ABSTRACT
Some bona fide adult adipocytes arise de novo from a bone marrow-derived myeloid lineage. These studies further demonstrate that adipose tissue stroma contains a resident population of myeloid cells capable of adipocyte and multilineage mesenchymal differentiation. These resident myeloid cells lack hematopoietic markers and express mesenchymal and progenitor cell markers. Because bone marrow mesenchymal progenitor cells have not been shown to enter the circulation, we hypothesized that myeloid cells acquire mesenchymal differentiation capacity in adipose tissue. We fabricated a 3-dimensional fibrin matrix culture system to define the adipose differentiation potential of adipose tissue-resident myeloid subpopulations, including macrophages, granulocytes and dendritic cells. Our data show that multilineage mesenchymal potential was limited to adipose tissue macrophages, characterized by the acquisition of adipocyte, osteoblast, chondrocyte and skeletal muscle myocyte phenotypes. Fibrin hydrogel matrices stimulated macrophage loss of hematopoietic cell lineage determinants and the expression of mesenchymal and progenitor cell markers, including integrin β1. Ablation of integrin β1 in macrophages inhibited adipocyte specification. Therefore, some bona fide adipocytes are specifically derived from adipose tissue-resident macrophages via an integrin β1-dependent hematopoietic-to-mesenchymal transition, whereby they become capable of multipotent mesenchymal differentiation. The requirement for integrin β1 highlights this molecule as a potential target for controlling the production of marrow-derived adipocytes and their contribution to adipose tissue development and function.
KEYWORDS: adipocyte, fibrin matrix, hematopoietic-to-mesenchymal transition, integrin, macrophage
Introduction
Cardiovascular disease, diabetes and other chronic metabolic disorders have long been linked to increases in total body fat mass,1,2 and more recently to increases in central adiposity.3,4 However, the mechanisms linking regional adiposity to chronic disease remain poorly understood, and current paradigms fail to explain several anomalies in the occurrence of fat-related chronic disease. First, approximately 10 to 25% of obese individuals are metabolically healthy,5 while a small percentage of lean individuals exhibit hallmarks of the metabolic syndrome and succumb to cardiovascular pathologies. Second, individuals who suffer from extreme loss of body fat due to under-nutrition or lipodystrophic disorders display the same metabolic complications as overweight and obese patients.6 Third, among the elderly, and people with chronic kidney disease, heart failure, or chronic obstructive pulmonary disease, those with an obese body mass index (BMI) between 30.0 and 34.9 counterintuitively have lower mortality than those with a normal BMI of less than 25 – an observation termed the “Obesity Paradox.”7–9 Fourth, the “Fit but Fat” model suggests that moderate-to-vigorous exercise abolishes differences in cardiovascular risk between overweight/obese and lean adults.10 These anomalies highlight fundamental deficiencies in current paradigms linking total body fat mass or central adiposity to pathological outcomes in patients with excess or diminished body fat.
The importance of variation in the cellular composition of adipose tissue has been underappreciated in previous attempts to understand the role of adipose tissue in causing pathophysiology. Exploiting green fluorescent protein (GFP)–labeled hematopoietic stem cells or lineage tracing with the myeloid-restricted lysozyme gene promoter (LysM) and Cre-Lox technology, we previously reported that a subpopulation of bona fide adipocytes in the major adipose depots of mice were generated, de novo, from bone marrow progenitor (BMP) cells.11–13 These BMP-derived adipocytes were produced in numbers sufficient (15–25% of total adipocytes) to contribute to adipose tissue function. High fat feeding increased the production of BMP-derived adipocytes, and they accumulated preferentially in visceral fat, and in greater numbers in female than male mice.12 We therefore proposed that a subpopulation of adipocytes arise from marrow progenitors that traffic to fat tissue and undergo adipogenic conversion. These observations were particularly noteworthy because global gene expression profiling demonstrated that BMP-derived adipocytes differ from conventional white or brown adipocytes, and possess a potentially detrimental phenotype including minimal expression of leptin and genes related to mitochondrial fuel oxidation, and elevated production of numerous inflammatory cytokines. Recently, Rydén et al.14 reported that BMP-derived adipocytes are produced in humans, an observation independently confirmed in our laboratory.15
Several important observations from ongoing murine studies demonstrated that BMP-derived adipocytes have a hematopoietic origin rather than the mesenchymal origin common to conventional white and brown adipocytes.12 Competitive bone marrow (BM) transplants initially revealed that BMP-derived adipocytes were produced from either CD45POS or lineage positive (LinPOS) marrow cells, but not CD45NEG or LinNEG (lack of expression of all differentiated hematopoietic lineage markers) populations. Since myeloid cells, but not lymphocytes, are able to generate phenotypes of skeletal muscle,16 vascular endothelium,17 and liver18 we tested whether BM myeloid cells could give rise to BMP-derived adipocytes. LacZPOS adipocytes were detected by flow cytometry and microscopy in adipose tissue from LysM-cre ROSAflox/STOP mice in which LacZ expression was controlled by the myeloid-restricted lysozyme gene promoter.12 We concluded that BMP-derived adipocytes arise from the hematopoietic lineage via myeloid intermediates.
Despite their hematopoietic origin, BMP-derived adipocytes are devoid of hematopoietic (CD45) and myeloid (CD11b, F4/80) markers.11–13,15,19 This suggests that the hematopoietic/myeloid cells from which BMP-derived adipocytes are derived lose their hematopoietic markers during differentiation to adipocytes. In support of this theory, we have also detected a population of BM-derived cells in adipose stroma that lack CD45 and CD11b, and are capable of adipogenic differentiation.15 Mesenchymal progenitor cells are not readily detectable in the circulation,20-24 thus these cells likely arose from hematopoietic cells that lost their hematopoietic markers while acquiring adipogenic potential. Cumulatively, our data support the existence of a novel “hematopoietic-to-mesenchymal” transition in the production of murine BMP-derived adipocytes.
Here we demonstrate that adipose tissue macrophages from adult mice, but not other myeloid cells, cultured in 3-dimensional (3D) fibrin matrices acquire the ability to differentiate into adipocytes and typical mesenchymal phenotypes including osteoblasts, chondrocytes and skeletal muscle myotubes. During culture in fibrin, the macrophages lose hematopoietic and myeloid markers and acquire markers common to mesenchymal progenitor cells. Myeloid cells from other tissues were not able to generate the complete repertoire of mesenchymal phenotypes when cultured in fibrin. The data suggest that the adipose tissue microenvironment induces a “hematopoietic-to-mesenchymal” transition in local macrophages. In addition, integrin β1 expression is upregulated during fibrin matrix culture, and knockdown of integrin β1 blocked the acquisition of adipogenic capacity. These observations establish a powerful in vitro model for exploring the developmental events that promote BMP-derived adipocyte production in the adult, and highlight integrin signaling as a potential target for controlling the cellular composition of adipose tissue.
