ABSTRACT
Hair follicle-associated-pluripotent (HAP) stem cells are located in the bulge area of the hair follicle, express the stem-cell marker, nestin, and have been shown to differentiate to nerve cells, glial cells, keratinocytes, smooth muscle cells, cardiac muscle cells, and melanocytes. Transplanted HAP stem cells promote the recovery of peripheral nerve and spinal cord injuries and have the potential for heart regeneration as well. In the present study, we implanted mouse green fluorescent protein (GFP)-expressing HAP stem-cell spheres encapsulated in polyvinylidene fluoride (PVDF)-membrane cylinders into the severed sciatic nerve of immunocompetent and immunocompromised (nude) mice. Eight weeks after implantation, immunofluorescence staining showed that the HAP stem cells differentiated into neurons and glial cells. Fluorescence microscopy showed that the HAP stem cell hair spheres promoted rejoining of the sciatic nerve of both immunocompetent and immunodeficient mice. Hematoxylin and eosin (H&E) staining showed that the severed scatic nerves had regenerated. Quantitative walking analysis showed that the transplanted mice recovered the ability to walk normally. HAP stem cells are readily accessible from everyone, do not form tumors, and can be cryopreserved without loss of differentiation potential. These results suggest that HAP stem cells may have greater potential than iPS or ES cells for regenerative medicine.
KEYWORDS: Cylinders, encapsulation, hair follicle, HAP stem cell, implantation, nerve regeneration, polyvinylidene
Introduction
In 2003,1 we reported that nestin, a protein marker for neural stem cells, also is expressed in hair follicle stem cells in transgenic mice in which GFP expression is driven by nestin regulatory elements in transgenic mice, and labeled these hair-follicle cells with GFP. These cells can differentiate into neurons, glia, keratinocytes, smooth muscle cells, and melanocytes and heart muscle cells2-9 in vitro and were therefore termed hair HAP stem cells. In vivo studies showed the HAP stem cells can differentiate into blood vessels and neurons after transplantation to the subcutis of nude mice. HAP stem cells implanted into the gap region of a severed sciatic nerve in mice enhanced regeneration and the restoration of nerve function and walking ability. The implanted HAP stem cells transdifferentiated largely into Schwann cells.10
Human HAP cells can also differentiate into neurons, glia, keratinocytes, smooth muscle cells, and melanocytes in vitro and when transplanted in the severed sciatic nerve of mice, they differentiated into Schwann cells and promoted the recovery of pre-existing axons, leading to nerve generation and functional recovery.11
Subsequently, we severed the thoracic spinal cord of C57BL/6 immunocompetent mice and implanted HAP stem cells to the injury site. Most of the implanted HAP stem cells also differentiated into Schwann cells and facilitated repair of the severed spinal cord. The rejoined spinal cord reestablished extensive hind-limb locomotor performance.12,13
In another study, HAP stem cells were implanted into rats with spinal cord injury. Immunohistochemical staining showed that HAP stem cells differentiated into oligodendrocytes and neuronal-like cells (βIII-tubulin-positive cells) at 3 weeks after transplantation. Recovery of hind limb locomotor function occurred in the HAP stem cell-implanted rats at 8 weeks following cell transplantation.14
In the present study, we demonstrate that mouse HAP stem cells encapsulated in polyvinylidene fluoride (PVDF)-membrane cylinders promote effective recovery of peripheral nerve injury when implanted in the severed sciatic nerve of immunocompetent and immunocompetent mice.
