Abstract
Minimally invasive thoracoscopic surgery has become an inevitable trend in the treatment of anterior mediastinal tumors and other thoracic diseases. Many surgical approaches may be used for video-assisted thoracoscopic surgery (VATS) thymectomy. Some researchers have proven that that VATS thymectomy using the subxiphoid approach is technically safe and feasible. Compared with the transthoracic approach, the subxiphoid approach is considered to provide a better view of the bilateral pleural cavities and to cause relatively less pain; it is thus considered a less invasive surgical approach. In this article, we summarize our experience with various incision designs, specific surgical procedures, and key operative manipulations that are crucial for successful performance of subxiphoid VATS extended thymectomy.
Keywords: Video-assisted thoracoscopic surgery (VATS), extended thymectomy, subxiphoid approach, thymoma
Introduction
Although thymectomy via a median sternotomy was historically considered the gold standard approach to thymoma or anterior mediastinal tumor dissection, improvements in minimally invasive thoracoscopic surgery have gradually changed this concept. In recent years, several less invasive methods have been applied in thymectomy (1-6). Among these, the lateral transthoracic approach is the most frequently performed. However, the main disadvantages of the lateral transthoracic approach are the poor operative view of the contralateral pleural cavity and the risk of intercostal nerve injury resulting in postoperative chronic incision pain and numbness. Hsu et al. (7) summarized the disadvantages of different minimally invasive approaches for thymectomy. They believed that a better view of the bilateral pleural cavities and a more radical thymectomy could be achieved only by the subxiphoid approach.
The subxiphoid approach in video-assisted thoracoscopic surgery (VATS) extended thymectomy can be divided into several techniques according to the incision design: the single-port subxiphoid approach (4), the subxiphoid and subcostal arch approach (5), and a combination of the transthoracic and subxiphoid approaches (3). Advantages of the single-port subxiphoid approach over the lateral transthoracic approach include the sufficient operative view of the cervical and bilateral pleural cavities (8), less pain (9), and a better cosmetic effect. Although the single-port subxiphoid approach was believed to be the least invasive approach for thymectomy, the need for specially designed instruments, decreased instrument maneuverability, and mutual interference among devices limited its promotion and prolonged the learning curve (4,10). The combination approach still has a risk of intercostal nerve injury due to the transthoracic incision that must be created for introduction of the thoracoscope (11). With our accumulated experience in more than 150 cases of transthoracic VATS thymectomy, we tend to perform VATS thymectomy using the subxiphoid and subcostal arch approach. The reason for this approach is that in addition to the merits of the single-port subxiphoid approach, it increases maneuverability and reduces interference among devices. Using several modifications, we routinely perform subxiphoid VATS extended thymectomy without sternum lifting, and the whole procedure is completed using traditional laparoscopic instruments. We herein summarize our institution’s experience with this technique.
Patient selection and workup
The indications for the subxiphoid and subcostal arch approach to VATS extended thymectomy are as follows: preoperative imaging reveals no involvement of surrounding tissues or organs, the patient’s physical condition meets the requirements for VATS, the tumor is ≤8 cm and has a complete capsule and clear border as shown by intraoperative exploration, and stable control of preoperative complications has been achieved. The preoperative examination includes blood testing, electrocardiography, echocardiography, and pulmonary function testing to ensure that the patient can tolerate the operation. Cervical and abdominal color doppler ultrasonography also helps to exclude lymph node metastasis. Finally, chest enhanced computed tomography reveals the tumor size, location, and relationship with surrounding tissues and organs.
Preoperative preparation
The specific preoperative preparation for subxiphoid VATS thymectomy is not different from that of the traditional VATS approach. For patients with myasthenia gravis, pyridostigmine bromide therapy is needed to decrease symptoms to a stable level suitable for the operation.
Equipment preference
The whole procedure is completed using conventional thoracoscopic instruments; no other special devices are applied. An ultrasonic scalpel is recommended as the main energy device in most procedures because of its good excision and hemostasis capabilities and ensuring nice operative field (Figure 1).
Figure 1.
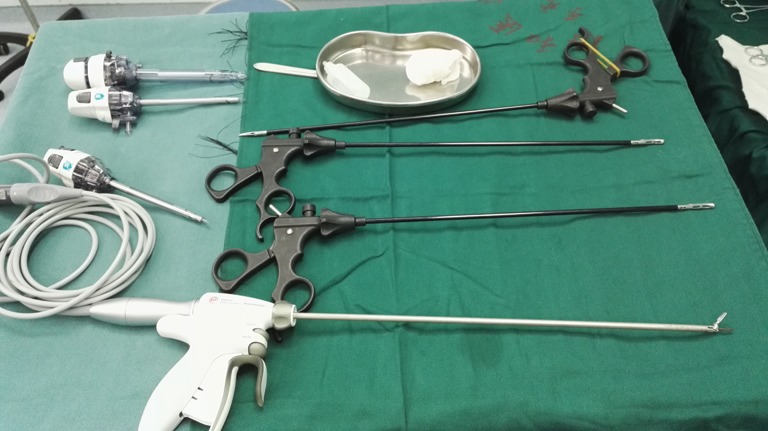
Instruments for video-assisted thoracoscopic surgery (VATS) extended thymectomy using the subxiphoid approach.