Results
Adipose tissue stroma contains BM-derived cells with adipogenic capacity
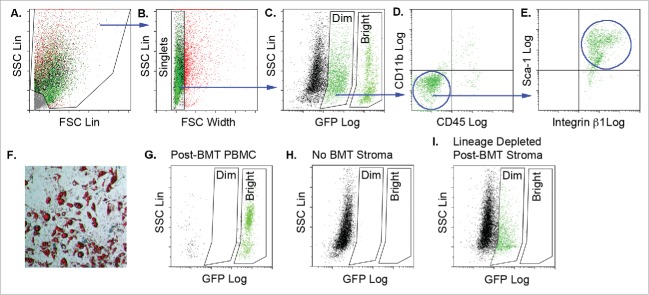
To date, mature adipocytes have not been detected in blood. Therefore, BMP-derived adipocytes are likely produced de novo in adipose tissue from marrow-derived progenitor cells that arrive via the circulation. We sought to identify marrow-derived adipocyte progenitor cells in adipose tissue from wild type mice transplanted with BM from donors ubiquitously expressing GFP. GFP expression in marrow-derived gonadal and dorsal adipose stromal cells was evaluated by flow cytometry using the gating strategy shown in Figure 1. Debris was excluded from the stromal population based on side scatter (SSC) versus forward scatter (FSC) analysis (Fig. 1A). Clusters and aggregates were excluded and single cells (singlets) retained based on the ratio of SSC signal height to FSC signal width (Fig. 1B). Singlets could be resolved into 3 populations based on GFP fluorescence: GFPNEG (GFP-negative) GFPDIM and GFPBright (Fig. 1C). Further evaluation of the GFPDIM population revealed that the majority of cells did not express the pan-leukocyte marker, CD45, or the myeloid marker, CD11b (Fig. 1D). However, these cells did express the mesenchymal marker integrin β1 and the progenitor cell marker, Sca-1 (Fig. 1E). No GFPDIM or GFPBright cells were detected in adipose tissue from transplant-naïve, wild type mice confirming that these populations expressed GFP and originated from the transplanted GFP-labeled BM (Fig. 1H). The existence of distinct GFPDIM and GFPBright populations was evident not only in the discontinuous spectrum of GFP fluorescence intensities shown in Figure 1C, but also by simultaneous flow cytometry analysis of peripheral blood mononuclear cells (PBMC) from mice transplanted with GFP-expressing BM, which revealed a high percentage of GFPBright, a small number of GFPNEG cells, but no GFPDIM events (Fig. 1G). Furthermore, when adipose stroma from mice transplanted with GFP-labeled BM was depleted of cells bearing hematopoietic lineage markers, only the GFPBright population was removed (Fig. 1I). These data would suggest that the GFPBright population in the tissue stroma was comprised differentiated hematopoietic cells expressing one or more of the lineage determinants CD11b, Gr-1, CD5 or B220. In contrast, the GFPDIM cells lacked the expression of hematopoietic lineage determinants, including CD45 and CD11b and represented a bone marrow derived ‘mesenchymal’ population present in the tissue stroma.
Figure 1.
Adipose tissue stroma contains bone marrow-derived cells with adipogenic capacity. Adipose tissue stromal cells from the gonadal and dorsal interscapular adipose depots were recovered from female wild type mice transplanted with bone marrow from male donor mice ubiquitously expressing GFP (cells from n = 3–5 animals pooled/experiment; independent experiments conducted in triplicate). The stromal cells were stained with fluorescent antibodies to CD45, CD11b, Sca-1 and integrin β1 and separated by flow cytometry using the gating strategy, which progresses from left to right (blue arrows). A) Debris was excluded based on the ratio of forward scatter (FSC) height to side scatter (SSC) height. B) Clusters and aggregates were excluded based on the ratio of SSC height to FSC width. C) Comparison of SSC to GFP fluorescence revealed GFPDIM and GFPBright populations as well as GFP-negative cells. D) The majority of GFPDIM cells did not express CD45 or CD11b, but E) did express Sca-1 and integrin β1. F) Flow cytometry purified adipose stromal GFPDIM cells were plated on plastic in MesenCult medium with Stem Cell Stimulatory Supplements (SCSS) until they reached confluency. The medium was switched to MesenCult medium plus adipogenic supplements, and the cells were stained with Oil Red O after 9 d. Representative image shows Oil Red O stained lipid droplets indicating adipogenic differentiation. Magnification 10x. G) Flow cytometry of circulating peripheral blood mononuclear cells (PBMCs) from wild type mice transplanted with GFPPOS bone marrow reveals primarily GFPBright cells with a small number of GFPNEG cells. No GFPDIM cells were observed. Representative image of n = 5 independent animals. H) Flow cytometry of adipose stromal cells from untransplanted mice revealed only GFPNEG cells. I) Adipose stromal cells from mice transplanted with GFP-expressing BM were depleted of LinPOS cells by magnetic bead lineage depletion. Flow cytometry shows loss of GFPBright cells. Representative image of n = 3 independent animals.
When plated on plastic in mesenchymal cell growth medium (MesenCult MSC Basal Medium plus Mesenchymal Stem Cell Stimulatory Supplements), the GFPDIM cells proliferated and underwent spontaneous adipogenic conversion (Fig. 1F). The GFPBright population did not adhere to plastic, proliferate or produce adipocytes in mesenchymal cell growth medium. This suggests that GFPDIM stromal cells represent a “mesenchymal-like” intermediate in the adipose stroma originating from the BM, which is capable of adipogenesis.
To test whether GFPDIM stromal cells were also capable of adipogenesis in vivo they were suspended in liquid Matrigel and injected subcutaneously in the abdomen of athymic mice. The use of athymic, or immunodeficient nude, mice was necessary to prevent the rejection of the Matrigel plug containing allogeneic cells. GFPNEG/LinNEG stromal cells, a population that contains tissue-resident preadipocytes, were implanted in the same manner in other mice as positive controls for in vivo adipogenesis. The solidified Matrigel plugs and adjacent regions of skin and muscle were recovered 4 weeks later, fixed in paraformaldehyde, embedded in paraffin and sectioned. Deparaffinization of the sections solubilized the triglycerides in adipocyte lipid droplets leaving cavities in the Matrigel, which were associated with hematoxylin-stained nuclei (Fig. 2A). Nuclei-associated cavities were observed in sections from mice implanted with either GFPDIM or GFPNEG/LinNEG stromal cells indicating that both populations contained cells capable of in vivo adipogenesis. Immunostaining revealed that the cavities from both populations were surrounded by perilipin, a lipid droplet surface protein expressed in adipocytes (Fig. 2B). As anticipated, no cytosolic GFP fluorescence was detected in adipocytes generated from GFPNEG/LinNEG stromal cells. However, GFP was present surrounding the lipid droplets of adipocytes that arose from implanted GFP-expressing BM cells confirming their BM origin (Fig. 2B). Thus, adipose tissue contains a population of LinNEG cells of bone marrow origin (GFPDIM) capable of adipogenic conversion in vitro and in vivo.
