Substitution of canonical histones by their variant counterparts is an important mean of epigenetic regulation.1 The essential and highly conserved histone variant H2A.Z, member of the H2A variant family, comprises of isoforms H2A.Z.1 and H2A.Z.2 and is involved in various DNA-related biological processes such as transcriptional regulation, cell cycle control and DNA repair.1 However, the mechanisms by which H2A.Z influences these processes are to date not entirely understood. Previously, together with the group of Emily Bernstein (Mount Sinai, New York) we reported involvement of H2A.Z.2 in metastatic melanoma progression, linking H2A.Z.2 to skin cancer and hinting towards H2A.Z as a possible cancer marker.2 To identify proteins that aid H2A.Z in chromatin-related processes, we recently determined the H2A.Z-nucleosome-interactome using quantitative mass spectrometry and identified PWWP2A as a novel and strong H2A.Z-nucleosome binder.3 PWWP2A is a vertebrate-specific protein that tightly binds to diverse chromatin features through a concerted multivalent binding mode using several different domains. While the eponymous PWWP domain mediates binding to DNA, the N-terminal part of an internal protein region binds nucleosomes whereas the C-terminal part specifically interacts with H2A.Z. On a genome-wide scope, PWWP2A predominantly binds H2A.Z-containing nucleosomes and preferentially sits at transcriptional start sites of highly transcribed genes.
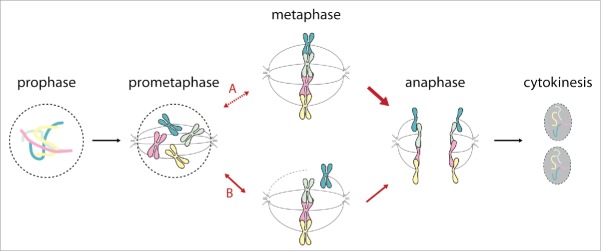
Intriguingly, RNAi-mediated depletion of PWWP2A in HeLa cells results in a severe mitotic progression defect (see Fig. 1) with cells shuffling back and forth between prometaphase and metaphase or halting in metaphase for up to 20 hours. Additionally, chromosomes are unable to properly align on the equatorial plate pointing towards a defect in chromosome congression. Single chromosomes are pulled from the metaphase plate towards the mitotic spindle poles but realign to the metaphase plate disabling the cell to successfully proceed to anaphase. Although cells presented the mentioned mitotic defects, eventually some cells fulfilled cytokinesis, in some cases even without previous separation of the sister chromatids. In general, mitosis is tightly controlled by a plethora of proteins belonging to complexes such as the mitotic checkpoint complex, spindle assembly checkpoint, chromosome passenger complex or anaphase-promoting complex ensuring flawless passage through mitosis.4,5 Testing many members of these complexes in PWWP2A knockdown mitotic cells, we did not find any obvious impairment. In addition, kinetochore assembly and localization appear unaffected by the loss of PWWP2A. On mitotic chromosomes PWWP2A localizes to distinct positions excluding the centromere region, therefore, incorrectly attached microtubuli are unlikely to be the cause of this phenotype.
Figure 1.
Consequences of PWWP2A depletion on mitosis. Mitosis consists of a series of phases starting from prophase, prometaphase, metaphase, anaphase, telophase (not shown) and cytokinesis. Depletion of PWWP2A causes back-and-forth shuffling between prometaphase and metaphase with many cells showing improperly aligned chromosomes at the equatorial plate (B).
One other possibility by which PWWP2A mediates mitotic progression might involve the regulation of genes that together ensure the formation of necessary cellular conditions for chromosome segregation. Indeed, PWWP2A depletion leads to the deregulation of around 700 genes, many being involved in metabolism or in actin- and tubulin-related processes. In line with this finding is the observation that mitotic tubulin filaments appear slightly abnormal in structure, indicating that regulation of the microtubule assembly network might be affected by the reduction in PWWP2A.