Procedure
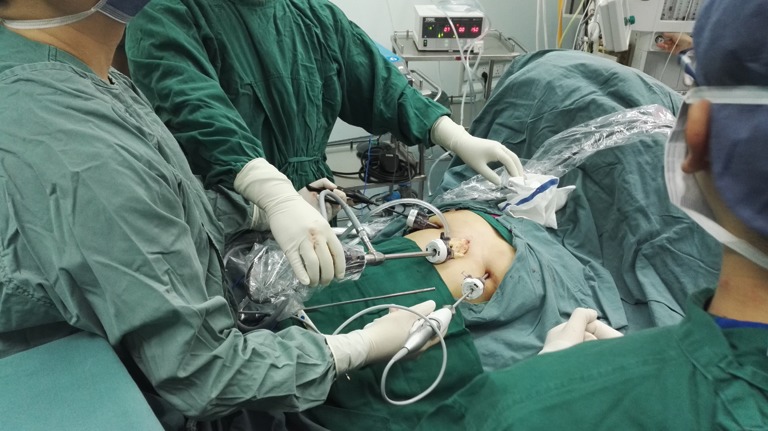
After the patient has been intubated with a single-lumen tube, the patient is placed in the supine position with the legs spread apart. A 3-cm observation port is made 1.5 cm inferior to the xiphoid. The retrosternal space is then bluntly separated with the surgeon’s finger to extent the working space. A 10-mm, 30-degree oblique thoracoscope is inserted through the observation port. Artificial pneumothorax is established at 6 to 8 cm H2O to amplify the anterior mediastinal space, after which two 0.5-cm operating ports are created under the costal arch at the bilateral midclavicular lines (Figure 2). An ultrasonic scalpel and thoracoscopic grasping forceps are introduced into the bilateral operating ports. To ensure capsule integrity and en bloc dissection of the tumor, all dissections should be performed an adequate distance away from the tumor. The main procedures are divided into the following steps. First, the anterior border of the thymus is gradually dissociated along the retrosternal space to the thoracic outlet vertically and toward the bilateral diaphragm attachment point transversely. The fat tissue at the bilateral cardiophrenic angle is then dissected. Second, the right mediastinal pleura are opened along the right phrenic nerve, and the posterior border of the thymus is meticulously separated from the front edge of the superior vena cava and bilateral innominate veins to the thoracic outlet. The thymic veins should be ligated and transected. The surrounding fat of the vessels is then removed. Third, the left mediastinal pleura are opened along the left phrenic nerve. The left subclavian artery, left common carotid artery, and left innominate vein are exposed and dissociated, and their surrounding fat is removed. The bilateral upper ports of the thymus are divided. Finally, after fully mobilizing the posterior border, the thymus together with the fat tissue is placed into a specimen bag and pulled out through the observation port (Figures 3,4). Two Abel tubes are respectively located in the bilateral sixth intercostal spaces of the anterior axillary line (Figure 5).
Figure 2.
Incision design for video-assisted thoracoscopic surgery (VATS) extended thymectomy using the subxiphoid approach.
Figure 3.
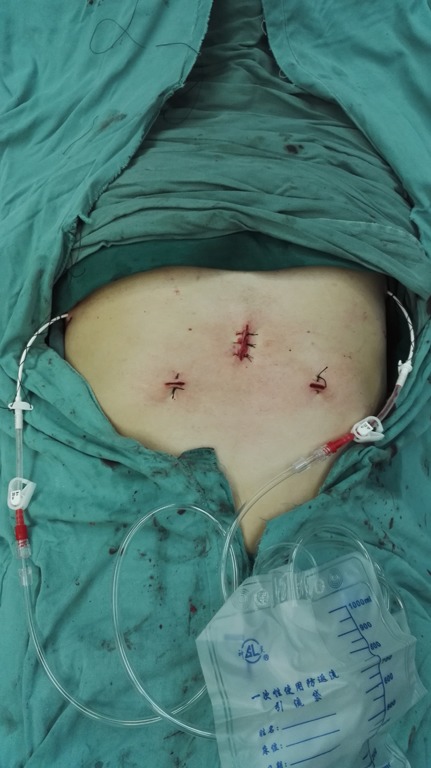
Location of drainage tubes: bilateral sixth intercostal spaces of anterior axillary line.