Results and discussion
Encapsulation of HAP stem-cell hair-spheres in polyvinylidene fluoride (PVDF)-membrane cylinders
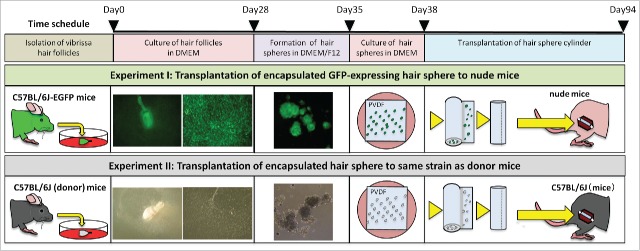
HAP stem cells from the upper parts of murine vibrissa hair follicles were cultured in 10% FBS DMEM for 4 weeks. Growing HAP stem cells that were detached were transferred to a non-adhesive culture dish in DMEM/F12 containing 2% B-27. After one week of culture, the detached cells formed hair spheres. Hair spheres were then cultured on sterilized PVDF-membranes in 10% FBS DMEM for 3 d. PVDF-membranes were encapsulated into cylinders with the hair spheres on the inside (Fig. 1, Fig. 2A1, Fig. 2A2).
Figure 1.
Encapsulation of HAP stem-cell hair-spheres in PVDF-membrane cylinders for implantation to the severed sciatic nerve in nude and immunocompetent mice. HAP stem cells from the upper parts of vibrissa hair follicles from C57BL/6J mice were cultured in 10% FBS DMEM for 4 weeks. Growing HAP stem cells detached and were transferred to a non-adhesive culture dish in DMEM/F12 containing 2% B-27. After one week of culture, the detached cells formed hair spheres. Hair spheres were cultured on sterilized PVDF-membranes in 10% FBS DMEM for 3 d. PVDF-membranes was rolled up into cylinders with the hair spheres on the inside. Schematic diagram shows 2 transplantation designs. Experiment I: GFP-expressing HAP stem-cell hair-spheres from GFP-mouse hair follicles in PVDF-membrane cylinders were implanted into the severed sciatic nerve of nude mice (Crlj: CD1-Foxn1nu). Experiment II: A HAP stem-cell hair-sphere-containing PVDF-membrane cylinder from C57BL/6J mice were transplanted into the severed sciatic nerve of C57BL/6J mice.
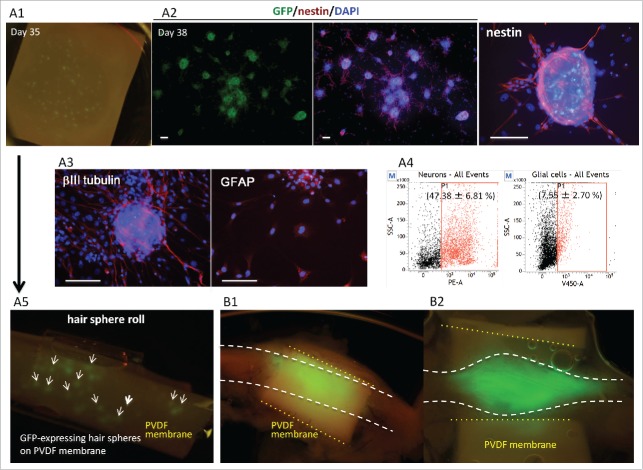
Figure 2.
HAP stem-cell hair-spheres were seeded on PVDF-membrane (A1), and then cultured in 10% FBS DMEM for 3 d. The HAP stem-cell hair-spheres differentiated to neurons and glial cells (A2, A3). Green = GFP; Red = nestin (A3); ßIII tubulin and GFAP; Blue = DAPI. Bar = 100 µm. Differentiated cells from HAP stem-cells hair-sphere-contained 47% neurons and 8% of glial cells (A4). The PVDF-membrane was rolled up into a cylinder with the HAP stem-cell hair-spheres on the inside (A5). HAP stem-cell hair-sphere-containing cylinders were implanted into the severed sciatic nerve of nude mice. After 8 weeks, the sciatic nerve of the implanted nude mice was directly observed by fluorescence microscopy. HAP stem cells differentiated and proliferated in the rejoining sciatic nerve (B1, 2).