Notably, a similar, but not identical, mitotic phenotype has been observed in chicken cells lacking both H2A.Z isoforms6 and involvement of H2A.Z in mitotic chromosome segregation was reported in yeast,7 suggesting that PWWP2A might be the mediator of H2A.Z-dependent mitotic progression in an yet mechanistically unknown fashion.
Besides its role in mitosis, PWWP2A appears to be crucial for proper embryonic development, as PWWP2A depletion in Xenopus causes severe head malformations and defects in neural crest stem cell differentiation and migration. As these cells are postmitotic, the functional link between the observed mitotic phenotype and the developmental defects in Xenopus must be crucial in diverse cellular functions. It will be interesting to see whether changes in metabolism and/or actin/tubulin biology might be the missing connection(s) between both phenotypes.
As neither PWWP2A depletion nor overexpression affects H2A.Z occupancy, but lack of H2A.Z leads to an impaired binding of PWWP2A to nucleosomes, it is tempting to speculate that PWWP2A serves as a mediator which recruits binders to H2A.Z-nucleosomes and fine-tunes expression of H2A.Z-containing genes. Future studies will aid in understanding the functional and mechanistic interplay of PWWP2A and H2A.Z and will shed light on their roles in mitosis and beyond.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Buschbeck M, Hake SB. Variants of core histones and their roles in cell fate decisions, development and cancer. Nat Rev Mol Cell Biol. 2017;18:299-314. doi: 10.1038/nrm.2016.166. PMID:28144029 [DOI] [PubMed] [Google Scholar]
- [2].Vardabasso C, Gaspar-Maia A, Hasson D, Pünzeler S, Valle-Garcia D, Straub T, Keilhauer EC, Strub T, Dong J, Panda T, Chung CY, Yao JL, Singh R, Segura MF, Fontanals-Cirera B, Verma A, Mann M, Hernando E, Hake SB, Bernstein E. . Histone variant H2A.Z.2 mediates proliferation and drug sensitivity of malignant melanoma. Mol Cell. 2015;59:75-88. doi: 10.1016/j.molcel.2015.05.009. PMID:26051178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pünzeler S, Link S, Wagner G, Keilhauer EC, Kronbeck N, Spitzer RM, Leidescher S, Markaki Y, Mentele E, Regnard C, Schneider K, Takahashi D, Kusakabe M, Vardabasso C, Zink LM, Straub T, Bernstein E, Harata M, Leonhardt H, Mann M, Rupp RA, Rupp RA, Hake SB.. Multivalent binding of PWWP2A to H2A.Z regulates mitosis and neural crest differentiation. EMBO J. 2017;36(15):2263-2279. doi: 10.15252/embj.201695757. PMID:28645917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Musacchio A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol. 2015;25:R1002−18. doi: 10.1016/j.cub.2015.08.051. PMID:26485365 [DOI] [PubMed] [Google Scholar]
- [5].Kitagawa M, Lee SH. The chromosomal passenger complex (CPC) as a key orchestrator of orderly mitotic exit and cytokinesis. Front Cell Dev Biol. 2015;3:14. doi: 10.3389/fcell.2015.00014. PMID:25798441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kusakabe M, Oku H, Matsuda R, Hori T, Muto A, Igarashi K, Fukagawa T, Harata M. Genetic complementation analysis showed distinct contributions of the N-terminal tail of H2A.Z to epigenetic regulations. Genes Cells. 2016;21:122-35. doi: 10.1111/gtc.12327. PMID:26833946 [DOI] [PubMed] [Google Scholar]
- [7].Hou H, Wang Y, Kallgren SP, Thompson J, Yates JR 3rd, Jia S. Histone variant H2A.Z regulates centromere silencing and chromosome segregation in fission yeast. J Biol Chem. 2010;285:1909-18. doi: 10.1074/jbc.M109.058487. PMID:19910462 [DOI] [PMC free article] [PubMed] [Google Scholar]