ABSTRACT
White adipose tissue is a remarkably expandable organ with results in the last decade showing that human white adipocytes are continuously turned over during the entire life-span. Data primarily in murine models have demonstrated that adipocytes are derived from precursors present mainly in the perivascular areas of adipose tissue but their precise origin remains unclear. Subsets of cells present in bone marrow display a multipotent differentiation capacity which has prompted the hypothesis that bone marrow-derived cells (BMDCs) may also contribute to the adipocyte pool present in peripheral fat depots. This notion was initially demonstrated in a murine transplantation model, however, subsequent animal studies have been conflicting resulting in a debate of whether BMDCs actually differentiate into adipocytes or just fuse with resident fat cells. This controversy was recently resolved in 2 studies of human subjects undergoing bone marrow transplantation. Using a combination of different assays these data suggest that bone marrow contributes to at least 10% of the adipocyte pool. This proportion is doubled in obesity, suggesting that BMDCs may constitute a reserve pool for adipogenesis, particularly upon weight gain. This review discusses the possible mechanisms and relevance of these findings for human pathophysiology.
KEYWORDS: adipogenesis, obesity, stem cells
White adipose tissue (WAT) is a remarkably expandable organ and obese individuals display twice the number of white fat cells compared with age-matched normal weight subjects.1,2 Despite this, it was for many years unclear whether fat cells are renewed in adulthood. The immergence of 14C-dating techniques could however conclusively demonstrate that adult human fat cells display an annual turnover of ∼10%.2 Adipocyte turnover is determined by the balance between fat cell generation and death. Although this is independent of WAT mass, the larger number of adipocytes in the obese state imply that the total sum of fat cells generated per year is significantly higher compared with lean individuals.2 This implies that there must be a constant supply of adipocyte precursors to allow generation of new fat cells and that these sources must be expanded in the obese state.
Inter-individual variations in the capacity to generate new fat cells may be of pathophysiological importance. Thus, irrespective of body fat mass, subjects with adipose hypertrophy (few but large fat cells), display significantly reduced adipocyte turnover rates compared with age and body weight matched subjects with hyperplasia (many small fat cells).3 Furthermore, adipose hypertrophy associates with insulin resistance/type 2 diabetes while hyperplasia is protective.4-6 This suggests that influencing adipogenesis and thereby adipocyte number could have therapeutic implications in common metabolic disorders, a hypothesis supported by the antidiabetic actions of thiazolidinediones. These agents improve systemic insulin sensitivity, in part by increasing the differentiation of adipocyte progenitor cells resulting in adipose hyperplasia.7 Unfortunately, side effects mediated via actions in non-adipose tissues (primarily fluid retention and osteoporosis), have limited their clinical use in recent years.8
A fundamental question in understanding fat cell formation relates to the origin of adipocytes. While it is clear that they differentiate from progenitor cells present in the perivascular stroma,9-12 it is not yet known from where, when or how these cells migrate into the tissue.13 Adipocyte precursors (APs) could in theory arise from different multipotent cell types. A major obstacle to the study of adipogenesis in vivo, is the fact that APs are not distinctly identifiable by cell surface markers. Several epitope panels have been suggested,14,15 indicating that a variety of progenitor cells with adipogenic capacity may operate within WAT. Furthermore, data in mice suggest that adipocytes arise from APs that are specific for different depots18 or developmental periods.16,17 To date, the majority of studies within this field have been performed in mice, therefore even less is known regarding human APs.19 Identification of the AP spectrum would allow for a much better understanding of how WAT mass expands and possibly also explain the inter-individual variations in metabolic phenotype observed upon changes in fat mass.