Figure 2.
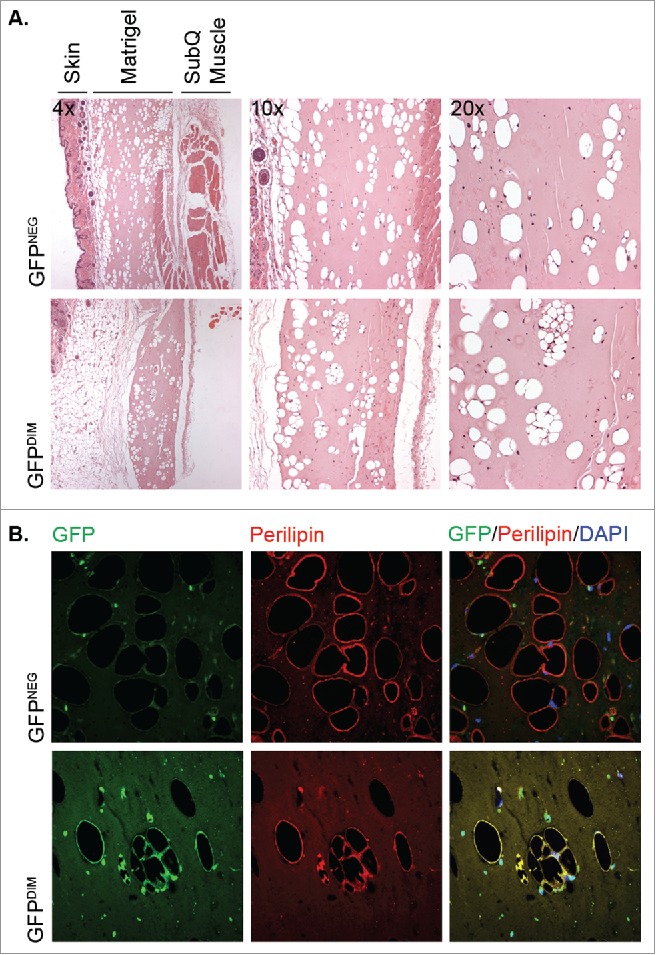
GFPDIM adipose stromal cells have adipogenic capacity in vivo. GFPDIM and GFPNEG adipose stromal cells were purified from female wild type mice transplanted with BM from male mice constitutively expressing GFP by flow cytometric sorting as in Figure 1 (cells from n = 3–5 animals pooled/experiment). The cells were pelleted and resuspended in cold Matrigel at 2 × 106 cells per 200 µl. The cell suspensions were injected subcutaneously in the abdomen anterior to the thigh of athymic female mice. Four weeks later the Matrigel plugs and adjacent skin and muscle were recovered, fixed in paraformaldehyde overnight and cut in half for embedding in paraffin. A) Five μm sections were deparaffinized and stained with Hematoxylin and Eosin. Figure shows images taken at 4x, 10x or 20x magnification of sections from mice receiving GFPNEG or GFPDIM cells. B) Additional sections were stained with a primary antibody to perilipin and Alexa 555-conjugated secondary antibody, and counterstained with DAPI. Fluorescence images taken at 40x show GFP and perilipin signals individually or overlaid with DAPI. Representative images of n = 3 independent experiments.
Adipose tissue macrophages are capable of differentiating into multiple mesenchymal phenotypes following culture in 3 dimensional fibrin matrices
We previously demonstrated that adipose stroma contained CD45NEG/CD11bNEG cells derived from the myeloid lineage (LacZ+ cells from lysM-cre ROSAfloxlysMcreROSAflox/STOP mice), which were capable of adipogenesis to varying efficiencies when cultured in 3D matrices, fibrin being highly efficient or Matrigel, low efficiency.12 Building on these studies, we tested whether specific subpopulations of adipose myeloid cells, including macrophages, neutrophils, eosinophils or dendritic cells, could be directed toward a “hematopoietic -to-mesenchymal” transition in engineered fibrin matrices in vitro, and acquire an enhanced ability to differentiate into adipocytes and other mesenchymal phenotypes.
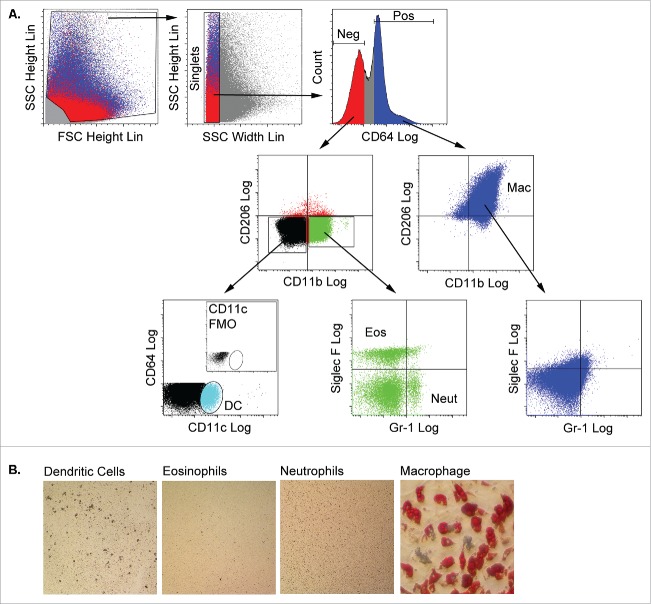
Myeloid subpopulations were purified from CD45POS adipose stroma using a flow cytometry sorting strategy recently reported by Lumeng and colleagues25 as shown in Figure 3A. Singlets were first separated based on their expression of the high affinity IgG receptor, CD64. The CD64POS population uniformly expressed the macrophage markers CD11b and CD206, but did not express markers for neutrophils (Gr-1) or eosinophils (Siglec-F) and was collected as the macrophage subpopulation. CD64NEG cells did not express CD206, but some were positive for CD11b. Distinct eosinophil (Siglec-FPOS) and neutrophil (Gr-1POS) subpopulations were identified in the CD64NEG/CD11bPOS population. Finally, CD11cPOS dendritic cells were sorted from the CD64NEG/CD11bNEG population.
Figure 3.
