Abstract
Phospholipases A2 (PLA2s) are enzymes found throughout the animal kingdom. They hydrolyze phospholipids in the sn-2 position producing lysophospholipids and unsaturated fatty acids, agents that can damage membranes. PLA2s from snake venoms have numerous toxic effects, not all of which can be explained by phospholipid hydrolysis, and each enzyme has a specific effect. We have earlier demonstrated the capability of several snake venom PLA2s with different enzymatic, cytotoxic, anticoagulant and antiproliferative properties, to decrease acetylcholine-induced currents in Lymnaea stagnalis neurons, and to compete with α-bungarotoxin for binding to nicotinic acetylcholine receptors (nAChRs) and acetylcholine binding protein. Since nAChRs are implicated in postsynaptic and presynaptic activities, in this work we probe those PLA2s known to have strong presynaptic effects, namely β-bungarotoxin from Bungarus multicinctus and crotoxin from Crotalus durissus terrificus. We also wished to explore whether mammalian PLA2s interact with nAChRs, and have examined non-toxic PLA2 from porcine pancreas. It was found that porcine pancreatic PLA2 and presynaptic β-bungarotoxin blocked currents mediated by nAChRs in Lymnaea neurons with IC50s of 2.5 and 4.8 μM, respectively. Crotoxin competed with radioactive α-bungarotoxin for binding to Torpedo and human α7 nAChRs and to the acetylcholine binding protein. Pancreatic PLA2 interacted similarly with these targets; moreover, it inhibited radioactive α-bungarotoxin binding to the water-soluble extracellular domain of human α9 nAChR, and blocked acetylcholine induced currents in human α9α10 nAChRs heterologously expressed in Xenopus oocytes. These and our earlier results show that all snake PLA2s, including presynaptically active crotoxin and β-bungarotoxin, as well as mammalian pancreatic PLA2, interact with nAChRs. The data obtained suggest that this interaction may be a general property of all PLA2s, which should be proved by further experiments.
Introduction
Phospholipases A2 (PLA2s, phosphatidylcholine 2-acylhydrolase, EC 3.1.1.4) hydrolyze predominantly phospholipids with polyunsaturated fatty acid residue in the sn-2 position; they are therefore essential participants in lipid digestion. In addition, they are involved in a range of other cell processes including inflammation, cell proliferation and signal transduction, largely because of their phospholipolytic activity [1]. The PLA2 superfamily includes 15 groups comprising four main types including the secreted, cytosolic, calcium-independent PLA2s, and platelet activating factor acetyl hydrolase/oxidized lipid lipoprotein-associated PLA2 [2]. Group I PLA2s are present in Elapidae snake venoms as group IA and in pancreatic juices of animals as group IB. The most obvious difference between them is the absence (group IA) or the presence (group IB) of an extra amino acid fragment, known as a pancreatic loop, next to the catalytic active site. Group II PLA2s are present in Viperidae snake venoms and in the synovial fluids of animals. These PLA2s, which are secreted by venomous glands of snakes, bees and other venomous animals, manifest various toxic actions. PLA2s from snake venoms have numerous toxic effects, not all of which can be explained by phospholipid hydrolysis, and each individual enzyme may have a specific effect. Some PLA2s are characterized by potent anticoagulant activity, for example PA11 from Pseudechis australis venom [3]; others manifest strong myotoxic properties, such as Lemnitoxin from Micrurus lemniscatus venom [4]. Among numerous PLA2 effects, neurotoxic action is one of the most important. Neurotoxicity is due to the block of neuromuscular transmission and proceeds in several steps: an initial weak inhibition of acetylcholine (ACh) release; a more prolonged facilitation of ACh secretion; and then a progressive decline of transmission leading to irreversible arrest [5–7].
There are several hypotheses of the mechanism of PLA2 neurotoxic action.
Phospholipolytic damage to the presynaptic membrane potentiates fusion of ready-to release synaptic vesicles in the active zone of neuroexocytosis, and inhibits vesicle retrieval [6]; consequently the ACh store is depleted.
Interaction of PLA2s with specific proteins: binding to these receptors facilitates a local enzyme-dependent or independent action [8]; the discovery of proteins that bind PLA2 with high affinity in different tissues supports this hypothesis [9–12].
Interaction of PLA2s with intracellular Ca2+ binding proteins after endocytosis or penetration through damaged membranes causing an increase in intracellular Ca2+ concentration, both leading to mitochondrial uncoupling [7, 13].
We have earlier reported antagonistic action of eight PLA2s from the venoms of snakes of Viperidae and Elapidae families (PLA2 groups IIA and IA, respectively) on nicotinic acetylcholine receptors (nAChRs) of different types [14, 15]. These enzymes, which differ in their enzymatic activities, competed with [125I]α-bungarotoxin (α-Bgt) for binding to the muscle-type nAChRs of Torpedo californica electric organ, to human α7 nAChRs expressed in GH4C1 cell line, and to ACh-binding protein (AChBP) from Lymnaea stagnalis. When tested on isolated neurons of L. stagnalis which contain α7 similar nAChRs [16, 17], PLA2s suppressed ACh- or cytisine-evoked currents under conditions that exclude hydrolysis of membrane phospholipids. These results indicate that binding of PLA2s to nAChRs affects their function.
To ascertain whether all types of PLA2s are able to interact with nAChRs, we have studied the action of three other phospholipases i.e. presynaptically active β-bungarotoxin (β-Bgt) from Bungarus multicinctus, crotoxin (Cro) from Crotalus durissus terrificus snake venom, and non-toxic mammalian PLA2 from porcine pancreas (PP PLA2, group IIB)—in binding assay and on Lymnaea neurons. β-Bgt is a heterodimeric protein in which a group IA PLA2 and a Kunitz type serine protease inhibitor are connected by a disulfide bond [18]. Cro is also a heterodimeric protein, and consists of a weakly toxic basic group IIA PLA2 and crotapotin, a non-enzymatic, non-toxic acidic component [19]. Mammalian porcine pancreatic PP PLA2 has been shown previously to induce presynaptic block of neuromuscular transmission in a mouse hemi-diaphragm preparation although it was much weaker than snake venom PLA2s [20]. β-Bgt and Cro have been previously shown to act presynaptically in a mammalian neuromuscular junction preparation [5, 21, 22].
We found that all PLA2s tested in this work interacted with nAChRs, with IC50 values ranging from hundreds of nM to tens of μM. The data from Cro revealed the presence of two sites both in muscle-type and α7 nAChRs with affinities differing by 1–3 orders of magnitude. Thus, we conclude that presynatically active PLA2s interact with muscle type nAChRs located postsynaptically. Moreover, it is not only snake venom PLA2s that are capable of binding to nAChRs, but mammalian pancreatic PLA2 also has this ability.