Bone Marrow (BM) contains different sets of stem cells, including haematopoietic stem cells and the less abundant, non-haematopoietic mesenchymal stem cells (MSCs).20 Following BM transplantation, several investigators have assessed the contribution of BM-derived cells (BMDCs) to different human tissues including brain, liver and buccal epithelium. These reports suggest that a significant proportion of the cells may be donor-derived.21-27 However, in most studies, the presence of donor-derived cells has been determined by assessing the presence of the Y-chromosomes in female recipients transplanted with BM from male donors, an approach which limits the study population to females receiving male BM. Moreover, virtually all investigators have analyzed sections and/or bulk cell preparations which cannot exclude leukocyte contamination (which by definition are all donor-derived) and/or cell/nuclear fusion events accounting for the Y-chromosome detection. In fact, several studies, primarily in animal models, have suggested that cell fusion is the major mechanism explaining why BM transplantation results in the presence of donor-derived sequences in neurons, hepatocytes and cardiomyocytes.28-32 With regard to WAT, several groups have used allogeneic BM transplantation in mice to study the contribution of BMDCs to selected WAT depots. Unfortunately, the use of BM from transgenic donor animals expressing green fluorescent protein (GFP) (albeit under different promoters) has failed to provide clarity with independent investigators coming to divergent conclusions. In the initial study, transplantation of GFP+ BMDCs into mice generated a small population (2–7%) of GFP expressing adipocytes which increased (up to 8–25%) in the presence of either pro-adipogenic compounds or high fat diet.33 In subsequent studies, the contribution of BMDCs was shown to be gender-, depot- and age-specific.34 Thus, the highest infiltrations rates were observed in the gonadal WAT of female mice, occasionally reaching a maximum of ∼25%. In contrast, other studies have reported no significant contribution of BMDCs to either rat35 or mouse36 WAT, resulting in an uncertainty regarding over the role of BMDCs in murine adipogenesis.
These conflicting results in murine models motivated 2 research groups to study BMDC contribution in human subcutaneous WAT from adult subjects previously transplanted with BM or mobilized peripheral blood stem cell (PBSC).37,38 Together, the studies included >70 men and women spanning a broad range in body mass index (BMI), thereby enabling an assessment of the possible influence of gender and body fat status on BMDC contribution. The investigators explored donor- and recipient-specific gene sequences within the nuclear DNA (microsatellites and/or single nucleotide polymorphisms) allowing the determination of donor cell infiltration irrespective of gender (of the recipient or donor). For these studies to be valid it was essential to establish that the fat cell preparations were free from donor leukocytes and/or other non-adipocyte cell types. This was confirmed by microscopic analyses and qPCR for different non-adipocyte markers and was further supported in both studies by the observation that there was a linear increase in donor cell infiltration following time since transplantation (up to ∼30 years). Moreover, in repeated biopsies from the same subjects, Gavin et al observed an increase in donor-derived fat cells over time.38 If leukocyte contamination would have been an issue, the proportion of donor-derived sequences would have been independent of time.
Using bulk preparations of fat cells, the proportion of donor-derived cells in the 2 studies was very similar ranging from 0.1–35% with an average of 5%37 and 14%,38 respectively. However, the percentage of BMDCs-derived adipocytes in WAT at a given time point is a rough estimate and does not consider the contribution over the entire life span. To evaluate the latter, Rydén et al developed a mathematical model to estimate the contribution of donor cells at steady-state. This “production ratio” was expressed as percent of the total fat cell pool and revealed that on average 10% of the fat cell population was BMDC-derived. While this proportion was not influenced by donor/recipient age, gender and/or different transplantation-related parameters (e.g. cell dose, irradiation, graft versus host reactions etc.), body weight exerted a significant effect as there was a linear relationship between BMI and the production ratio. In fact, the production ratio was more than 2-fold higher in obese compared with lean subjects. Taken together, these findings indicate that BMDCs constitute a significant, but albeit not major reservoir for developing fat cells in non-obese individuals. However, BMDCs become important in obesity, a condition where increased AP demand is met with a doubling in the production ratio. It should be pointed out that the donor cell proportion varied significantly even between BMI-matched subjects. Several factors may dictate this, including the degree of vascularity in WAT which could impact on the ability of BMDCs to access the tissue. In addition, it is also possible that other intrinsic properties of WAT related to the microenvironment (e.g., inflammation, hypoxia, adipokine secretion, leukocyte infiltration) may influence BMDC migration/differentiation.