Adipose tissue macrophages are capable of adipogenic differentiation following culture in 3 dimensional fibrin matrices. A) Stromal cells from collagenase-digested gonadal adipose tissue of male mice (n = 3 pooled; independent experiments conducted in duplicate) were separated from adipocytes by centrifugation. Stromal cells were stained with fluorescent antibodies to CD64, CD11b, CD206, CD11c, Siglec-F and Gr-1, and myeloid subpopulations (macrophages, eosinophils, neutrophils and dendritic cells) were purified by flow cytometry using the gating strategy shown. Debris was excluded based on the ratio of FSC height to SSC height. Clusters and aggregates were excluded based on the ratio of SSC height to FSC width. CD64 fluorescence revealed distinct positive and negative populations. The CD64POS fraction expressed CD11b and CD206, but were negative for eosinophil (Siglec-F) and neutrophil (Gr-1) markers, and were recovered as the adipose tissue macrophage (Mac) population. The CD64NEG fraction contained cells expressing CD11b, but not CD206, and neutrophils (Neut, Gr-1POS) and eosinophils (Eos, Siglec-FPOS) were recovered from this population. Finally, the CD64NEG/CD11bNEG/CD206NEG fraction contained a CD11c-expressing population, which was recovered as dendritic cells (DC). DCs were not detected in stromal fractions stained with all antibodies minus the CD11c antibody (fluorescence minus one or FMO, inset). Each fraction was cultured in fibrin matrices overlaid with MesenCult medium containing SCSS for 5 d. B) Cells were recovered from the clots by plasmin hydrolysis of the fibrin and plated in plastic wells with MesenCult medium with SCSS until confluency. The cells were then switched to MesenCult medium with adipogenic supplements for 14–21 d. Cells were then stained with Oil Red O to assess adipogenic differentiation.
Each of the myeloid subpopulations was suspended in fibrin matrices overlaid with mesenchymal cell growth medium for 5 d after which they were recovered by digestion of the matrix with plasmin. Plastic adherent, proliferating cells were recovered from all 4 fibrin-treated myeloid subpopulations (Fig. 3B), but only fibrin-treated adipose tissue macrophages were capable of spontaneous adipogenic conversion.
Adipose tissue macrophages cultured in fibrin hydrogels decrease expression of myeloid markers, acquire mesenchymal and progenitor cell markers, and are capable of differentiating to multiple mesenchymal fates
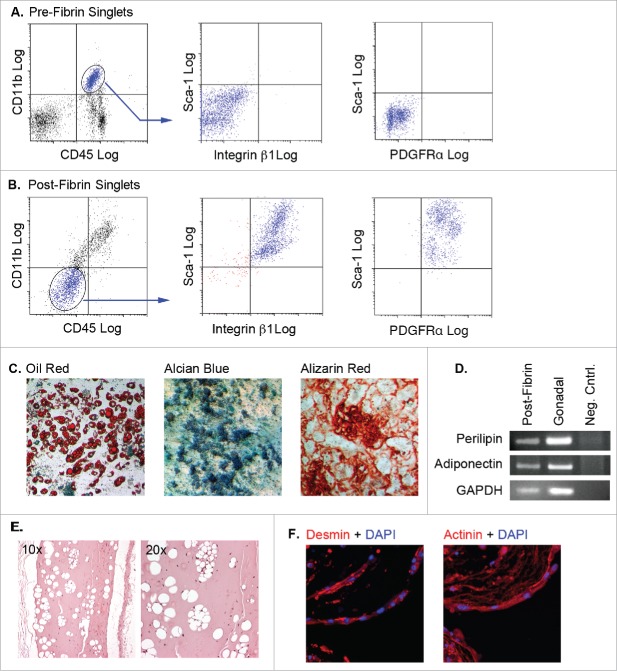
Flow cytometry analysis of adipose tissue myeloid cells before (Fig. 4A) and following (Fig. 4B) culture in fibrin matrices showed that the cells lost pan-hematopoietic (CD45) markers, while acquiring mesenchymal (integrin β1 or CD29) and progenitor cell (Sca-1) markers. The post-fibrin cells were reminiscent of the GFPDIM cells presented in Figure 1A–E. Post-fibrin cells also expressed PDGFRα (Fig. 4B). The post-fibrin population was plastic adherent, proliferated in mesenchymal cell growth medium, and was capable of adipogenic, osteogenic and chondrogenic differentiation (Fig. 4C). RT-PCR analysis of RNA from post-fibrin adipocytes and adipose tissue showed expression of adiponectin and perilipin in both samples confirming that post-fibrin cells with lipid droplets were bona fide adipocytes (Fig. 4D). Adipocytes were also produced from post-fibrin cells implanted subcutaneously in athymic mice, indicating their in vivo adipogenic capacity (Fig. 4E).
Figure 4.
Adipose tissue myeloid cells lose expression of hematopoietic/myeloid markers, acquire mesenchymal and progenitor markers, and are capable of differentiating to multiple mesenchymal fates following 3D fibrin culture. (A & B) Pre-fibrin LinPOS gonadal and dorsal (pooled n = 3–5) adipose stromal cells (A) or post-fibrin “myeloid” cells (B) were stained with fluorescently-labeled antibodies to CD45, CD11b, integrin β1, PDGFRα and Sca-1 as indicated. Cells were analyzed and separated by flow cytometry. A) Pre-fibrin stroma contained cells expressing both CD45 and CD11b, but lacking integrin β1, PDGFRα and Sca-1. B) The majority of the post-fibrin cells were CD45NEG and CD11bNEG, but expressed integrin β1, PDGFRα and Sca-1. C) The CD45NEG/CD11bNEG post-fibrin cells were plated in plastic wells with MesenCult medium with SCSS until confluency. The cells were then switched to MesenCult medium with adipogenic supplements, or STEMPRO medium containing either osteogenic or chondrogenic supplements for 14–21 d. Cells were then stained with Oil Red O, Alcian Blue or Alizarin Red to assess adipogenic, chondrogenic or osteogenic differentiation, respectively. Analyses were performed twice independently. D) Semiquantitative RT-PCR for perilipin, adiponectin or GAPDH RNA extracted from post-fibrin CD45NEG/CD11bNEG cells treated with adipogenic agents as in (C). RNA from whole gonadal adipose tissue was included as a positive control. E) Post-fibrin CD45NEG/CD11bNEG cells were suspended in cold Matrigel and injected subcutaneously into female athymic mice (n = 2). After 4 weeks, the Matrigel plugs and adjacent tissue were recovered for fixation, embedding and sectioning. Five μm sections were stained with hematoxylin and eosin. Representative images show the presence of cavities in the Matrigel formed by adipocyte lipid droplets. F) Filaments of multinucleated cells spontaneously formed in cultures of post-fibrin CD45NEG/CD11bNEG cells in MesenCult medium as shown by DAPI staining. When stained with primary antibodies to either actinin or desmin and Alexa 555-conjugated secondary antibodies, the filaments exhibited intense fluorescence indicative of myocyte differentiation.
Under adipogenic conditions, we frequently observed foci of tangled “fibers” among adipocytes and undifferentiated cells (Fig. 4F). Staining with diamidinophenylindole (DAPI) confirmed the presence of multiple nuclei within the fibers, and immunocytochemistry revealed the expression of skeletal muscle markers including desmin and actinin. Over time many of the fibers exhibited spontaneous contraction (Suppl. Movie 1). Thus, adipose myeloid cells passaged through fibrin matrices acquired multiple mesenchymal phenotypes of adipocyte, chondrocyte, osteocyte and skeletal myocyte fibers under appropriate conditions.