HAP stem cells encapsulated in PVDF-membrane cylinders differentiate into neurons and glial cells after implantation in the severed sciatic nerve in nude mice
Immunofluorescence showed that nestin-expressing HAP stem cells hair spheres on PVDF-membranes differentiated to neurons and glial cells (Fig. 2A3). Flowcytometory analysis showed that the differentiated cells from the hair spheres on the PVDF-membranes contained 47% neurons and 8% glial cells (Fig. 2A4). The PVDF-membranes were rolled into cylinders with the HAP stem cells hair spheres on the inside (Fig. 2A5). Nude mice had their sciatic nerve severed under anesthesia. The mice were then implanted with HAP stem cell-hair sphere-containing PVDF-membrane cylinders into the sciatic nerve. Eight weeks after implantation, the sciatic nerve of the transplanted nude mice was directly observed by fluorescence microscopy which showed that GFP-expressing HAP stem cells extended from the cylinder and joined the severed sciatic nerve (Fig. 2B)
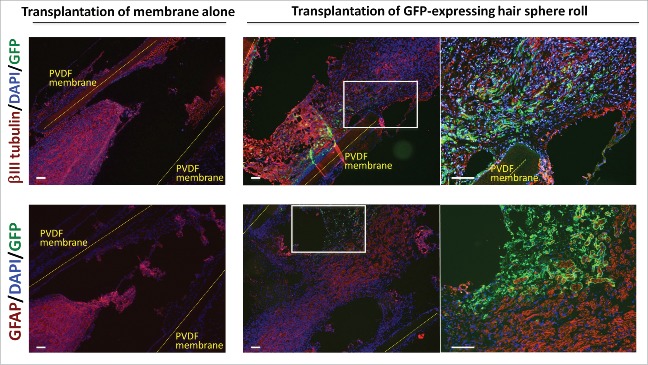
Immunofluorescence staining also showed that the nerves joined and HAP stem cells differentiated to neurons and glial cells. In contrast, the nerves in the nude mice implanted with empty cylinders remained separated (Fig. 3)
Figure 3.
HAP stem-cell hair-spheres differentiated to neurons and glial cells after encapsulation in PVDF-membrane cylinders and implantation to the severed sciatic nerve. Immunofluorescence images of frozen sections 8 weeks after implantation of the HAP stem-cell hair-sphere-containing PVDF-membrane cylinders transplanted between the served sciatic nerve fragments, showed that the HAP stem cell spheres differentiated to neurons and glial cells. Red = βIII tubulin (upper) or GFAP (lower); Green = GFP; Blue = DAPI. Right panels = high magnification images of white boxes. Bar = 100 µm.
Implantation of the HAP stem-cell-spheres encapsulated in PVDF-membrane cylinders promotes rejoining of the severed sciatic nerve in C57BL/6J mice
Eight weeks after implantation of the HAP stem cell sphere cylinders between the served sciatic nerve fragments of C57BL/6J mice, the severed sciatic nerves rejoined. Hematoxylin and eosin (H&E) stained slides showed that a large number of spindle cells grew in the severed part of the sciatic nerve. (Fig. 4A) Furthermore immunostaining shows that hair spheres differentiated to neurons and glial cells after implantation (Fig. 4B, C)
Figure 4.
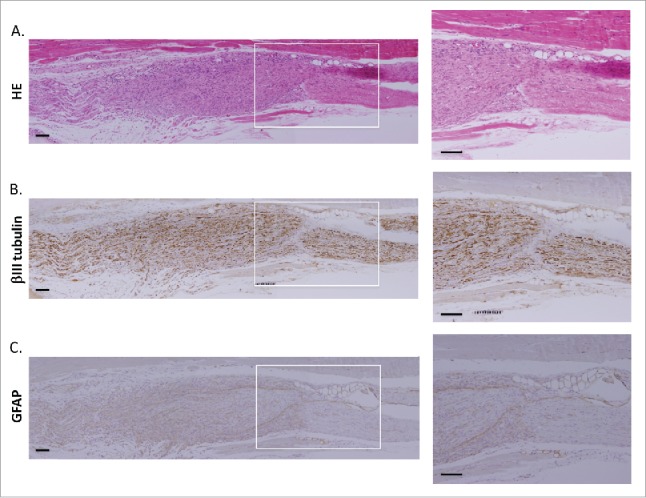
Implantation of HAP stem-cell hair-sphere-containing PVDF-membrane cylinders to C57BL/6J mice rejoined severed sciatic nerves. H&E staining of the severed part of sciatic nerve in implanted mice (A). Immunostained images of paraffin sections, 8 weeks after implantation of HAP stem-cells hair-sphere-containing PVDF-membrane cylinders between the severed sciatic nerve fragments, showed that the HAP stem cells differentiated into neurons (B), and glial cells (C). Right panels = high-magnifications of white boxes, individually. Bar = 100 µm.