The results discussed so far were based on bulk analyses of short stretches of donor-derived sequences. As discussed previously, this does not exclude the possibility that donor-derived cells (e.g., leukocytes) had fused with recipient fat cells, resulting in the detection of donor-derived sequences in the purified fat cells. To exclude this possibility Rydén et al developed techniques to retrieve individual mature fat cells and analyze their full content of donor/recipient DNA.37 A major obstacle when working with adipocytes is their fragility and buoyancy once in suspension which makes them notoriously difficult to study at the single cell level. By embedding fat cell suspensions in low-temperature melting agarose, individual adipocytes containing a single nucleus could be isolated by laser capture microdissection. Single cells were subjected to exome sequencing of homozygous single nucleotide polymorphisms (SNPs) unique for either the donor or the recipient. These genomic variations were then called in the exome data as either donor, recipient or mixed genotypes. As expected, the majority of the cells contained only recipient-specific SNPs. Nevertheless, some cells displayed entirely donor-derived SNPs, demonstrating that the nuclear DNA originated only from the donor. Interestingly, some other cells displayed mixed genotypes with both donor- and recipient-derived sequences. The presence of both donor- and mixed sequences was confirmed by genome-wide sequencing. Altogether, this supports the notion that BMDCs can indeed differentiate into mature fat cells, at least in the setting of BM/PBSC transplantation. However, the mixed cells are somewhat more difficult to explain. In theory, BMDCs could fuse with recipient cells which after reduction divisions, results in mononuclear cells with heterokaryons containing sequences from both the donor and the recipient. Ploidy analyses of isolated fat cells were performed in the study by Gavin et al.38 Using 2 independent methods, flow cytometry or fluorescence in situ hybridization, they found no evidence of polysomy suggesting that the presence of donor-derived adipocytes cannot simply be explained by cell fusion resulting in tetra- or aneuploid cells. Thus, adipocytes with a mixed genetic profile may be generated via more complex mechanisms, e.g. involving reduction division. Another possibility is that the mixed and donor-derived cells derive from different cell types, whereby the former result from recipient cell fusion with BMDCs that lack the capacity to differentiate into adipocytes while the latter derive from BMDCs with adipogenic potential.
Neither of the human studies could establish whether the fat cell phenotype differed between donor-derived or recipient cells. This is relevant given that data in mice suggest that BM-derived adipocytes, in comparison with recipient fat cells, display higher expression of pro-inflammatory genes and lower expression of genes involved in mitochondrial biogenesis and lipid oxidation.34 It would therefore be of interest to compare the global gene expression in fat cells of donor, recipient or mixed origins. Unfortunately, it is currently still a major challenge to analyze both the genome and transcriptome from the same single cell.
Another relevant issue is to identify the BMDC subset that differentiates into the adipocyte lineage. It is currently a matter of debate whether haematopoietic stem cells can develop into cells outside the haematopoietic lineage32,39-45 and most investigators suggest that haematopoietic stem cells cannot cross lineage boundaries.42 In accordance with this, bulk preparations of human fat cells from BM-transplanted subjects expressed no detectable amounts of haematopoietic markers.37,38 Another type of multipotent stem cells are the MSCs which have the capacity to develop into functional cells of the mesenchymal lineage e.g., osteocytes, chondrocytes and adipocytes.46,47 As MSCs can be found in both BM and PBSC48,49 it could be speculated that these cells may constitute “adipogenic” BMDCs. Based on data from murine models, additional cell sources could be endothelial cells.50
Admittedly, both the murine and human results discussed herein were obtained in transplanted subjects and may not reflect normal physiology. However, the time-dependent increase in donor cell infiltration,37,38 in the absence of immunosuppressant therapy,37 suggests that BMDC-derived adipogenesis is a continuous process that may be relevant also outside the setting of transplantation. The recent findings in mice, indicating that fat cells may arise from distinct precursor pools, would be in line with a notion that BM constitutes one of several progenitor pools contributing to WAT mass growth (Fig. 1). Future development of techniques allowing identification of cellular origin also under non-transplanted conditions will hopefully resolve these issues. Although speculative, BMDCs with adipogenic potential could be of value in future approaches targeting genetically dysfunctional WAT, e.g., in severe forms of lipodystrophy.
Figure 1.