Resident myeloid cells from non-adipose tissues are incapable of complete mesenchymal differentiation after culture in fibrin matrices
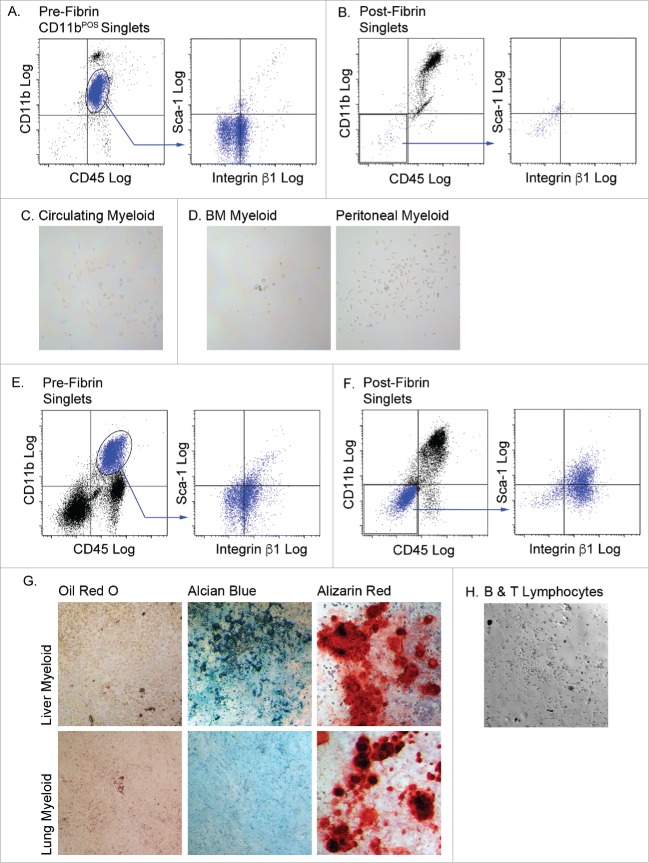
Do myeloid cells from non-adipose tissues have the capacity to differentiate to mesenchymal fates following 3D fibrin culture? To answer this question, myeloid cells from bone marrow, blood, the peritoneal cavity, lung and liver were cultured in fibrin matrices for 5 d and then plated on plastic in mesenchymal cell growth medium. Myeloid cells from bone marrow, blood and the peritoneal cavity retained myeloid (CD11b) and pan-hematopoietic (CD45) marker expression, and failed to acquire mesenchymal (integrin β1) and progenitor cell (Sca-1) markers during fibrin culture (results for circulating myeloid cells shown in Fig. 5A and B). Moreover, they did not adhere to plastic following fibrin culture, and therefore could not be analyzed for multipotent mesenchymal differentiation potential (Fig. 5C and D). A small percentage (∼30%) of liver or lung myeloid cells lost expression of CD11b and CD45 and exhibited a modest increase in the expression of integrin β1 and Sca-1 (results for liver myeloid cells shown in Fig. 5E and F). These cells were plastic adherent and proliferated in mesenchymal cell growth medium (Fig. 5G). Post-fibrin liver cells were capable of chondrogenic and osteogenic differentiation, but not adipogenic or skeletal muscle differentiation. Post-fibrin lung cells were restricted to osteogenic differentiation. Finally, we tested the ability of a mixed lymphoid population from adipose tissue to acquire mesenchymal phenotypes following passage through 3D fibrin matrices. Adipose tissue stromal B and T lymphocytes did not adhere to plastic following fibrin culture (Fig. 5H). The data show that acquisition of mesenchymal lineage differentiation is restricted to myeloid cells, and that multilineage mesenchymal potential is restricted to adipose tissue-resident macrophages.
Figure 5.
Myeloid cells from other tissues are incapable of complete mesenchymal differentiation after fibrin culture. Myeloid (CD45POS/CD11bPOS) cells were purified from the circulation (A, B & C), bone marrow (BM) and peritoneal fluid, (D), liver and lung (E, F & G), and B and T lymphocytes (H). Cells from n = 3 male and female animals were pooled experimented; independent experiments conducted in duplicate. The cells were cultured in fibrin clots for 5 days, recovered by plasmin hydrolysis of the clots and plated on plastic surfaces in MesenCult medium with SCSS. A) Flow analysis of pre-fibrin circulating myeloid cells. B) Flow cytometry analysis of post-fibrin “myeloid” cells. C) Post fibrin circulating cells or D) BM or peritoneal cells exhibited minimal plastic adherence and failed to proliferate. Flow cytometry analysis of E) pre-fibrin or F) post-fibrin liver “myeloid” cells. G) Post-fibrin cells from liver or lung were plastic adherent and proliferated. At confluence they were exposed to adipogenic, chondrogenic or osteogenic conditions for 14–21 d and stained with Oil Red O, Alcian Blue or Alizarin Red to assess adipogenesis, chondrogenesis or osteogenesis, respectively. H) Post-fibrin lymphocytes exhibited limited plastic adherence and failed to proliferate in SCSS medium. All images 20x magnification.
Blockade of integrin β1 expression prevents transition of adipose myeloid cells to “mesenchymal” progenitor cells
Following passage of adipose tissue myeloid cells through fibrin clots, a portion of the cells lost hematopoietic/myeloid markers, concurrent with increased expression of integrin β1, PDGFRα and Sca-1 (Fig. 4). Evaluation of integrin β1 RNA expression in adipose myeloid cells before and during fibrin culture revealed minimal expression before fibrin exposure (Fig. 6A). However, expression increased approximately 20-fold by day 2 in fibrin (day 3 post-siRNA), declining thereafter on day 5 and 8 in fibrin (days 6 and 9 post-siRNA).
Figure 6.