Figure 4.

Subxiphoid approach video-assisted thoracoscopic extended thymectomy (12). Available online: http://www.asvide.com/articles/1140
Figure 5.
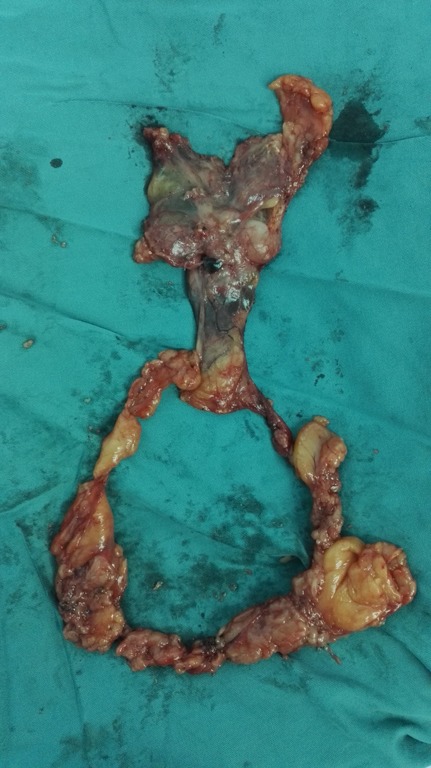
Resected thymus and surrounding fat tissue.
Roles of team members
The surgeon is positioned between the patient’s legs while the camera holder stands at the patient’s left or right side. The camera holder must maintain a steady surgical view without interfering with the surgeon’s action. When dissociating the front border of thymus along the retrosternal area, the camera holder should slightly retreat the camera according to the specific situation to avoid lens blurring secondary to the narrow operative space. Coordination between the surgeon and camera holder is the key factor in the success of the operation (Figure 6). The nurse stands on the side opposite the camera holder, passing appropriate surgical instruments and removing eschar from the ultrasonic scalpel to maintain the fluency of the operation. The anesthesiologist sits cranial to the patient; monitors the blood pressure, oxyhemoglobin saturation, and tidal volume; and communicates with the surgeon when abnormal parameters are detected. When performing this operation on patients with myasthenia gravis, the anesthesiologist should keep the airway unobstructed by aspirating sputum and reducing the dose of muscular relaxants (Figure 7).
Figure 6.
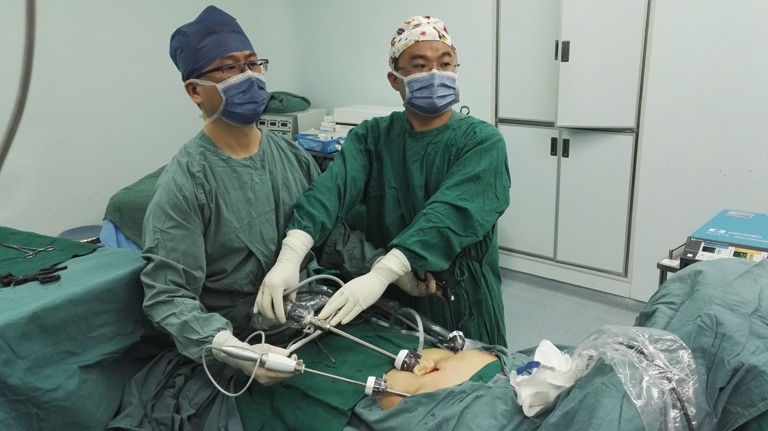
Standing positions of the surgeon and camera holder.
Figure 7.
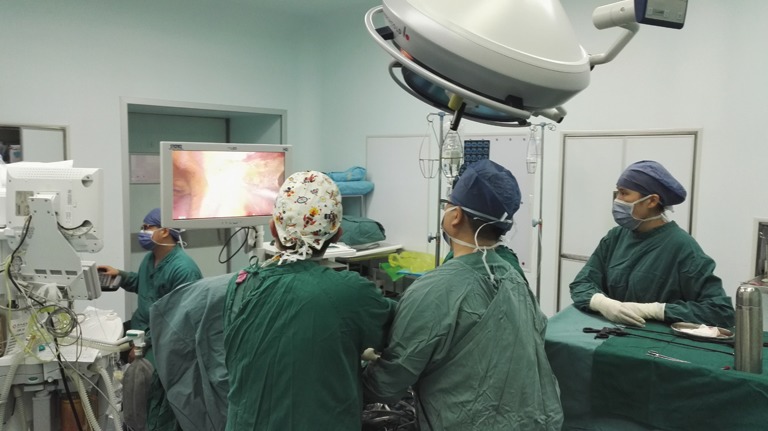
Standing positions of the whole surgical team.