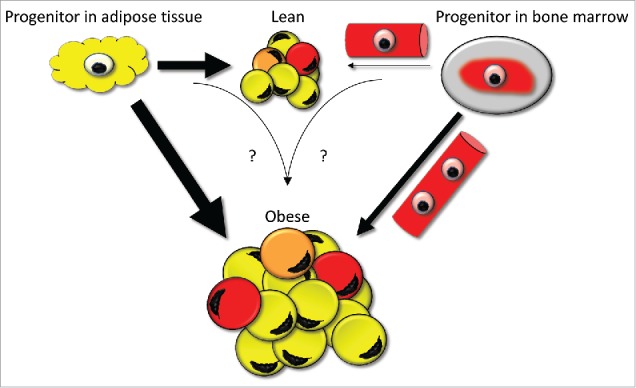
The contribution of bone marrow-derived cells to the human fat cell population. In the lean state, progenitor cells (light yellow) resident in the perivascular areas of WAT constitute the primary source of adipocytes (indicated by thick arrow and many yellow adipocytes). The contribution of bone marrow-derived progenitor cells transported via the circulation (light red) and subsequently differentiated into fat cells is probably only minor (indicated by thin arrow and few red fat cells). Upon increases in fat mass, the number of fat cells is increased which requires an input from additional adipocyte precursor sources. In this setting, the contribution of bone marrow-derived cells becomes relatively more significant (indicated by a thicker arrow). In the transplanted individuals, some cells display mixed genotypes containing both donor and recipient sequences. This suggests the possibility that a limited amount of recipient and donor cells may, via unclear mechanisms (highlighted by question marks), result in fused cells with diploid DNA content (orange).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Alastair Kerr for reading and his constructive advice on the text.
References
- [1].Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest 1979; 63:239-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, et al.. Dynamics of fat cell turnover in humans. Nature 2008; 453:783-7; PMID:18454136; http://dx.doi.org/ 10.1038/nature06902 [DOI] [PubMed] [Google Scholar]
- [3].Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 2010; 59:105-9; PMID:19846802; http://dx.doi.org/ 10.2337/db09-0942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lonn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. Faseb J 2010; 24:326-31; PMID:19741173; http://dx.doi.org/ 10.1096/fj.09-133058 [DOI] [PubMed] [Google Scholar]
- [5].Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000; 43:1498-506; PMID:11151758; http://dx.doi.org/ 10.1007/s001250051560 [DOI] [PubMed] [Google Scholar]
- [6].Arner P, Arner E, Hammarstedt A, Smith U. Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS One 2011; 6:e18284; PMID:21532749; http://dx.doi.org/ 10.1371/journal.pone.0018284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Frayn KN, Tan GD, Karpe F. Adipose tissue: a key target for diabetes pathophysiology and treatment? Horm Metab Res 2007; 39:739-42; PMID:17952837; http://dx.doi.org/ 10.1055/s-2007-990270 [DOI] [PubMed] [Google Scholar]
- [8].Della-Morte D, Palmirotta R, Rehni AK, Pastore D, Capuani B, Pacifici F, De Marchis ML, Dave KR, Bellia A, Fogliame G, et al.. Pharmacogenomics and pharmacogenetics of thiazolidinediones: role in diabetes and cardiovascular risk factors. Pharmacogenomics 2014; 15:2063-82; PMID:25521362; http://dx.doi.org/ 10.2217/pgs.14.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al.. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3:301-13; PMID:18786417; http://dx.doi.org/ 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- [10].Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev 2008; 17:1053-63; PMID:18597617; http://dx.doi.org/ 10.1089/scd.2008.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol 2008; 214:413-21; PMID:17654479; http://dx.doi.org/ 10.1002/jcp.21210 [DOI] [PubMed] [Google Scholar]
- [12].Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell 2008; 135:240-9; PMID:18835024; http://dx.doi.org/ 10.1016/j.cell.2008.09.036 [DOI] [PubMed] [Google Scholar]