HAP stem-cell hair-sphere PVDF-membrane cylinder-implanted C57BL/6J mice recovered the ability to walk
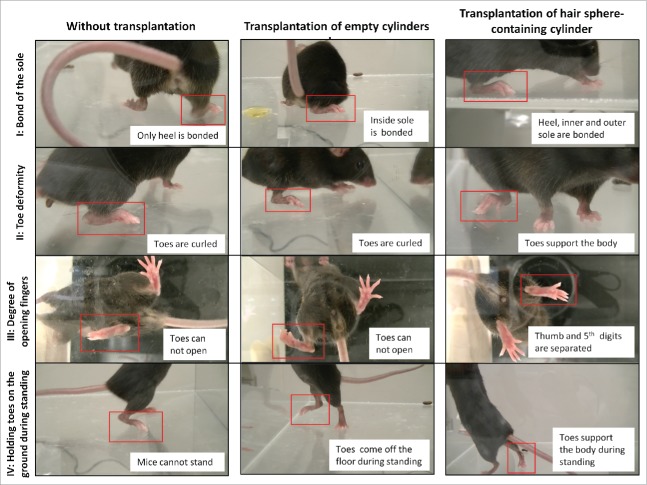
Eight weeks after transplantation of HAP stem-cell hair-sphere-containing PVDF-membrane cylinders to C57BL/6J mice between the severed sciatic nerve fragments, locomotion of the mice was evaluated by the sum of 4 points; (i) bond of the sole, (ii) toe deformity, (iii) degree of opening toes and (iv) holding toes on the ground during standing. Mice implanted with the HAP stem-cell hair-sphere-containing PVDF-membrane cylinders recovered the above 4 functions. On the other hand, 7 mice without implantation or 7 mice implanted with empty PVDF-membrane cylinders, did not recover all these functions (Fig. 5, Supplemental Videos 1, 2). A locomotion score, based on the above parameters, of the mice implanted with HAP stem-cell hair-sphere-containing PVDF-membrane cylinders had a significantly higher score compared with mice without implantation or the mice implanted with the empty cylinder alone. The mice without implantation had a score of 4.4 ± 1.7, the mice implanted with the empty cylinder had a score of 4.4 ± 1.9, the mice implanted with the HAP stem-cells hair-sphere-containing PVDF-membrane cylinders had a score of 10.1 ± 3.8 (p = 0.002, compared with no transplantation or transplantation of empty cylinders) (Table. 1).
Figure 5.
Implantation of HAP stem-cell hair-sphere-containing PVDF-membrane cylinders enabled recovery of the ability of the mice to walk. C57BL/6J mice with servered sciatic nerves were treated under 3 conditions: without implantation (left); implantation of empty PVDF-membrane cylinders (middle); and implantation of HAP stem cell-sphere-containing PVDF-membrane cylinders (right). Eight weeks after implantation, bonding of the soles, toe deformity, degree of opening toes and holding toes on the ground during standing were determined. The locomotion of the mice implanted with HAP stem-cells hair-sphere-containing PVDF-membrane cylinder recovered compared with the mice transplanted with empty PVDF-membrane cylinders.
Table 1.