Materials and methods
PLA2 from porcine pancreas (PP PLA2), Trizma-HCl, EGTA, HEPES, β-lactoglobulin, Pronase E, acetylcholine iodide, cytisine, choline chloride, and all chloride salts were purchased from Sigma (USA). RNAse was from P-L Biochemicals, Inc. (USA), soybean trypsin inhibitor from Boehringer Mannheim GmbH (Germany), cytochrome C from Ferak Berlin. Crotoxin from Crotalus durissus terrificus venom was purified as previously described [23, 24]. β-Bgt was isolated from Bungarus multicinctus venom by procedure described in [25]. Mono-iodinated (3-[125I]iodotyrosyl54)-α-Bgt (~2000 Ci/mmol) was from GE Healthcare. nAChR-enriched membranes from the electric organs of T. californica ray were kindly provided by Prof. F. Hucho (Free University of Berlin, Germany), GH4C1 cells transfected with human α7 nAChR were a gift from Eli-Lilly (USA). The expressed acetylcholine binding protein (AChBP) from L. stagnalis was kindly provided by Prof. T. Sixma (Netherlands Cancer Institute, Amsterdam, the Netherlands); the extracellular domain (ECD) of the human neuronal α9 nAChR was expressed, enzymatically deglycosylated and purified as described [26]. Plasmid pT7TS constructs of human nAChR α9 and α10 subunits were kindly provided by Prof. D.J.Adams (University of Wollongong, Wollongong, Australia).
Electrophysiological measurements
Identified L. stagnalis giant neurons
Pond snails L. stagnalis (3–4 cm long) were collected from lakes near the Oka River (Pushchino, Moscow region) and kept in tap water at 4–6°C until use. L. stagnalis has the conservation status “Least Concerned” and does not require a special permission for use. The experiments were carried out on identified giant neurons (LP1,2,3, RPV2,3; according to the map of L. stagnalis ganglia [27]) isolated from the left and right parietal ganglia as described [17]. Neurons were internally perfused with internal solution (in mM: CsCl 95, CaCl2 0.3, EGTA 2, HEPES 10, pH 7.2) and voltage-clamped at –60 mV [28]. Constant flow of the external solution (in mM: NaCl 92, KCl 1.6, BaCl2 2, MgCl2 1.5, Trizma-HCl 4, pH 7.6; Ba2+ was used instead of Ca2+ to avoid phospholipolytic action of the PLA2s on the cell membrane) was maintained, except the time of application of an agonist or neuron incubation with PLA2s. In the experiments with proteins lacking phospholipolytic activity, the CaCl2-containing extracellular solution was used. Acetylcholine (ACh), cytisine (Cyt) or choline were applied on the whole cell surface using 4 s pulses with intervals not less than 6 min. Agonist-induced currents were monitored and digitized with a patch-clamp amplifier A-M Systems (USA), the data acquisition was performed using Digidata1200 B interface and pClamp6 software (Axon Instruments Inc., USA). Aliquots of solutions of PLA2s in water were kept in the refrigerator and diluted using extracellular solution to the desired concentration immediately before use.
The effects of PLA2s were determined by measuring the changes in peak current amplitude induced by the agonist after 5-min incubation with PLA2 compared to the control responses before treatment and after prolonged washing. IC50 values were calculated using Sigma plot 11.0 software using the Hill plot analysis.
Xenopus oocytes
Plasmid pT7TS constructs of human nAChR α9 and α10 subunits were linearized with XbaI restriction enzymes (NEB, USA). Linearized plasmid constructs were subjected to in vitro cRNA transcription using T7 mMessagemMachine® transcription kit (AMBION, USA).
Mature Xenopus laevis female frogs used in this study were obtained commercially (NASCO, Fort Atkinson, WI, USA) and housed in a facility with 12:12 hours light:dark cycles, 18–20°C ambient temperature. Animals were fed twice a week and maintained according to supplier recommendations (https://www.enasco.com/page/xen_care). All the appropriate actions were taken to minimize discomfort to frogs. The World Health Organization’s International Guiding Principles for Biomedical Research Involving Animals were followed during experiments on animals. Oocytes were prepared from mature female frogs by following the standard procedure described elsewhere [29]. Stage V-VI oocytes were defolliculated with 2 mg/mL collagenase Type I (Life Technologies, USA) at room temperature (21−24°C) for 2 h in ND96 solution composed of (in mM) 96 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2 and 5 HEPES at pH 7.4. Oocytes were injected with 9.2 ng of human nAChR α9 and α10 cRNA (in a ratio 1:1) and incubated at 18°C in Barth’s solution composed of (in mM) 88 NaCl, 1.1 KCl, 2.4 NaHCO3, 0.3 Ca(NO3)2, 0.4 CaCl2, 0.8 MgSO4 and 15 HEPES-NaOH at pH 7.6, supplemented with 40 μg/mL gentamicin and 100 μg/mL ampicillin for 4 days before electrophysiological recordings.
Two-electrode voltage clamp recordings at a holding potential of -60 mV were made using turbo TEC-03X amplifier (Npi electronic, Germany) and WinWCP recording software (University of Strathclyde, UK). Oocytes were briefly washed with Ba2+ Ringer’s solution (in mM: 115 NaCl, 2.5 KCl, 1.8 BaCl2, 10 HEPES at pH 7.2) followed by 3 applications of 25 μM ACh. Washout with Ba2+ Ringer’s solution was done for 5 min between ACh applications. Oocytes were incubated with PP PLA2 for 5 min followed by its co-application with ACh. Peak current amplitudes of ACh-induced responses were measured before and after preincubation of oocytes with PP PLA2. The ratio between these two measurements was used to assess the activity of PLA2 on human α9α10 nAChR.
Receptor binding studies
Radioligand analysis
For competition binding assays, suspensions of nAChR-rich membranes from T. californica ray electric organ (1.25 nM α-Bgt binding sites) in 20 mM Tris-HCl buffer, pH 8.0, containing 1 mg/ml bovine serum albumin (BSA) (binding buffer), human α7 nAChR transfected GH4C1 cells (0.4 nM α-Bgt binding sites) in binding buffer, or a solution of heterologously expressed AChBP from L. stagnalis (2.4 nM in binding buffer) were incubated for 3 h with various amounts of the PLA2s, followed by an additional 5 min incubation with 0.4 nM [125I]α-Bgt. Nonspecific binding was determined by preliminary incubation of the preparations with 20 μM α-cobratoxin. The membrane and cell suspensions were applied to glass GF/C filters (Whatman, Little Chalfont, UK) presoaked in 0.25% polyethylenimine, and the unbound radioactivity was removed from the filter by washing (3 × 3 ml) with 20 mM Tris-HCl buffer, pH 8.0, containing 0.1 mg/ml BSA (washing buffer). The AChBP solutions were applied to two layers of DE-81 filters presoaked in PBS-T buffer, and washed (3 × 3 ml) with washing buffer. The bound radioactivity was determined using a Wizard 1470 Automatic Gamma Counter (Perkin Elmer). The binding results were analyzed using ORIGIN 7.5 (OriginLab Corporation, Northampton, MA, USA) fitting to a one-site or two-site dose-response competition curve.
For competition binding assays on the extracellular domain (ECD) of human α9 nAChR, PP PLA2 in the concentration range 0.3–30 μM was incubated for 2 h at room temperature with the ECD (final concentrations of 30 μg/ml) in 50 μL of a 20 mM Tris–HCl buffer, pH 8.0, containing 1 mg/ml of the bovine serum albumin (binding buffer). Then [125I]α-Bgt was added to the reaction mixtures to a final concentration of 0.2 nM. Simultaneously 15 μL Ni-NTA-agarose (QIAGEN) pre-washed in reaction buffer was added. After 6 min, the reaction was stopped by a rapid filtration on GF/C filters (Whatman) pre-soaked in 0.25% polyethylenimine and the unbound radioactivity was removed from the filters by washes (3×4 ml) with the 20 mM Tris–HCl buffer. Nonspecific binding was determined by preliminary incubation of the ECD with 10 μM α-cobratoxin. The bound radioactivity was determined using Wizard 1470 Automatic Gamma Counter (Perkin Elmer). The data were analyzed using ORIGIN 7.5 as a one-site dose-response curve.
