E (Eds.), Stored Grain in Australia 2003, Proceedings of the Australian Postharvest Technical Conference, 25–27 June 2003, Canberra, CSIRO Stored Grain Research Laboratory, Canberra, Australia. 2003; Pp 230–234.
- 7.Liska A, Rozman, V, Kalinovic I, Ivezic M, Balicevic R. Contact and fumigant activity of 1,8-cineole, eugenol and camphor against Tribolium castaneum (Herbst). Proceedings of the 10th International Working Conference on Stored Product Protection. 2010; Pp. 716–720.
- 8.Kamatou GPP, Vermaak I, Viljoen AM, Lawrence BM. Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry. 2013; 96: 15–25. doi: 10.1016/j.phytochem.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 9.Harwood SH, Moldenke AF, Berry RE. Toxicity of Peppermint monoterpenes to the variegated cutworm (Lepidoptera: Noctuidae). J Econ Entomol. 1990; 83:1761–1767. [Google Scholar]
- 10.Isman MB, Grieneisen ML. Botanical insecticide research: many publications, limited useful data. Trends Plant Sci. 2014; 19:140–145. doi: 10.1016/j.tplants.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 11.CIBRC (Central Insecticides Board & Registration Committee) Insecticides / Pesticides Registered under section 9(3) of the Insecticides Act, 1968 for use in the Country. 2016; http://cibrc.nic.in/
- 12.Rajendran S, Sriranjini V. Plant products as fumigants for stored-product insect control. J Stored Prod Res. 2008; 44:126–135. [Google Scholar]
- 13.Prakash B, Kiran S; Essential oils: a traditionally realized natural resource for food preservation. Current Science. 2016; 110:1890–1892. [Google Scholar]
- 14.Manivannan S, Swati AP, Hemalatha P, Gisha EK, Roopa RS. Phosphine gas generated from an aluminium phosphide tablet exhibits early knock down effects on tamarind pod borer. RSC Advances. 2016; 6:90024–90030. [Google Scholar]
- 15.Rajendran S. Benchmarking—what makes a good fumigation?. In Donahaye EJ, Navarro S, Bell C, Jayas D, Noyes R, Phillips TW (Eds.), Proceedings of International Conferences of Controlled Atmosphere and Fumigation in Stored Products, Gold-Coast Australia. 8-13th August 2004. FTIC Ltd. Publishing, Isreal. 2007; Pp. 345–365.
- 16.Reddy PV, Rajashekar Y, Begum K, Leelaja BC, Rajendran S. The relation between phosphine sorption and terminal gas concentrations in successful fumigation of food commodities. Pest Manag Sci. 2007; 63:96–103. doi: 10.1002/ps.1298 [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TT, Collins PJ, Ebert PR. Inheritance and characterization of strong resistance to phosphine in Sitophilus oryzae. PLoS ONE. 2015; 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925; 18:265–267. [Google Scholar]
- 19.Finney JD. Probit analysis; Cambridge University Press: London, UK: 1971; Pp333. [Google Scholar]
- 20.Khani M, Awang RM, Omar D. Insecticidal effects of peppermint and black pepper essential oils against rice weevil, Sitophilus oryzae L. and rice moth, Corcyra cephalonica (St.). J Med Plants. 2012; 11:97–110. [Google Scholar]
- 21.Shaaya E, Kostjukovski M, Eilberg J, Sukprakarn C. Plant oils as fumigants and contact insecticides for the control of stored-product insects. J Stored Prod Res. 1997; 33:7–15. [Google Scholar]
- 22.Rajendran S, Sriranjini V. Use of fumigation for managing grain quality. Stewart Postharvest Review. 2007; 6:1–9. [Google Scholar]
- 23.Krishnarajah SR, Ganesalingam VK, Senanayake UM. Repellancy and toxicity of some plant oils and their terpene components to Sitotroga cerealella (Olivier), (Lepidoptera, Gelechiidae). Trop Sci. 1985; 25:249–252. [Google Scholar]
- 24.Manivannan S, Koshy G E, Patil SA. Response of phosphine-resistant mixed-age cultures of lesser grain borer, Rhyzopertha dominica (F.) to different phosphine-carbon dioxide mixtures. J Stored Prod Res. 2016b; 69:175–178. [Google Scholar]
- 25.Nattudurai G, Paulraj MG, Ignacimuthu S. Fumigant toxicity of volatile synthetic compounds and natural oils against red flour beetle Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J King Saud Univ—Sci. 2012; 24:153–159. [Google Scholar]
- 26.Lee SE, Lee BH, Choi WS, Park BS, Kim JG, Campbell BC. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L.). Pest Manag Sci. 2001; 57:548–553. doi: 10.1002/ps.322 [DOI] [PubMed] [Google Scholar]
- 27.Palm K, Luthman K, Ungell AL, Strandlund G, Artursson P. Correlation of drug absorption with molecular surface properties. J Pharm Sci. 1996; 85:32–9. doi: 10.1021/js950285r [DOI] [PubMed] [Google Scholar]
- 28.Belitz H-D, Grosch W, Schieberle P; Aroma compounds In Food Chemistry. Springer publications; 2009; pp 340–402. [Google Scholar]
- 29.Krishnaswamy NR. Learning organic chemistry through natural products. Resonance. 1996; 1:40–46. [Google Scholar]
- 30.Pecar D, Gorsek A. Oxidation of menthol: reaction-rate determination based on thermodynamic profiles. Chem Eng Commun. 2014; 11:1548–1554. [Google Scholar]
- 31.Cartalade D, Vernhet A. Polar interactions in Flavan-3-ol adsorption on solid surfaces. J Agri Food Chemistry. 2006; 54:3086–3094. [DOI] [PubMed] [Google Scholar]
- 32.Manivannan S. Toxicity of phosphine on the developmental stages of rust-red flour beetle, Tribolium castaneum Herbst over a range of concentrations and exposures. J Food Sci Technol. 2015; 52:6810–6815. doi: 10.1007/s13197-015-1799-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams TB, Hallagan JB, Putnam JM, Gierke TL, Doull J, Munro IC, et al. The FEMA GRAS assessment of alicyclic substances used as flavour ingredients. Food Chem Toxicol. 1996; 34:763–828. [DOI] [PubMed] [Google Scholar]
- 34.SCF (Scientific Committee on Food). Opinion of the Scientific Committee on Food on eucalyptol. European Commission Health & Consumer Protection Directorate-General. SCF/CS/FLAV/FLAVOUR/20 ADD2 Final. 2002; Pp1-10 http://europa.eu.int/comm/food/fs/sc/scf/index_en.html.
- 35.USFDA (U.S. Food and Drug Administration). Essential oils, oleoresins (solvent-free), and natural extractives (including distillates). Substances generally recognized as safe. Code of Federal Regulations. Title 26, volume. 1998; 6:485–487 https://www.libertynatural.com/info/eoinfo/FDA_EO_GRAS.htm
- 36.USFDA (U.S. Food and Drug Administration) GRAS substances SCOGS databases 2015; www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm261254.htm
- 37.Isman MB. Plant essential oils for pest and disease management. Crop Prot. 2000; 19:603–608. [Google Scholar]
- 38.USEPA (U.S. Environmental Protection Agency). Exemption of certain pesticide substances from federal insecticide, fungicide, and rodenticide act requirements, Washington DC, USA. 1996; 61:8876–8879 http://www.nj.gov/dep/enforcement/pcp/bpo/min_risk.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.