Postoperative management
Analgesia (except for myasthenia gravis patients), reduction of airway secretions, and gastric mucosal protection should be ensured postoperatively. The patient should start oral feeding and off-bed activity on the first day postoperatively. For patients with myasthenia gravis, pyridostigmine bromide therapy should be continued and its dosage and administration should be under the guidance of the neurology department to most effectively control postoperative symptoms. The subxiphoid tubes can be removed when the drainage volume is <50 mL.
Tips and tricks
The incision for the observation port should be made vertically on the abdominal rectus fascia 1.5 cm caudal to the inferior margin of the xiphoid to prevent incision disunion;
To create a sufficient visual field and operative space, after blunt dissection of the retrosternal space, the surgeon should actively release the surrounding tissue toward the bilateral diaphragm attachment point, then open the bilateral mediastinal pleura;
Important anatomical landmarks, namely the bilateral phrenic nerves, innominate vein, and superior vena cava, should be accurately identified to ensure effective en bloc dissection of the tumor;
Dissection of fat around the left innominate vein is the most technically demand step in this operation. To ensure the safety of this process, the surgeon should carefully dissect the surrounding fat from the bilateral end to the middle point of the left innominate vein. The distal tissue can thus be well exposed, ensuring the safety of the procedure;
When using an ultrasonic scalpel, the surgeon should keep the active surface of the blade in the field of view of the video-assisted thoracoscope at all times to prevent unexpected injury to the posterior tissues;
A standardized surgical technique is crucial for the successful performance of the subxiphoid and subcostal arch approach to VATS extended thymectomy. The whole process should follow the principle of “from proximal to distal, from superficial to deep”.
Conclusions
Subxiphoid video-assisted thoracoscopic extended thymectomy is safe and feasible for thymic tumor. Its merits over the lateral transthoracic approach contain better operative view, less pain and cosmetic effect. For our experience, it is technically feasible to conduct partial pericardiotomy and wedge resection of lung when facing pericardiac and pulmonary invasion. But due to the change of surgical view and operative manner, sufficient training is needed. And median sternotomy should be transferred when tumor’s en bloc resection cannot be ensured by subxiphoid approach.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Calhoun RF, Ritter JH, Guthrie TJ, et al. Results of transcervical thymectomy for myasthenia gravis in 100 consecutive patients. Ann Surg 1999;230:555-9; discussion 559-61. 10.1097/00000658-199910000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiratsuka M, Iwasaki A, Shirakusa T, et al. Role of video-assisted thoracic surgery for the treatment of myasthenia gravis: extended thymectomy by median sternotomy versus the thoracoscopic approach with sternal lifting. Int Surg 2006;91:44-51. [PubMed] [Google Scholar]
- 3.Hsu CP, Chuang CY, Hsu NY, et al. Subxiphoid approach for video-assisted thoracoscopic extended thymectomy in treating myasthenia gravis. Interact Cardiovasc Thorac Surg 2002;1:4-8. 10.1016/S1569-9293(02)00003-8 [DOI] [PubMed] [Google Scholar]
- 4.Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. 10.1016/j.athoracsur.2011.08.047 [DOI] [PubMed] [Google Scholar]
- 5.Zhao J, Wang J, Zhao Z, et al. Subxiphoid and subcostal arch thoracoscopic extended thymectomy: a safe and feasible minimally invasive procedure for selective stage III thymomas. J Thorac Dis 2016;8:S258-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu CP, Chuang CY, Hsu NY, et al. Comparison between the right side and subxiphoid bilateral approaches in performing video-assisted thoracoscopic extended thymectomy for myasthenia gravis. Surg Endosc 2004;18:821-4. 10.1007/s00464-003-9146-1 [DOI] [PubMed] [Google Scholar]
- 8.Suda T. Single-port thymectomy using a subxiphoid approach-surgical technique. Ann Cardiothorac Surg 2016;5:56-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i54-8. [DOI] [PubMed] [Google Scholar]
- 10.Wu L, Lin L, Liu M, et al. Subxiphoid uniportal thoracoscopic extended thymectomy. J Thorac Dis 2015;7:1658-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zieliński M, Rybak M, Wilkojc M, et al. Subxiphoid video-assisted thorascopic thymectomy for thymoma. Ann Cardiothorac Surg 2015;4:564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Xu G, Chen C, et al. Subxiphoid approach video-assisted thoracoscopic extended thymectomy. Asvide 2016;3:371. Available online: http://www.asvide.com/articles/1140 [DOI] [PMC free article] [PubMed]