Surface plasmon resonance (SPR) experiments
SPR experiments were performed at 20°C, using a Biacore® 2000 system (GE Healthcare, Biacore AB). AChBP was covalently immobilized to a CM5 sensor chip at acidic pH. For binding experiments, the running and dilution buffer was composed of 20 mM Tris (pH 7.4), 150 mM NaCl, and 0.005% Surfactant P20 (GE Healthcare, Biacore AB). The concentrations of Cro ranged from 5.5 to 46 μg/ml, and solutions were injected at a flow rate of 30 μl/min. Background signals were obtained by injection of samples to a blank-immobilized flow cell and these signals were subtracted from the sample signals. At the end of each run, a 10 s injection of 10 mM Gly/HCl pH 1.5 was performed to restore the complete binding capacity of the AСhBP coupled to the CM5 sensor chip. The kinetic constants, ka (association rate constant), and kd (dissociation rate constant), for the interaction between Cro and AChBP were calculated using Biacore BIAEVALUATION 3.1 software (Biacore AB). The curves were fitted according to the simple two-component model of interaction. The apparent dissociation constant (KDapp) was obtained as the ratio of kd and ka (KDapp = kd/ka).
Results
Electrophysiological experiments
Suppression of acetylcholine- or cytisine-induced currents in L. stagnalis neurons
Under the experimental conditions used, ACh and cytisine elicited inward currents in identified L. stagnalis neurons LP1,2,3 and RPV2,3 due to an increase in chloride permeability [30]. Previously this conductance was shown to be mediated by two subtypes of nAChRs with low and high affinity for α-conotoxin ImI (ImI) and reversed relative affinities for ACh [15, 17]. A further distinction between two subtypes is in the kinetics of receptor desensitization in response to ACh. The nAChRs with a higher sensitivity to ImI and faster desensitization, in spite of possessing chloride ion conductance, are more similar to vertebrate α7 nAChRs; furthermore cytisine is a full agonist at this subtype whereas it is a weak partial agonist at other nAChR subtype. In most experiments, we used cytisine or choline instead of ACh because of their more selective actions on α7 nAChRs. To exclude a possible contribution of phospholipolytic activity, Ca2+ was replaced with Ba2+ in the extracellular solution.
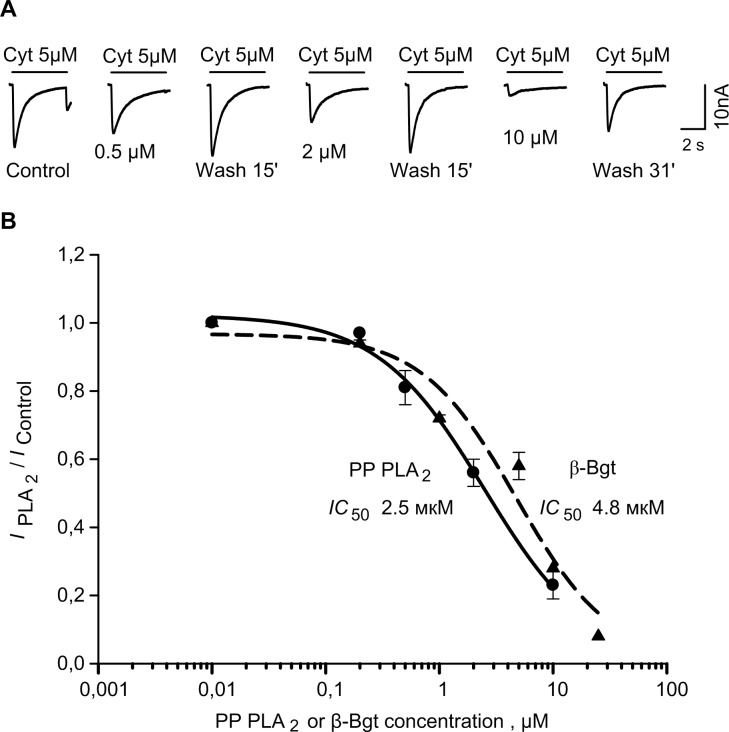
It was found that 5 min treatment of a neuron with PP PLA2 or β-Bgt resulted in a decrease of ACh- or cytisine-induced currents (Fig 1A). Peak response suppression was dependent on PLA2 concentration (Fig 1B) and reversed slowly after PLA2 wash out. IC50 values for PP PLA2 and β-Bgt inhibition of cytisine-induced current were 2.5 ± 0.4 (n = 7) and 4.8 ± 1.6 (n = 4) μM, respectively.
Fig 1. Inhibition of acetylcholine or cytisine-elicited current in L.stagnalis neurons by PP PLA2 and β-Bgt.
(A) Representative recordings from a neuron in control, after a 5-min incubation with PP PLA2 at three different concentrations, and after PP PLA2 wash out. (B) Dependence of ACh- or cytisine-evoked current suppression on PP PLA2 (n = 7) and β-Bgt (n = 4) concentration.
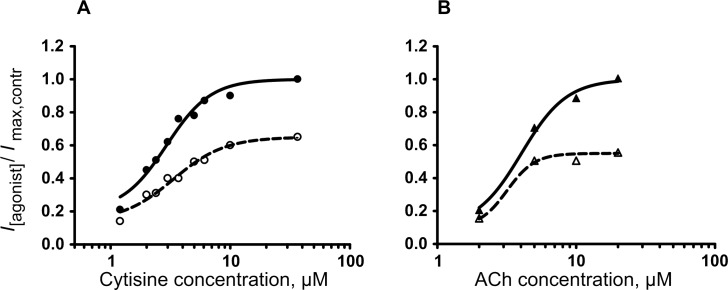
For PP PLA2 the type of antagonism was determined. For this purpose, a set of cytisine or ACh concentrations including saturating ones were applied to a neuron before and after treatment with PP PLA2 at 3 μM (a concentration slightly higher than its IC50). As can be seen in Fig 2A and 2B, the curves of the current dependence on agonist concentration shifted rightward after incubation of the neurons with PP PLA2 solution. EC50 values for cytisine were 2.9 in control and 3.3 μM after PP PLA2 treatment (n = 4) and for ACh—4.0 and 3.2 μM (n = 1), respectively. However, the maximal responses to both cytisine and ACh were reduced by 40–50% (Fig 2). These data indicate non-competitive antagonism and support our previous results obtained with enzymatically inactive Vur-S49 from Vipera ursinii renardi venom [15].
Fig 2. Determination of antagonism type for PP PLA2.
Dependence of cytisine (A) or acetylcholine (B) induced currents on agonist concentration in control (closed circles and triangles) and after 5 min treatment with PP PLA2 at 3 μM (open symbols), (n = 5 and 1, respectively).