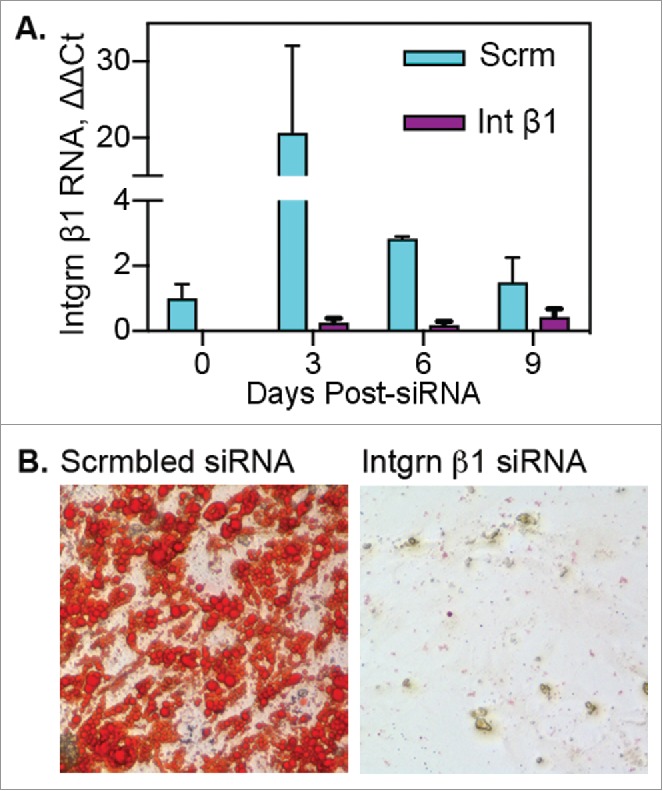
Blockade of integrin β1 expression prevents transition of adipose myeloid cells to “mesenchymal” progenitor cells. Adipose myeloid cells (pooled adipose tissue stroma, n = 7 independent donors) were treated with scrambled (Scrm) or integrin β1-specific siRNA 24 hours before culture in fibrin. A) Following transfection, cells were cultured in fibrin clots for 0, 3, 6 or 9 d and then recovered for RNA isolation and subsequent qRT-PCR analysis. RNA samples were run in triplicate and are presented as mean fold change ± SE as compared with day 0 scrambled control. B) Following transfection, cells were cultured 5 d in fibrin, recovered from clots and cultured on plastic for differentiation. Cells treated with scrambled siRNA acquired adipogenic capacity as shown by Oil Red O staining of lipid droplets. Cells treated with integrin β1-specific siRNA failed to adhere to plastic or proliferate following culture in fibrin clots.
Integrin β1 has been implicated in production of endothelial phenotypes from circulating myeloid cells26 and adipocyte vs. osteocyte lineage commitment by mesenchymal stem cells.27 Therefore, the role of integrin β1 in the transition of myeloid cells to adipocyte progenitor cells was tested via siRNA-mediated blockade of integrin β1 expression. Figure 6A shows efficient suppression of integrin β1 expression by specific siRNA during fibrin culture compared with expression in cells treated with scrambled siRNA. Cells exposed to scrambled siRNA were capable of spontaneous adipogenesis after removal from fibrin, whereas integrin β1 siRNA-treated cells showed minimal plastic adherence, were not proliferative, and were incapable of adipogenesis (Fig. 6B). The data indicate that integrin β1 plays a central role in the transition of myeloid cells to cells with adipogenic capacity.
Discussion
Bone marrow-derived mesenchymal stromal cells (MSC) are virtually absent in the circulation.20–24 Thus, the discovery of bone marrow-derived adipocytes in the adipose tissue of mouse and human transplant recipients raised the prospect that these cells were generated from hematopoietic progenitors rather than from tissue-resident MSCs.11 A hematopoietic origin for BMP-derived adipocytes was subsequently confirmed by competitive adoptive transfer and fat mapping studies in mice.12 In the current studies, we detected CD45NEG/CD11bNEG cells of BM origin (GFPDIM) in adipose tissue stroma capable of differentiation to bona fide adipocytes. Their origin in the BM, lack of hematopoietic markers and adipogenic capacity suggest that these cells may be a putative “mesenchymal-like” intermediate in the production of BMP-derived adipocytes (Fig. 7). Indeed, we propose that marrow-derived hematopoietic/myeloid cells traffic to adipose tissue via the circulation where they became mesenchymal adipocyte progenitors via a hematopoietic -to-mesenchymal transition.
Figure 7.
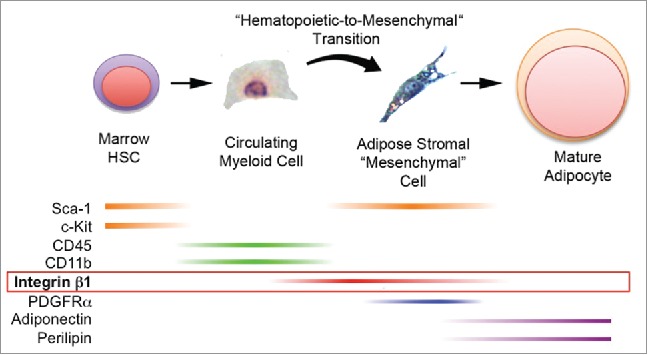
Production of bona fide adipocytes from bone marrow-derived hematopoietic progenitor cells via a hematopoietic-to-mesenchymal transition. Current and previous results support the production of adipocytes from bone marrow hematopoietic stem cells that express Sca-1 and c-Kit. These cells or their progeny enter the circulation as myeloid cells that express CD45 and CD11b (but now lack Sca-1 and c-Kit) and travel to adipose tissue. There they undergo a hematopoietic-to-mesenchymal transition during which they lose CD45 and CD11b and acquire integrin β1, Sca-1 and PDGFRα. Terminal adipogenesis is accompanied by loss of integrin β1, Sca-1 and PDGFRα and expression of mature adipocyte markers including adiponectin and perilipin 1.
In vitro production of adipocytes and other mesenchymal fates from adipose tissue macrophages
Here we also sought to establish in vitro conditions to recapitulate the production of adipocytes from adipose tissue myeloid cells, validate the hematopoietic-to-mesenchymal transition, better understand the developmental processes that promote the production of BM-derived adipocytes, and facilitate their phenotypic characterization. Our previously published studies used myeloid lineage specific tracing to define that bone marrow derived cells differentiated to multilocular adipocytes, highlighting this lineage for further analyses.11–13 The current studies demonstrate that the adipogenic capability of the myeloid lineage resides in the macrophage, not neutrophil or dendritic cell. Monocytes and macrophages are highly promiscuous cells with significant diversity in their lineage specifications based on their tissue resident niches.28,29 While there is significant plasticity between myeloid cells during hematopoietic differentiation, neutrophils are released as differentiated granulocytes whereas monocytes are less differentiated precursors, able to circulate for up to 3 d before homing to a tissue and further differentiating.30 It is likely that the differentiated functions and state of neutrophils prevent their mesenchymal conversion to non-hematopoietic cell types. The functional specification of these cells may coincide with silencing of transcription factors and genes required for lineage plasticity to occur.
Differentiation of adipose stromal macrophages to adipocytes was achieved using a strategy originally reported by Gerard et al.31 who observed adipogenic conversion of a subset of CD45POS non-adherent bone marrow cells in 3 dimensional fibrin clots. We modified this technique to recover cells from fibrin matrices by digestion of the clot with plasmin. This modification allowed us to assess plastic adherence, proliferation and mesenchymal differentiation of the post-fibrin treated cells.