- [13].Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab 2014; 19:8-20; PMID:24239569; http://dx.doi.org/ 10.1016/j.cmet.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 2013; 15:302-8; PMID:23434825; http://dx.doi.org/ 10.1038/ncb2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Church CD, Berry R, Rodeheffer MS. Isolation and study of adipocyte precursors. Methods Enzymol 2014; 537:31-46; PMID:24480340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol 2015; 17:376-85; PMID:25730471; http://dx.doi.org/ 10.1038/ncb3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep 2014; 9:1007-22; PMID:25437556; http://dx.doi.org/ 10.1016/j.celrep.2014.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 2012; 61:1691-9; PMID:22596050; http://dx.doi.org/ 10.2337/db11-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Raajendiran A, Tsiloulis T, Watt MJ. Adipose tissue development and the molecular regulation of lipid metabolism. Essays Biochem 2016; 60:437-50; PMID:27980094; http://dx.doi.org/ 10.1042/EBC20160042 [DOI] [PubMed] [Google Scholar]
- [20].Stoltz JF, de Isla N, Li YP, Bensoussan D, Zhang L, Huselstein C, Chen Y, Decot V, Magdalou J, Li N, et al.. Stem cells and regenerative medicine: Myth or Reality of the 21th century. Stem Cells Int 2015; 2015:734731; PMID:26300923; http://dx.doi.org/ 10.1155/2015/734731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, Scott EW. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet 2004; 363:1432-7; PMID:15121406; http://dx.doi.org/ 10.1016/S0140-6736(04)16102-3 [DOI] [PubMed] [Google Scholar]
- [22].Crain BJ, Tran SD, Mezey E. Transplanted human bone marrow cells generate new brain cells. J Neurol Sci 2005; 233:121-3; PMID:15949500; http://dx.doi.org/ 10.1016/j.jns.2005.03.017 [DOI] [PubMed] [Google Scholar]
- [23].Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci U S A 2003; 100:1364-9; PMID:12538864; http://dx.doi.org/ 10.1073/pnas.0336479100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol 2003; 5:959-66; PMID:14562057; http://dx.doi.org/ 10.1038/ncb1053 [DOI] [PubMed] [Google Scholar]
- [25].Tran SD, Pillemer SR, Dutra A, Barrett AJ, Brownstein MJ, Key S, Pak E, Leakan RA, Kingman A, Yamada KM, et al.. Differentiation of human bone marrow-derived cells into buccal epithelial cells in vivo: a molecular analytical study. Lancet 2003; 361:1084-8; PMID:12672312; http://dx.doi.org/ 10.1016/S0140-6736(03)12894-2 [DOI] [PubMed] [Google Scholar]
- [26].ten Hove WR, Verspaget HW, Barge R, Lamers CB, van Hoek B. Liver chimerism after allogeneic blood stem cell transplantation. Transplant Proc 2007; 39:231-6; PMID:17275511; http://dx.doi.org/ 10.1016/j.transproceed.2006.10.022 [DOI] [PubMed] [Google Scholar]
- [27].Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology 2000; 32:11-6; PMID:10869283; http://dx.doi.org/ 10.1053/jhep.2000.9124 [DOI] [PubMed] [Google Scholar]
- [28].Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 2003; 425:968-73; PMID:14555960; http://dx.doi.org/ 10.1038/nature02069 [DOI] [PubMed] [Google Scholar]
- [29].Nern C, Wolff I, Macas J, von Randow J, Scharenberg C, Priller J, Momma S. Fusion of hematopoietic cells with Purkinje neurons does not lead to stable heterokaryon formation under noninvasive conditions. J Neurosci 2009; 29:3799-807; PMID:19321776; http://dx.doi.org/ 10.1523/JNEUROSCI.5848-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY, Steinman L, Rossi FM, Blau HM. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol 2008; 10:575-83; PMID:18425116; http://dx.doi.org/ 10.1038/ncb1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature 2003; 422:901-4; PMID:12665833; http://dx.doi.org/ 10.1038/nature01539 [DOI] [PubMed] [Google Scholar]
- [32].Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003; 422:897-901; PMID:12665832; http://dx.doi.org/ 10.1038/nature01531 [DOI] [PubMed] [Google Scholar]