We also explored the ability of the proteins unrelated to PLA2 to interact with nAChRs. Ribonuclease A (RNAse), β-lactoglobulin and soybean trypsin inhibitor were tested on Lymnaea neurons. We found that RNAse decreased the peak of the cytisine-induced current but the effect (IC50 > 50 μM, n = 3) was more than an order of magnitude weaker than that for PP PLA2 or β-Bgt. A decrease in the response to choline caused by β-lactoglobulin or soybean trypsin inhibitor at concentrations of 10 and 50 μM was not more than 8 and 12%, respectively (n = 7 and 7), and did not depend on the concentration of these proteins (data not shown).
Suppression of acetylcholine-induced current mediated by human α9α10 nAChR heterologously expressed in Xenopus oocytes
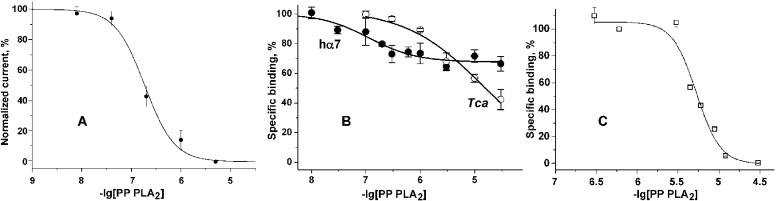
The activity of PP PLA2 was tested in electrophysiological experiments on human α9α10 nAChR heterologously expressed in Xenopus oocytes. It was found that a 5 min treatment of oocytes with PP PLA2 resulted in a decrease of the ACh-induced current (Fig 3A). Peak response suppression was dependent on PP PLA2 concentration (Fig 3A) and the IC50 value was 0.19 ± 0.03 μM (n = 3).
Fig 3. Inhibition experiments with PP PLA2.
(A) Dose-response curve of PP PLA2 inhibitory action on the ACh-evoked (25 μM ACh) ionic currents mediated by human α9α10 nAChR heterologously expressed in Xenopus oocytes. (B) Inhibition by PP PLA2 of the initial rate of specific [125I]-α-Bgt binding to T. californica and human hα7 nAChRs expessed in GH4C1 cells. Only 30% of binding sites in hα7 nAChRs could be protected from [125I]-α-Bgt binding. (C) Inhibition by PP PLA2 of specific [125I]-α-Bgt binding to ECD of human α9 nAChR. IC50 5.5 μM.
Competition of PLA2s with [125I]α-bungarotoxin in binding assay
The capability of PLA2s to interact with nAChRs was studied using muscle-type nAChRs of T. californica electric organ and human neuronal α7 nAChRs (hα7 nAChRs) heterologously expressed in cells of GH4C1 line. ACh-binding protein (AChBP) from L. stagnalis, a structural analog of the extracellular ligand-binding domain of all nAChR subtypes, and the extracellular domain (ECD) of human α9 nAChR were also used for this purpose. The affinities of PLA2s for nAChRs, AChBP and ECD were evaluated using a radioligand competition binding assay with [125I]-labeled α-Bgt. The data obtained showed that all these PLA2s inhibited the initial rate of [125I]-labeled α-Bgt binding, although their potencies differed.
At Torpedo nAChR, PP PLA2 inhibited [125I]-α-Bgt binding with low efficiency (IC50 about 15 μM) with tend of complete inhibition at greater than 100 μM (Fig 3B). However, the inhibition of α-Bgt binding to hα7 nAChRs by PP PLA2 reached a plateau at approximately 70% of binding sites, the affinity to 30% of binding sites being fairly high (IC50 = 120 nM) (Fig 3B). Comparison of these results with the data on suppression of the cytisine-evoked current in L. stagnalis neurons indicates that both the affinities of PP PLA2 for hα7 and α7-similar nAChRs in Lymnaea neurons and the degree of inhibition of these receptors differed greatly. The interaction of PP PLA2 with ECD of human α9 nAChR showed one binding site with IC50 of 5.5 μM (Fig 3C).
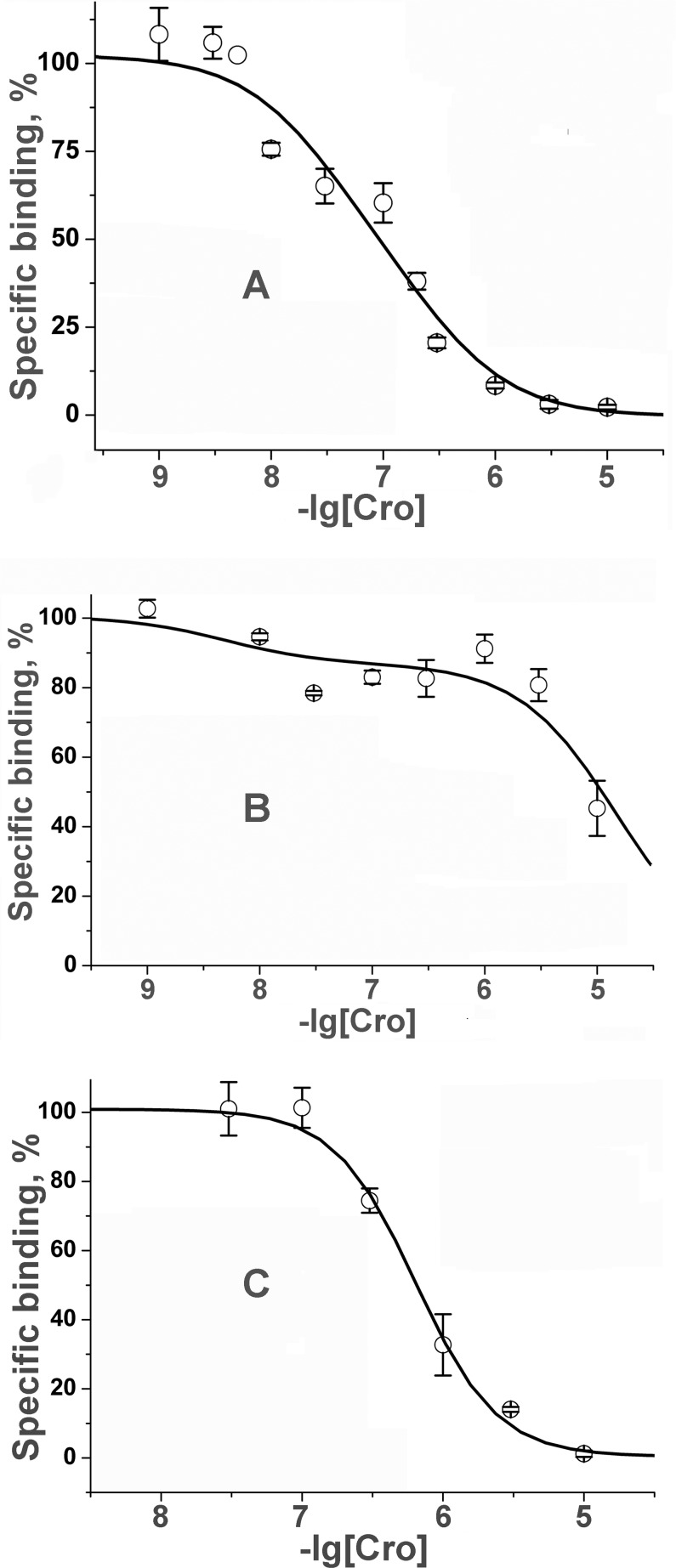
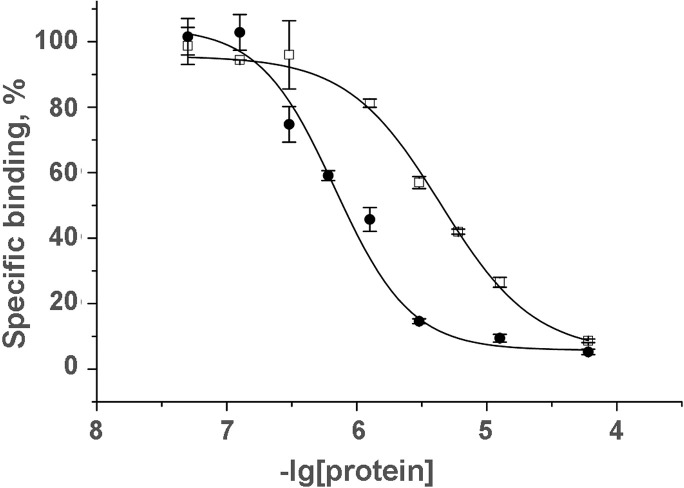
Cro completely inhibited [125I]-α-Bgt binding to all three targets (Fig 4). The experimental points for Torpedo and hα7 nAChRs were best approximated by a two-site model, with the difference in affinities between the two sites being about an order of magnitude in Torpedo nAChRs (30 and 260 nM) and more than 3 orders of magnitude in hα7 nAChRs (4.9 nM and 15 μM). For both the high affinity binding sites accounted for 20–30% of all binding sites. The interaction of Cro with AChBP showed one binding site with an IC50 of 640 nM (Fig 4C).