During fibrin culture, adipose tissue macrophages lost hematopoietic and myeloid markers and acquired mesenchymal markers including integrin β1, PDGFRα and the murine stem cell marker Sca-1. Post-fibrin cells were capable of spontaneous adipogenic and skeletal myocyte differentiation, and the number of cells generating adipocytes and the rate of adipogenesis could be enhanced by the addition of adipogenic supplements. Osteogenic and chondrogenic differentiation were also observed under conditions that promote these terminal phenotypes, indicating that fibrin-treated adipose myeloid cells are capable of multiple mesenchymal phenotypes in vitro, while in vivo this differentiation is likely specified by the specific microenvironmental niche. For example, similar stromal populations present in bone and cartilage would likely differentiate to their respective tissue fates to promote tissue function, repair or regeneration. Interestingly, myeloid cells from other tissues were either incapable of any mesenchymal differentiation (BM, peripheral blood or peritoneal myeloid cells) or exhibited a limited repertoire of mesenchymal fates (liver or lung myeloid cells). This suggests that the adipose tissue microenvironment is unique in its ability to render myeloid cells capable of mesenchymal multipotency. Ongoing studies are being conducted to determine the specific factors within the adipose tissue microenvironment that support this capacity.
Human adipose tissue macrophages also exhibit adipogenic capacity
Our data are consistent with observations by Chazenbalk et al.32 who reported that CD14POS or CD68POS human adipose tissue macrophages acquire expression of the preadipocyte marker DLK when co-cultured with free-floating human adipocytes. These macrophage-derived preadipocytes could undergo adipogenic differentiation with upregulation of C/EBPα and PPARγ, lipid accumulation and gradual loss of macrophage markers. Their data suggest that soluble factors or direct contact with floating adipocytes can stimulate proadipogenic processes in macrophages.
Eto et al.33 also reported a subset of human adipose tissue-resident macrophages with mesenchymal multipotency. Unlike the mouse adipose myeloid cells in our studies, which are CD34NEG, their cells expressed CD45, CD14, CD206 and CD34, and were plastic adherent without fibrin culture. These cells were capable of adipogenic, chondrogenic and osteogenic differentiation in culture, but whether expression of hematopoietic/myeloid markers was diminished or lost was not examined. Circulating human CD14POS cells also acquire mesenchymal multipotency following culture on fibronectin-coated plates.34 However, the multipotent cells express CD14, CD45 and collagen I, and continue to express CD45 after differentiation to cells with adipocyte, chondrocyte, osteoblast and myocyte characteristics. The simultaneous expression of CD14, CD45 and collagen I is typical of fibrocytes, cells with hematopoietic and mesenchymal features that are also capable of adipogenic conversion in vitro.35
Differences between our results and those of Chazenbalk et al.32 or Eto et al.33 may simply reflect variations between hematopoietic development and adipose tissue biology and microenvironment between mice and humans. Undoubtedly, the vagaries of tissue digestion and culture conditions also contribute to differences between our results and those of the other laboratories. However, it seems evident that adipose tissue macrophages possess a unique mesenchymal multipotency, which likely underlies our previous discovery of adipocytes generated de novo from bone marrow progenitor cells. Whether these cells ever leave adipose tissue and contribute to tissue development and regeneration elsewhere remains unexplored. Our finding that adipose tissue myeloid cells are capable of differentiating to multiple mesenchymal fates suggests this may be an important area of future study.
The hematopoietic-to-mesenchymal transition
The production of mesenchymal cell types from hematopoietic progenitors has been reported in animal models.16,18,36-39 This is often, as in the cases of hepatocytes or skeletal myocytes, the result of cell fusion and phenotypic reprogramming rather than transdifferentiation.16,18 However, hematopoietic cells are capable of generating cardiomyocytes, vascular endothelium and hepatic stellate cells via direct differentiation, although their contribution to these cell populations is modest even in injury/repair models.37,39 Evidence for cross-lineage cell differentiation is seldom reported in humans during tissue homeostasis or following tissue injury and regeneration. This paucity of information reflects the absence of specific markers or methods to lineage trace or follow stem cell fate in humans. The most convincing evidence to support hematopoietic tissue specification is demonstrated in the production of myofibroblasts from circulating mononuclear cells during liver fibrosis and repair.40,41 However, in this case the hematopoietic /myeloid identity of these cells was retained and evident in the presence of hematopoietic cell surface markers including CD45, CD11b and CD14. The marrow-derived adipocytes we discovered in mice and humans, and recovered from fibrin culture do not express myeloid or other hematopoietic lineage markers. Therefore, we conclude that some adipocytes are generated via hematopoietic-to-mesenchymal transdifferentiation in humans. Importantly, adipose tissue macrophage accumulation is increased in obesity42 and Rydén et al. report that the contribution of BMP-derived adipocytes is 2.5-fold higher in patients with obesity compared with those of normal weight.14 Thus, it will be important to understand if adipose tissue macrophages in humans are indeed capable of undergoing “hematopoietic-to-mesenchymal” transition and are thereby the source of BMP-derived adipocytes. Studies to answer these questions are currently underway in our laboratory.
The 3D environment in promoting the hematopoietic-to-mesenchymal transition
3D matrices are commonly used to facilitate expansion of mature hematopoietic stem cells, confirm anchorage-independent proliferation of non-adherent cells, and in colony forming assays.43,44 In our studies, 3D matrices appear to be essential for the conversion of adipose myeloid cells to “mesenchymal” progenitors. Plastic adherence, proliferation and mesenchymal differentiation were not observed when myeloid cells were seeded directly on plastic substrates, or on 2-dimensional surfaces coated with gelatin, Matrigel or fibrinogen. Thus, architectural, mechanical and/or biochemical cues specific to the 3D environment appear to play a key role in promoting the hematopoietic-to-mesenchymal transition of adipose tissue macrophages. This idea is supported by the upregulation of integrin β1 during 3D culture, and its requisite role in promoting adipogenic conversion of adipose myeloid cells. However, the 3D environment alone was insufficient to elicit mesenchymal multipotency since culture of non-adipose myeloid cells in fibrin clots failed to stimulate their conversion to multipotent mesenchymal progenitors. Clearly, a combination of adipose tissue-specific factors and architectural/mechanical cues are necessary for this unique developmental process.