Comparison of locomotion evaluation score.
| Locomotion function | Without implantation | Implantation of PVDF-membrane cylinder | Implantation of hair sphere roll |
|---|---|---|---|
| I: Bond of the sole | 1.6 ± 0.5 | 1.3 ± 0.5 | 3.0 ± 1.0 |
| II: Toe deformity | 1.1 ± 0.9 | 1.0 ± 0.8 | 2.3 ± 1.3 |
| III: Degree of opening toes | 1.0 ± 1.0 | 0.9 ± 0.9 | 2.4 ± 1.9 |
| IV: Holding toes on the ground during standing | 0.7 ± 0.8 | 1.3 ± 0.8 | 2.4 ± 1.1 |
| Sum | 4.4 ± 1.7 | 4.4 ± 1.9 | *10.1 ± 3.8 |
The locomotion of mice was evaluated as follows: (I) bonding of the sole; (1 point = bonding at any one point of inner sole, outer sole, or heel, 2 points = bonding at 2 points. 3 points – bonding at 3 points. 5 points = bonding of entire sole); (II) toe deformity (0 points = toes are curled up. 1 point = toes are rounded and nails come off the floor. 2 points = toes are rounded and nails bond floor. 5 points = flatfoot); (III) degree of opening of toes (0 points = closed, 1 point = big toes separate from other toes, 2 points = big toe and 5th digit separate. 5 points = 5 toes separate individually); and (iv) holding toes on ground during rearing (0 points = cannot rear, 1 point = toes come off the floor, 2 points = supports the body with clenched back foot, 5 points = supports the body with flat open foot).
In conclusion, in the present study, we developed HAP stem-cell hair-spheres encapsulated in a PVDF-membrane cylinder for implantation in severed peripheral nerves which effect functional repair. Future use of human hair follicles to produce HAP stem cells for encapsulation in PVDF-membrane cylinders have potential to regenerate nerve injury in the clinic.
Materials and methods
Animals
Transgenic C57BL/6J-EGFP mice (GFP mice) were obtained from the Research Institute for Microbial Diseases (Osaka University, Osaka).15 C57BL/6J mice were obtained from Nihon CLEA (Kawasaki, Japan). Crlj: nu/nu mice (nude mice) were obtained from Oriental BioService, Inc. (Tokyo, Japan). All animal experiments were conducted according to the Guidelines for Animal Experimentation at Kitasato University.
Isolation of vibrissa hair follicles
The vibrissa hair follicles from C57Bl/6j GFP mice were isolated as described previously.3,16 To isolate the vibrissa follicles, parts of left or right vibrissa pads were dissected under a binocular microscope.
Transplantation HAP stem cells encapsulated in polyvinylidene fluoride membrane cylinders
HAP stem cells from the upper parts of vibrissa hair follicles and hair spheres were cultured as described previously.3,16 Hair spheres were subsequently cultured on sterilized PVDF-membranes (Millipore, Darmstadt, Germany) in DMEM (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (FBS), 50 µg/ml gentamycin (Gibco, Grand Island, NY), 2 mM L-glutamine (Gibco) and 10 mM HEPES (MP Biomedicals, Santa Ana, CA) for 3 d. PVDF-membranes were rolled up into cylinders with the HAP stem cell hair spheres on the inside.
Implantation of the HAP stem cell spheres encapsulated in polyvinylidene fluoride membrane cylinders to the severed sciatic nerve
The mouse sciatic nerve was severed under anesthesia. HAP stem cell spheres encapsulated in PVDF-membrane cylinders were implanted between the served sciatic nerve fragments and the incision was closed with nylon sutures. After 8 weeks, the sciatic nerve of the transplanted mice was directly observed by fluorescence microscopy. Nerve samples of the implanted mice were excised under anesthesia and embedded in O.C.T. Compound (Sakura Finetek, Torrance, CA) in liquid N2. Frozen sections, 3 µm thick, were sectioned with a cryostat (Bright Instruments, Bedfordshire, UK) and air-dried. The C57BL/6J nerve samples were fixed in 4% paraformaldehyde and were embedded in paraffin using Tissue Tek® VIP® (Sakura Finetek). Paraffin sections, 3 µm thick, were sliced with a YAMATO ROM-380 (Yamato Kohki Industrial, Saitama, Japan).