Fig 4. Interaction of Cro with nAChR of T. californica electric organ, human neuronal α7 nAChR and AChBP.
(A) Inhibition of the initial rate of specific [125I]-α-Bgt binding to T.californica nAChRs by Cro. Points were fit to a 2-site model with affinity for Cro of 30 nM and 260 nM. (B) Inhibition of the initial rate of specific [125I]-α-Bgt binding to human α7 nAChRs by Cro. Two binding sites with affinity for Cro differing more than 3 orders of magnitude were revealed. (C) Inhibition of the initial rate of specific [125I]-α-Bgt binding to acetylcholine-binding protein from L. staganlis by Cro.
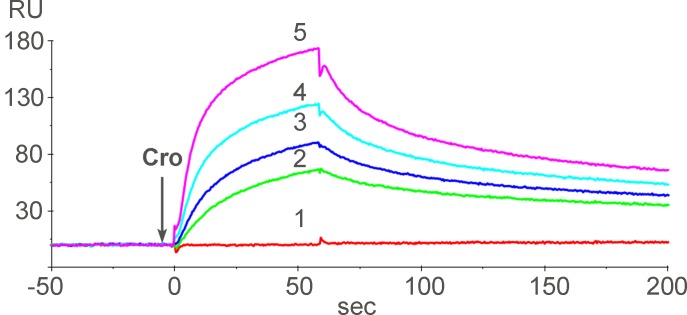
To observe the direct binding of Cro to AChBP, SPR measurements were performed (Fig 5). SPR recordings demonstrated that Cro interacted with immobilized AChBP and formed a stable complex. Quantitative analysis allowed the determination of the apparent dissociation constant (KDapp) for this interaction which was 120 nM.
Fig 5. SPR recordings of Cro interaction with AChBP from L. stanalis.
An arrow indicates injection of the analyte. Line 1 corresponds to injection of buffer solution. Curve 2–5 correspond to injections of solutions with Cro at concentrations of 5.5, 11.5, 23 and 46 μg/ml, respectively.
Several other proteins were checked for inhibition of α-Bgt binding. The proteins chosen have molecular masses close either to monomer PLA2 (RNAse, cytochrome C) or to heterodimer (soybean typsin inhibitor). Soybean trypsin inhibitor and β-lactoglobulin were inactive at concentration up to 60 μM. These data coincide with the results on Lymnaea neurons. RNAse inhibited α-Bgt binding to Torpedo nAChRs fairly well (IC50 5 μM) and cytochrome C was slightly more potent (IC50 1.12 μM) (Fig 6). However, these proteins even at high concentrations had only marginal effects on function of nAChRs in Lymnaea neurons.
Fig 6. Interaction of non-venom proteins with T.californica nAChR.
Inhibition of the initial rate of specific [125I]-α-Bgt binding to T.californica nAChR by RNAse (squares) and cytochrome C (circles).
Discussion
Snake venom PLA2s are multi-functional proteins evolved to affect multiple biological targets in prey organisms. They possess different toxic activities including presynaptic neurotoxicity, myotoxicity, cardiotoxicity, anticoagulant, and haemolytic activity. Usually each individual enzyme manifests its own specific effect, although other weaker activities can also be observed. For example, the presynaptically acting neurotoxin Cro has analgesic actions, and also immunomodulatory and anti-inflammatory effects [19], with no direct correlation between these activities and the catalytic activity of Cro. The PLA2 effects could be manifested through direct binding to membrane-bound receptors, and several binding proteins and glycoproteins, the so-called M- and N-receptors, which are tissue specific and bind certain PLA2s, have been discovered [9–12]. These receptors were shown to have high affinity binding sites for PLA2s with IC50 ranging from several pM to about 100 nM, i.e. significantly higher than the affinity of PLA2s for phospholipids [8]). One receptor protein isolated from porcine cerebral cortex bound not only neurotoxic PLA2s from snake venoms but also non-toxic PP PLA2 with similar affinities [12]. High affinity binding of mouse non-toxic IIA and IB PLA2s to M-type receptors in mouse colon has also been reported [31]. These data indicate that PLA2s possess the capacity to interact with receptor proteins.
In our previous paper, we reported on the ability of PLA2s from two families of snakes to antagonize an ACh-elicited current in L. stagnalis neurons containing α7-like receptors, and to compete with α-Bgt binding to recombinant human α7 nAChRs, T.californica nAChRs and AChBP from L. stagnalis [15]. Using Ca2+-free solution and experiments with a natural non-enzymatic analog of PLA2 from V. ursinii renardi venom (Vur-S49) allowed us to explore the type of interaction with nAChRs of different types. Although PLA2s completely inhibited α-Bgt binding to nAChRs and AChBP, characteristic changes of current-agonist concentration curves after neuron treatment with Vur-S49 indicated a non-competitive interaction.
In this paper, we present additional evidence for interaction with nAChRs of two dimeric presynaptic PLA2 toxins, i.e. Cro from C. durissus terrificus and β-Bgt from B. multicinctus, as well as non-toxic mammalian PLA2 from porcine pancreas. PP PLA2 and β-Bgt suppressed current responses of neurons to agonists with IC50 values of 2.5 and 4.8 μM, respectively (Fig 1). These values are in the same range as those obtained earlier for other PLA2s (0.4–10 μM [15]) which are monomeric enzymes. In heterodimeric β-Bgt the PLA2 subunit is connected to a Kunitz type inhibitor subunit by a disulfide bond, but this does not interfere with β-Bgt binding to nAChR. PP PLA2 suppressed the current responses of heterologously expressed human α9α10 nAChR to ACh with an IC50 value of 0.19 μM (Fig 3A). This is the highest affinity observed in electrophysiological experiments on current suppression by PLA2s.