- [33].Crossno JT Jr, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest 2006; 116:3220-8; PMID:17143331; http://dx.doi.org/ 10.1172/JCI28510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Majka SM, Fox KE, Psilas JC, Helm KM, Childs CR, Acosta AS, Janssen RC, Friedman JE, Woessner BT, Shade TR, et al.. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc Natl Acad Sci U S A 2010; 107:14781-6; PMID:20679227; http://dx.doi.org/ 10.1073/pnas.1003512107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tomiyama K, Murase N, Stolz DB, Toyokawa H, O'Donnell DR, Smith DM, Dudas JR, Rubin JP, Marra KG. Characterization of transplanted green fluorescent protein+ bone marrow cells into adipose tissue. Stem Cells 2008; 26:330-8; PMID:17975222; http://dx.doi.org/ 10.1634/stemcells.2007-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest 2007; 117:3684-95; PMID:18060029; http://dx.doi.org/ 10.1172/JCI32504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ryden M, Uzunel M, Hard JL, Borgstrom E, Mold JE, Arner E, Mejhert N, Andersson DP, Widlund Y, Hassan M, et al.. Transplanted Bone Marrow-Derived Cells Contribute to Human Adipogenesis. Cell Metab 2015; 22:408-17; PMID:26190649; http://dx.doi.org/ 10.1016/j.cmet.2015.06.011 [DOI] [PubMed] [Google Scholar]
- [38].Gavin KM, Gutman JA, Kohrt WM, Wei Q, Shea KL, Miller HL, Sullivan TM, Erickson PF, Helm KM, Acosta AS, et al.. De novo generation of adipocytes from circulating progenitor cells in mouse and human adipose tissue. Faseb J 2016; 30:1096-108; PMID:26581599; http://dx.doi.org/ 10.1096/fj.15-278994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Porada CD, Atala AJ, Almeida-Porada G. The hematopoietic system in the context of regenerative medicine. Methods 2016; 99:44-61; PMID:26319943; http://dx.doi.org/ 10.1016/j.ymeth.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002; 416:542-5; PMID:11932747; http://dx.doi.org/ 10.1038/nature730 [DOI] [PubMed] [Google Scholar]
- [41].Wurmser AE, Nakashima K, Summers RG, Toni N, D'Amour KA, Lie DC, Gage FH. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature 2004; 430:350-6; PMID:15254537; http://dx.doi.org/ 10.1038/nature02604 [DOI] [PubMed] [Google Scholar]
- [42].Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med 2001; 7:393-5; PMID:11283651; http://dx.doi.org/ 10.1038/86439 [DOI] [PubMed] [Google Scholar]
- [43].Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001; 105:369-77; PMID:11348593; http://dx.doi.org/ 10.1016/S0092-8674(01)00328-2 [DOI] [PubMed] [Google Scholar]
- [44].Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 2002; 297:2256-9; PMID:12215650; http://dx.doi.org/ 10.1126/science.1074807 [DOI] [PubMed] [Google Scholar]
- [45].Sera Y, LaRue AC, Moussa O, Mehrotra M, Duncan JD, Williams CR, Nishimoto E, Schulte BA, Watson PM, Watson DK, et al.. Hematopoietic stem cell origin of adipocytes. Exp Hematol 2009; 37:1108-20, 20 e1-4; PMID:19576951; http://dx.doi.org/ 10.1016/j.exphem.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143-7; PMID:10102814; http://dx.doi.org/ 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- [47].Kobolak J, Dinnyes A, Memic A, Khademhosseini A, Mobasheri A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods 2016; 99:62-8; PMID:26384580; http://dx.doi.org/ 10.1016/j.ymeth.2015.09.016. [DOI] [PubMed] [Google Scholar]
- [48].Fernandez M, Simon V, Herrera G, Cao C, Del Favero H, Minguell JJ. Detection of stromal cells in peripheral blood progenitor cell collections from breast cancer patients. Bone Marrow Transplant 1997; 20:265-71; PMID:9285540; http://dx.doi.org/ 10.1038/sj.bmt.1700890 [DOI] [PubMed] [Google Scholar]
- [49].Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant 2006; 37:967-76; PMID:16670702; http://dx.doi.org/ 10.1038/sj.bmt.1705358 [DOI] [PubMed] [Google Scholar]
- [50].Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, et al.. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 2012; 15:222-9; PMID:22326223; http://dx.doi.org/ 10.1016/j.cmet.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