Hematoxylin and eosin (H&E) staining
Paraffin sections were de-paraffinized and washed. They were stained with H&E and were observed by Olympus BX 51 (Olymupus, Tokyo, Japan).
Immunostaining
Paraffin sections were incubated with anti-βIII tubulin mouse monoclonal antibody (1:500, Tuj1 clone; Covance, CA) and then were treated with the Dako ChemMateEnvision kit/ HRP (Dako Japan, Tokyo, Japan). Paraffin sections were incubated with anti-glial fibrillary acidic protein (GFAP) chicken polyclonal antibody (1:300; abcam, UK) and then were incubated with goat anti-chicken IgY biotinylated (1:1000; R&D Systems, Minneapolis, MN). The paraffin sections were then treated with VECTASTAIN ABC kit (Funakoshi, Tokyo, Japan). The paraffin sections were then developed using DAB and were stained with Mayer's hematoxylin solution for nuclear staining.
Immunofluorescence staining
Frozen sections and HAP stem-cell hair-sphere-containing cylinders were incubated with anti-nestin mouse monoclonal antibody (1:200, rat401 clone; Millipore, Tokyo, Japan); anti-βIII tubulin mouse monoclonal antibody (1:500) (Covance, CA) or anti-glial fibrillary acidic protein (GFAP) chicken polyclonal antibody (1:300) (Abcam, UK) in blocking buffer (4% BSA, 0.5% Triton X100, 0.04% NaN3 in PBS) and then were incubated with goat anti-mouse IgG conjugated with Alexa Flour 568® (1:400, Molecular Probes, Eugene, Oregon) or goat anti-chiken IgG conjugated with Alexa Flour 568® (1:1500, Molecular Probes) and 4′, 6-diamino-2-phenylindole, dihydrochloride (DAPI) (Molecular Probes) in blocking buffer. Fluorescence staining was visualized using an Olympus BX51 microscope.
Flow cytometry
Cells differentiated for HAP stem cells hair-spheres on PVDF-membranes were detached and incubated with anti-βIII tubulin mouse monoclonal antibody (1:500) or anti-GFAP chicken polyclonal antibody (1:300) as the primary antibody. Secondary antibodies were goat anti-mouse IgG H&L phycoerythrin (1:500; Abcam, Cambridge, UK) or goat anti-chicken IgY biotinylated (1:1000) and Brilliant Violet ™ streptavidin (1:1000; BioLegend, CA, USA). The cells were measured by FACS Verse (BD Bioscience, NJ, USA), were analyzed by FACS suite™ software (BD Bioscience). Flow cytometry analyses were repeated in triplicate.
Walking analysis
Mice were observed walking in a transparent case (30 × 30 cm) and recoded with an Everio Hi-Vision Memory Movie GZ-E265 (JVC, Kanagawa, Japan). Their locomotion was scored by 4 criteria: bond of the soles, toe deformity, degree of opening toes and holding their toes on the ground during standing. I: bond of the sole (1 point = bond at any one point of inner sole, outer sole or heel. 2 points = bond at 2 points. 3 points = bond at 3 points. 5 points = bond of entire sole); II: toe deformity (0 points = toes are curled up. 1 point = toes are rounded and nails come off the floor. 2 points = toes are rounded and nails bond floor. 5 points = flatfoot.); III: degree of opening toes (0 points = closed, 1 point = large toe separate from other toes, 2 points = large toe and 5th digits separate, 5 points = 5 toes separate individually); and IV: holding toes on the ground during standing (0 points = cannot rear; 1 point = toes come off the floor; 2 points = supports the body with clenched back foot; 5 points = support of body with flat open foot).
Statistical Analysis
The experimental data are expressed as the mean ± SD. Statistical analyses were performed with unpaired Student's t test.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest.