In the binding assay, Cro and PP PLA2 competed with α-Bgt for binding to nAChR and AChBP. Interestingly, PP PLA2 at α7 nAChR competed with α-Bgt for binding to only about 30% of the total binding sites. The inhibition reached a plateau at about 3 μM and remained at the same level up to 30 μM (Fig 3B). The affinity for this 30% of sites was fairly high, the IC50 value being 150 nM. It should be noted that PP PLA2 completely inhibited the acetylcholine-induced current in Lymnaea neurons in a noncompetitive manner, albeit with a higher IC50 value (2.5 μM). The nAChR α7 subtype contains five α-Bgt binding sites [32], but α-Bgt binding to only one site is enough to block ion currents [33]. The bound α-Bgt locks the agonist-binding site in an inactive conformation and the dominant mechanism of antagonism is non-competitive, originating from conformational arrest of the binding sites [33]. Given these data, we suggest that at α7 nAChR, PP PLA2 competes with α-Bgt and binds to only one (or possibly two) binding site(s). However, PP PLA2 completely inhibits receptor ionic conductance in Lymnaea neurons. Therefore, binding of PP PLA2 to one or two sites could be sufficient to block Lymnaea receptor similar to the inhibition of α7 nAChR by α-Bgt.
PP PLA2 inhibited α-Bgt binding to the ECD of the human α9 nAChR with an IC50 value of 5.5 μM (Fig 3C), which is higher than that (0.19 μM) observed for inhibition of the ACh-induced current in oocytes expressing human α9α10 nAChR. Thus, for α9 nAChR we have observed receptor inhibition both in the ECD binding experiments and in electrophysiological experiments on whole receptors. It should be noted that signaling via α9α10 nAChRs is involved in the expression of pain [34] and inhibition of this receptor prevents neuropathic pain [35]. Consistent with these data, type IIA PLA2 has been localized by immunohistochemistry to the spinal trigeminal and facial motor nuclei and dorsal- and ventral-horns of the spinal cord [36], implying an important role of CNS sPLA2 in nociceptive transmission. It has also been shown that the treatment of mice with bee venom PLA2 might prevent oxaliplatin-induced neuropathic pain [37]. Given our data shows an interaction of PP PLA2 with α9α10 nAChR, we suggest that the PLA2 interaction with this receptor may be involved in the pain transmission pathway.
Interaction of Cro with muscle-type nAChRs has been previously studied [38, 39]. The toxin or phospholipolytically active basic component blocked depolarization and the Na+ permeability increase induced by carbamylcholine in membrane preparations from electric organs of Electrophorus electricus and T. marmorata. Although Cro reduced the initial velocity of labeled α-toxin from Naja nigricollis binding to postsynaptic membranes by about 30%, the authors concluded that Cro did not interfere with binding of α-toxin to nAChRs. These data along with Cro evoked enhancement of affinity to agonist was considered as a sign of non-competitive interaction of Cro with nAChR, leading to stabilization of the desensitized state [38]. In support of this, Cro decreased depolarization of the guinea-pig end-plate and the frequency of miniature end-plate postsynaptic potentials [39].
In this work, we found that Cro could compete with α-Bgt for binding to nAChRs. It is interesting to note that two binding sites with different affinities for Cro were revealed in both Torpedo and hα7 nAChRs: IC50 were of 30 and 260 nM for the first and 4.9 nM and 15 μM for the second (Fig 4). In Torpedo nAChR the ratio of low and high affinity binding sites was 1:1, corresponding to the presence of two agonist/competitive antagonist binding sites. Non-equivalence in the affinity of two binding sites of Torpedo nAChR was earlier shown for d-tubocurarine and α-conotoxins [40, 41]. In hα7 nAChR the high affinity binding sites represented about 20% of total sites. This finding could be explained by the assumption that the high affinity Cro binding to one site out of five ones resulted in some changes in the receptor which were responsible for a decrease in affinity to Cro in the other four sites.
In experiments with water soluble AChBP, Cro binding to one binding site was observed (Figs 4C and 5). The binding to water soluble protein observed both in competition with radioactive α-Bgt and direct SPR experiment allowed complete exclusion of membrane effects in its interaction.
Inhibition of α-Bgt binding to Torpedo nAChRs found in this study is consistent with Cro inhibition of responses to carbamylcholine observed in membrane preparations from electric organs and in guinea-pig diaphragm, although competitive binding of Cro and α-Bgt was not reported [38]. Our finding of muscle type nAChR inhibition by Cro demonstrates the postsynaptic activity of this toxin, although the block of hα7 nAChR by Cro may contribute to its presynaptic activity as the participation of α7 nAChR in acetylcholine release in mouse motor synapses was suggested [42].
According to our electrophysiological data, antagonism of PLA2s (Fig 2 here and Fig 4B in [15]) of nAChRs was non-competitive. This fact seems contradictory to the ability of all PLA2s to inhibit α-Bgt binding (Figs 3 and 4 here and Fig 6 in [15]). The reason of the discrepancy might be structural differences between α7 similar Lymnaea nAChRs and Torpedo or human α7 receptors. For another explanation of the PLA2 competition with α-Bgt, one should consider the existing model of α-neurotoxin-nAChR interaction: it is accepted that the tip of the toxin central loop is inserted into the receptor at the interfaces between two subunits and the toxin molecule is placed almost equatorially to the extracellular domain of the nAChR. Recently, a possible participation of the membrane in which nAChR is embedded in neurotoxin-receptor interaction was suggested [43, 44]. It was found that a snake venom neurotoxin can bind membrane and this binding can be considered as a first interaction step facilitating the receptor recognition by the toxin. Thus, it can be suggested that any disturbance of toxin interaction with the membrane can constrain its binding to the receptor. At the extreme, this may result in inhibition of toxin binding to the receptor. Interestingly, in competition experiments the interaction of Cro with the water soluble AChBP (Fig 4C) was weaker than with the membrane-bound nAChRs which could be due to participation of membrane in toxin-receptor interaction. Finally, competition with α-Bgt might be explained by PLA2 binding to nAChR in close vicinity to the agonist/competitive antagonist binding site that leads to steric hindrance of α-Bgt binding.
Here we found that PLA2s interact with nAChRs with different affinities, with IC50 ranging from tens of nM to tens of μM. The fairly low affinities in the micromolar range raise the question about specificity of interaction, and thus four proteins lacking phospholipolytic activity—RNAse, β-lactoglobulin, soybean trypsin inhibitor, and cytochrome C—were tested for their ability to interact with nAChRs. Unexpectedly, RNAse and cytochrome C could compete with α-Bgt for binding to Torpedo nAChR with IC50s of 1.12 and 5 μM, respectively (Fig 6), while soybean trypsin inhibitor and β-lactoglobulin did not compete with α-Bgt at concentrations up to 60 μM. However, all these proteins were practically inactive in functional tests on Lymnaea neurons: RNAse only slightly suppressed nAChR-mediated current with an IC50 greater than 50 μM, while soybean trypsin inhibitor and β-lactoglobulin were ineffective at this concentration. It is well documented that both RNAse [45] and cytochrome C [46] interact with cellular membranes, and in the view of the above consideration they might interfere with the α-Bgt binding to the membrane-associated nAChR. Indeed, such inhibition was observed in competition experiments with radioactive α-Bgt (Fig 6). However, the addition of RNAse had only marginal effect on the current elicited by agonist in Lymnaea neurons. The competition of the PLA2s with α-Bgt for binding to membranes could also explain the higher enzyme affinities observed in radioligand experiments as compared to electrophysiological data obtained in this and previous [15] work. PLA2s studied here not only competed with radioactive α-Bgt for binding to nAChRs but also blocked acetylcholine elicited ion currents.
In summary, we have revealed the ability of two toxic heterodimeric snake PLA2s and non-toxic PP PLA2 to interact with different types of nAChRs. These data indicate that the interaction with nAChR may be a general property of all PLA2s and defines a novel activity that can be attributed to these proteins.
Acknowledgments
We thank Prof. S.Lummis for the help with preparation of the manuscript, Prof. P.Bregestovski for help with PP PLA2 purchase and Prof. S.Tzartos for useful discussions.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by a grant of Russian Foundation for Basic Research (http://www.rfbr.ru/rffi/eng) No. 15-04-01843 to YU; Grant of the President of the Russian Federation (https://grants.extech.ru/) No. МК-6216.2016.4 to IS.
References
- 1.Murakami M, Lambeau G. Emerging roles of secreted phospholipase A(2) enzymes: an update. Biochimie. 2013;95: 43–50. doi: 10.1016/j.biochi.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 2.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111: 6130–6185. doi: 10.1021/cr200085w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du QS, Trabi M, Richards RS, Mirtschin P, Madaras F, Nouwens A, et al. Characterization and structural analysis of a potent anticoagulant phospholipase A2 from Pseudechis australis snake venom. Toxicon. 2016;111: 37–49. doi: 10.1016/j.toxicon.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 4.Casais-E-Silva LL, Teixeira CF, Lebrun I, Lomonte B, Alape-Girón A, Gutiérrez JM. Lemnitoxin, the major component of Micrurus lemniscatus coral snake venom, is a myotoxic and pro-inflammatory phospholipase A2. Toxicol Lett. 2016;257: 60–71. doi: 10.1016/j.toxlet.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 5.Rowan EG. What does β-bungarotoxin do at the neuromuscular junction? Toxicon. 2001;39: 107–118. [DOI] [PubMed] [Google Scholar]
- 6.Montecucco C, Rossetto O, Caccin P, Rigoni M, Carli L, Morbiato L, et al. Different mechanisms of inhibition of nerve terminals by botulinum toxin and snake presynaptic neurotoxins. Toxicon. 2009;54: 561–564. doi: 10.1016/j.toxicon.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 7.Križaj I. Ammodytoxin: A window into understanding presynaptic toxicity of secreted Phospholipases A2 and more. Toxicon. 2011;58: 219–229. doi: 10.1016/j.toxicon.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 8.Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42: 827–840. doi: 10.1016/j.toxicon.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 9.Lambeau G, Barhainin H, Schweitz H, Qar J, Lazdunski M. Identification and properties of very high affinity brain membrane-binding sites for a neurotoxic phospholipase from the taipan venom. J Biol Chem. 1989;264: 11503–11510. [PubMed] [Google Scholar]
- 10.Lambeau G, Scmid-Alliana A, Lazdunsli M, Barhainin H. Identification and purification of a very high affinity binding protein for toxic phospholipases A2 in skeletal muscle. J Biol Chem. 1990;265: 9526–9532. [PubMed] [Google Scholar]
- 11.Križaj I, Faure G, Gubenšek F, Bon C. Neurotoxic phospholipases A2 ammodytoxin and crotoxin bind to distinct high-affinity protein acceptors in Torpedo marmorata electric organ. Biochemistry. 1997;36: 2779–2787. doi: 10.1021/bi9612374 [DOI] [PubMed] [Google Scholar]
- 12.Čopič A, Vučemilo N, Gubenšek F, Križaj I. Identification and purification of a novel receptor for secretory phospholipase A2 in porcine cerebral cortex. J Biol Chem. 1999;274: 6315–26320. [DOI] [PubMed] [Google Scholar]
- 13.Rigoni M, Pizzo P, Schiavo G, Weston AE, Zatti G, Caccin P, et al. Calcium influx and mitochondrial alterations at synapses exposed to snake neurotoxins or their phospholipids hydrolysis products. J Biol Chem. 2007;282: 11238–11245. doi: 10.1074/jbc.M610176200 [DOI] [PubMed] [Google Scholar]
- 14.Vulfius CA, Gorbacheva EV, Starkov VG, Osipov AV, Kasheverov IE, et al. An unusual phospholipase A2 from puff adder Bitis arietans venom–a novel blocker of nicotinic acetylcholine receptors. Toxicon. 2011;57: 787–793. doi: 10.1016/j.toxicon.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 15.Vulfius CA, Kasheverov IE, Starkov VG, Osipov AV, Andreeva TV, Filkin SYu, et al. Inhibition of nicotinic acetylcholine receptors, a novel facet in the pleiotropic activities of snake venom phospholipases A2. PLoS One. 2014;9: e115428 doi: 10.1371/journal.pone.0115428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vulfius CA, Krasts IV, Utkin YuN, Tsetlin VI. Nicotinic receptors in Lymnaea stagnalis neurons are blocked by neurotoxins from cobra venoms. Neurosci Lett. 2001;309: 189–192. [DOI] [PubMed] [Google Scholar]
- 17.Vulfius CA, Tumina OB, Kasheverov IE, Utkin YuN, Tsetlin VI. Diversity of nicotinic receptors mediating Cl− current in Lymnaea neurons distinguished with specific agonists and antagonist. Neurosci Lett. 2005;373: 232–236. doi: 10.1016/j.neulet.2004.10.010 [DOI] [PubMed] [Google Scholar]
- 18.Kwong PD, McDonald NQ, Sigler PB, Hendrickson WA. Structure of beta -bungarotoxin: potassium channel binding by Kunitz modules and targeted phospholipase action. Structure. 1995;3: 1109–1119. [DOI] [PubMed] [Google Scholar]
- 19.Sampaio SC, Hyslop S, Fontes MR, Prado-Franceschi J, Zambelli VO, Magro AJ, et al. Crotoxin: novel activities for a classic beta-neurotoxin. Toxicon. 2010;55: 1045–1060. doi: 10.1016/j.toxicon.2010.01.011 [DOI] [PubMed] [Google Scholar]
- 20.Rigoni M, Caccin P, Gschmeissner S, Koster G, Postle AD, Rossetto O, et al. Equivalent effects of snake PLA2 neurotoxins and lysophospholipid–fatty acid mixtures. Science. 2005;310: 1678–1680. doi: 10.1126/science.1120640 [DOI] [PubMed] [Google Scholar]
- 21.Chang CC, Lee CY. Crotoxin, the neurotoxin of South American rattlesnake venom is a presynaptic toxin acting like beta-bungarotoxin. Naunyn-Schemiedebergs Arch Pharmacol. 1977;296: 159–168. [DOI] [PubMed] [Google Scholar]
- 22.Prasarnpun S, Walsh J, Harris JB. Beta-bungarotoxin-induced depletion of synaptic vesicles at the mammalian neuromuscular junction. Neuropharmacology. 2004;47: 304–314. doi: 10.1016/j.neuropharm.2004.04.012 [DOI] [PubMed] [Google Scholar]