Funding
This work was supported by Grant-in-Aid for Scientific Research (C) 16K10173 from the Ministry of Education, Science, Sports, and Culture of Japan, a grant from the Ministry of Education, Culture, Sports, Science, and Technology of the Japan Government (Assistance for Strategic Creation of Research Basis, 2014–2016), and the Terumo Life Science Foundation (to Y. Amoh).”
References
- [1].Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci USA. 2003;100:9958-9961. doi: 10.1073/pnas.1733025100. PMID:12904579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle-bulge stem cells can form neurons. Proc Natl Acad Sci USA. 2005;102:5530-5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y. From hair to heart: hair follicle stem cells differentiate to beating cardiac muscle cells. Cell Cycle. 2015;14:2362-2366. doi: 10.1080/15384101.2015.1042633. PMID:25970547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tohgi N, Obara K, Yashiro M, Hamada Y, Arakawa N, Mii S, Aki R, Hoffman RM, Amoh Y. Human hair-follicle associated puripotent (hHAP) stem cells differentiate to cardiac-muscle cells. Cell Cycle. 2017;16:95-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shirai K, Iiamada Y, Arakawa N, Yamazaki A, Tohgi N, Aki R, Mii S, Hoffman RM, Amoh Y. Hypoxia enhances differentiation of hair follicle-associated-puripotent (HAP) stem cells to cardiac muscle cells. J Cell Biochem. 2017;118:554-558. [DOI] [PubMed] [Google Scholar]
- [6].Yamazaki A, Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Hoffman RM, Amoh Y. Isoproterenol directs hair follicle-associated puripotent (HAP) stem cells to differentiate in vitro to cardiac muscle cells which can be induced to form beating heart muscle tissue sheets. Cell Cycle. 2016;15:760-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yamazaki A, Hamada Y, Arakawa N, Yashiro M, Mii S, Aki R, Kawahara K, Hoffman RM, Amoh Y. Early-age-dependent selective decrease of differentiation potential of hair-follicle-associated pluripotent (HAP) stem cells to beating cardiac muscle cells. Cell Cycle. 2016;15:2619-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hoffman RM. Nestin-expressing hair follicle-accessible-pluripotent (HAP) stem cells for nerve and spinal cord repair. Cells Tissues Organs. 2014;200:42-47. [DOI] [PubMed] [Google Scholar]
- [9].Hoffman RM, editor Multipotent Stem Cells of the Hair Follicle: Methods and Protocols. Methods in Molecular Biology 1453. Walker John M., series editor Humana Press (Springer Science+Business Media New York), 2016. doi: 10.1007/978-1-4939-3786-8. [DOI] [Google Scholar]
- [10].Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman RM. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci USA. 2005;102:17734-17738. doi: 10.1073/pnas.0508440102. PMID:16314569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, Hoffman RM, Katsuoka K. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: An advantageous alternative to ES and iPS cells. J Cell Biochem. 2009;107:1016-1020. doi: 10.1002/jcb.22204. PMID:19507228 [DOI] [PubMed] [Google Scholar]
- [12].Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle. 2008;7:1865-1869. doi: 10.4161/cc.7.12.6056. PMID:18583926 [DOI] [PubMed] [Google Scholar]
- [13].Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N, Mii S, Amoh Y, Katsuoka K, Hoffman RM. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle. 2011;10:830-839. [DOI] [PubMed] [Google Scholar]
- [14].Najafzadeh N, Nobakht M, Pourheydar B, Golmohammadi MG. Rat hair follicle stem cells differentiate and promote recovery following spinal cord injury. Neural Regen Res. 2013;8:3365-3372. PMID:25206658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313-319. doi: 10.1016/S0014-5793(97)00313-X. PMID:9175875 [DOI] [PubMed] [Google Scholar]
- [16].Kajiura S, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Li L, Katsuoka K, Hoffman RM, Amoh Y. Cryopreservation of the hair follicle maintains pluripotency of nestin-expressing hair follicle associated pluripotent stem cells. Tissue Eng. 2015;21:825-31. doi: 10.1089/ten.tec.2014.0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.