- 23.Faure G, Bon C. Crotoxin, a phospholipase A2 neurotoxin from the South American Rattlesnake, Crotalus durissus terrificus: purification of several isoforms and comparison of their molecular structure and of their biological activities. Biochemistry. 1988;27: 730–738. [DOI] [PubMed] [Google Scholar]
- 24.Faure G, Xu H, Saul F. Crystal structure of crotoxin reveals key residues involved in stability and toxicity of this potent heterodimeric beta-neurotoxin. J Mol Biol. 2011;412: 176–191. doi: 10.1016/j.jmb.2011.07.027 [DOI] [PubMed] [Google Scholar]
- 25.Utkin YN, Gantsova EA, Andreeva TV, Starkov VG, Ziganshin RH, Anh HN, et al. Venoms of kraits Bungarus multicinctus and Bungarus fasciatus contain anticoagulant proteins. Dokl Biochem Biophys. 2015;460: 53–58. doi: 10.1134/S1607672915010159 [DOI] [PubMed] [Google Scholar]
- 26.Zouridakis M, Giastas P, Zarkadas E, Chroni-Tzartou D, Bregestovski P, Tzartos SJ. Crystal structures of free and antagonist-bound states of human alpha9 nicotinic receptor extracellular domain. Nat Struct Mol Biol. 2014;21: 976–980. doi: 10.1038/nsmb.2900 [DOI] [PubMed] [Google Scholar]
- 27.Benjamin PR, Ings CT. Golgi-Cox studies on the central nervous system of a gastropod mollusk. Z Zellforsch. 1972;128: 564–582. [DOI] [PubMed] [Google Scholar]
- 28.Kostyuk PG, Krishtal OA, Pidoplichko VI. Intracellular perfusion. J. Neurosci Methods. 1981;4: 201–210. [DOI] [PubMed] [Google Scholar]
- 29.Kudryavtsev DS, Shelukhina IV, Son LV, Ojomoko LO, Kryukova EV, Lyukmanova EN, et al. Neurotoxins from snake venoms and α-conotoxin ImI inhibit functionally active ionotropic γ-aminobutyric acid (GABA) receptors. J Biol Chem. 2015;290: 22747–22758. doi: 10.1074/jbc.M115.648824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chemeris NK, Kazachenko VN, Kislov AN, Kurchikov AL. Inhibition of acetylcholine responses by intracellular calcium in Lymnaea stagnalis neurons. J Physiol (London). 1982;323: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cupillard L, Mulherkar R, Gomez N, Kadam S, Valentin E, Lazdunski M, et al. Both group IB and group IIA secreted phospholipases A2 are natural ligands of the mouse 180 kDa M-type receptor. J Biol Chem. 1999;274: 7043–7051. [DOI] [PubMed] [Google Scholar]
- 32.Simonson PD, Deberg HA, Ge P, Alexander JK, Jeyifous O, Green WN, et al. Counting bungarotoxin binding sites of nicotinic acetylcholine receptors in mammalian cells with high signal/noise ratios. Biophys J. 2010;99: L81–L83. doi: 10.1016/j.bpj.2010.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.daCosta CJ, Free CR, Sine SM. Stoichiometry for α-bungarotoxin block of α7 acetylcholine receptors. Nat Commun. 2015;6: 8057 doi: 10.1038/ncomms9057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Bufalo A, Cesario A, Salinaro G, Fini M, Russo P. Alpha9 alpha10 nicotinic acetylcholine receptors as target for the treatment of chronic pain. Curr Pharm Des. 2014;20: 6042–6047. [DOI] [PubMed] [Google Scholar]
- 35.Romero HK, Christensen SB, Di Cesare Mannelli L, Gajewiak J, Ramachandra R, Elmslie KS, et al. Inhibition of α9α10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc Natl Acad Sci U S A. 2017;114: E1825–E1832. doi: 10.1073/pnas.1621433114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma MT, Nevalainen TJ, Yeo JF, Ong WY. Expression profile of multiple secretory phospholipase A(2) isoforms in the rat CNS: enriched expression of sPLA(2)-IIA in brainstem and spinal cord. J Chem Neuroanat. 2010;39: 242–247. doi: 10.1016/j.jchemneu.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 37.Li D, Kim W, Shin D, Jung Y, Bae H, Kim SK. Preventive effects of bee venom derived phospholipase A₂ on oxaliplatin-induced neuropathic pain in mice. Toxins (Basel). 2016;8: E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bon C, Changeux J-P, Jeng TW, Fraenkel-Conrat H. Postsynaptic effects of crotoxin and of its isolated subunits. Eur. J Biochem. 1979;99:471–481. [DOI] [PubMed] [Google Scholar]
- 39.Brazil OV, Fontana MD, Heluany NF. Nature of the postsynaptic action of crotoxin at guinea-pig diaphragm end-plates. J Nat Toxins. 2000;9: 33–42. [PubMed] [Google Scholar]
- 40.Kreienkamp HJ, Utkin YN, Weise C, Machold J, Tsetlin VI, Hucho F. Investigation of ligand-binding sites of the acetylcholine receptor using photoactivatable derivatives of neurotoxin II from Naja naja oxiana. Biochemistry. 1992;31: 8239–8244. [DOI] [PubMed] [Google Scholar]
- 41.Utkin YN, Kobayashi Y, Hucho F, Tsetlin VI. Relationship between the binding sites for an alpha-conotoxin and snake venom neurotoxins in the nicotinic acetylcholine receptor from Torpedo californica. Toxicon. 1994;32: 1153–1157. [DOI] [PubMed] [Google Scholar]
- 42.Gaydukov AE, Bogacheva PO, Tarasova EO, Balezina OP. The mechanism of choline-mediated inhibition of acetylcholine release in mouse motor synapses. Acta Naturae. 2014;6: 110–115. [PMC free article] [PubMed] [Google Scholar]
- 43.Lesovoy DM, Bocharov EV, Lyukmanova EN, Kosinsky YA, Shulepko MA, Dolgikh DA, et al. Specific membrane binding of neurotoxin II can facilitate its delivery to acetylcholine receptor. Biophys J. 2009;97: 2089–2097. doi: 10.1016/j.bpj.2009.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shenkarev ZO, Lyukmanova EN, Paramonov AS, Panteleev PV, Balandin SV, Shulepko MA, et al. Lipid-protein nanodiscs offer new perspectives for structural and functional studies of water-soluble membrane-active peptides. Acta Naturae. 2014;6: 84–94. [PMC free article] [PubMed] [Google Scholar]
- 45.Sundlass NK, Eller CH, Cui Q, Raines RT. Contribution of electrostatics to the binding of pancreatic-type ribonucleases to membranes. Biochemistry. 2013;52: 6304–6312. doi: 10.1021/bi400619m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramanian M, Jutila A, Kinnunen PK. Binding and dissociation of cytochrome c to and from membranes containing acidic phospholipids. Biochemistry. 1998;37: 1394–1402. doi: 10.1021/bi9716581 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.