Integrin regulation of the hematopoietic-to-mesenchymal transition
Integrin ligation and signaling is crucial at several points in adipocyte production. Integrins α5 and α6 are coordinately regulated during terminal adipogenesis of 3T3-L1 cells, with α5 expression gradually suppressed and α6 increased.45,46 Ectopic overexpression of integrin α5 blocked adipogenic differentiation and stimulated preadipocyte proliferation. These integrins also play a role in the decision of MSCs to pursue adipogenic vs. osteogenic differentiation in response to changes in extracellular matrix composition.27 Likewise, osteopontin activation of the integrin αv/β1 complex promotes osteogenesis and inhibits adipogenesis of MSCs, at least in part via blockade of C/EBP function. Recently, Volloch and Olsen47 proposed that differences in integrin-mediated signaling through p38, β-catenin and ERK account for the ability of mechanical or thermal stress to repress adipogenesis in skeletal muscle but not in adipose tissue. In our model, integrin β1 appears to function during conversion of adipose myeloid cells to mesenchymal progenitors. Integrin β1 regulates hematopoietic cell migration and adhesion to basement membrane, while proliferation and differentiation to blood lineages is independently controlled.48,49 Monocyte subpopulations, including macrophage and dendritic precursors, home to tissues in the absence of chemokine stimulation, while integrin mediated extravasation elicits signaling cascades which subsequently regulate their tissue specific phenotypes.30,50 The integrin mediated mechanisms of the precursor recruitment and differentiation are not well understood. Our data demonstrate the requirement for integrin β1 in the adipose specification of myeloid precursors and tissue resident macrophages, which is absent in other tissue resident macrophages. Interestingly, β1 (and α5) expression is also upregulated during TNF-α-induced transdifferentiation of monocytes to endothelial cells,51 and the transformation of avian lens epithelial cells to mesenchyme on collagen gels.52 We propose integrin β1 plays a common role in directing diverse cells toward mesenchymal fates. The specific mechanisms downstream of integrin signaling are the focus of ongoing investigation.
Summary
We have devised in vitro culture conditions that promote the production of bona fide adipocytes from adipose tissue macrophages. The results support our previous observations that some adipocytes in adipose depots throughout the mouse are generated from myeloid cells arising from bone marrow. The primary feature of our in vitro strategy is culture of adipose tissue macrophages in 3D fibrin matrices that elicit a hematopoietic-to-mesenchymal transition requiring transient upregulation of integrin β1. Without 3D fibrin culture, adipose tissue myeloid cells do not acquire mesenchymal potency, and non-adipose tissue myeloid cells exhibit no or limited mesenchymal differentiation capacity. This suggests that biochemical and architectural features of the adipose tissue microenvironment are required for the acquisition of mesenchymal potency by macrophages. The data highlight key differences in the developmental pathways for conventional and BMP-derived adipocytes (Fig. 7). By exploiting these differences it may be possible to target the production of pro-inflammatory and leptin-deficient BMP-derived adipocytes to prevent or reverse adipose tissue-related metabolic disorders. Experiments such as these may also lead to more mechanistic insight into the development of the chronic inflammation associated with obesity.
Methods
Animals, tissue harvest and isolation of myeloid cells
All procedures and treatments were approved by the Institutional Animal Care and Use Committee at the University of Colorado Anschutz Medical Campus. Bone marrow transplant experiments were performed in wild type, C57BL6/J (The Jackson Laboratory, #000664), female mice that received GFPPOS BM from male, GFP donors (The Jackson Laboratory, #004353). Mice were killed by CO2 asphyxiation and cervical dislocation. Gonadal and dorsal (interscapular) adipose depots were recovered from killed mice, combined due to limited tissue volume, and minced into pieces of 1–3 mm. Tissue fragments were digested for 1 hour with gentle shaking at 37°C in digestion buffer [Krebs-Ringers-HEPES (KRH) + 2.5 mM glucose + 2% fetal bovine serum (FBS) (Gemini Bio-products, #100–500) + 200 nM adenosine (Sigma Aldrich, #A4036) + 1 mg/ml collagenase (type VIII, Sigma Aldrich, C2139), pH 7.4] using 4 ml buffer/gram of fat. Any remaining undigested fragments were removed by filtration through a 150 μm Celltrics filter (Sysmex Partec GmbH, Germany, #04–004–2324). Between one half and one digestion volume of wash buffer (Hanks Balanced Salt Solution (Mediatech, #21–022-CV) + 2% FBS + 200 nM adenosine, pH 7.4) was filtered into the collection tube to stop digestion. Following centrifugation at 500 g x 10 minutes the stromal cell pellet was resuspended in 5 ml Wash Buffer then re-pelleted.
Lung and liver were collected from killed wild type mice and digested similarly with the following modifications. Lung digestion buffer was KRH + 2.5 mM glucose + 2% FBS + 2 mg/ml collagenase, pH 7.5 (Worthington Type 2, #LS004202). Digestion proceeded for 80 minutes. Liver digestion buffer was KRH + 2.5 mM glucose + 2% FBS + 1 mg/ml collagenase + 0.2 mg/ml dispase, pH 7.4 (Worthington Biochemical, #LS02104). Peritoneal macrophages were collected following euthanasia of wild type mice. The ventral skin of the abdomen was dissected away from the peritoneal wall and 5 ml of cold HBSS + 2% FBS was injected into the peritoneal cavity. The abdomen was massaged gently for 1 minute and the cell suspension aspirated from the cavity via needle and syringe. Bone marrow cells were collected from the same animals by dislocation and removal of the hind leg. The lower leg and tissue were dissected from the femurs and the epiphyses cut off. Using a 27 gauge needle the marrow was flushed with 5 ml cold HBSS + 2% FBS into a collection tube then filtered through a 100 µm cell strainer. Both cell types were pelleted and washed with HBSS + 2% FBS. For all cell samples red blood cells were lysed using 1X RBC lysis buffer (eBioscience, #00–4300–54) per manufacturer's instructions. Lineage depletion beads were used to select for LINNEG cells (Miltenyi, # 130–110–470). The kit included a cocktail of CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), 7–4, Ter-119 antibodies.
Staining and flow sorting of stromal cells
Antibodies to CD11b-PE (BD Biosciences, # 557397), CD45-APC (BioLegend, # 103112), Sca-1-Alexa488 (BioLegend, # 122515), integrin β1-PE/Cy7 (BioLegend, # 102221), PDGFRα-BV421 (BD Biosciences # 562774), CD64-PEDazzle (BioLegend, # 139319), CD11b-PerCP/Cy5.5 (eBiosciences, # 45–0112–80), CD206-BV421 (BioLegend, # 141717), Siglec-F-PE (BD Biosciences, # 552126), Gr-1-PE/Cy7 (BD Biosciences, # 560601), CD11c-APC/Cy7 (BD Biosciences, # 561241) were added to the resuspended stromal cells at 0.25 µg / 106 cells. Samples were incubated at 4°C in the dark for 25 minutes. Following incubation samples were centrifuged to remove unbound antibodies, and the cells were resuspended in PBS containing 5% FBS. Stromal cells were sorted within 15 minutes using a MoFlo XDP cell sorter with Summit 4.3 software. The sheath fluid was IsoFlow. The sample and collection tubes were maintained at 4°C. The sample flow rate was set to a pressure differential of less than 0.4 psi. Sort mode was set to Purify 1. Appropriate signal compensation was set using single color control samples.
Three-Dimensional fibrin culture
Sorted myeloid cells were plated on plastic overnight in DMEM (Mediatech, #10–013-CV) + 20% FBS to remove remaining adherent cells. Non-adherent cells were resuspended in 10 µl MesenCult Basal medium (StemCell Technologies, #05501) + Stem Cell Stimulatory Supplements (SCSS, StemCell Technologies, #05502) at 40,000 cells/µl. Fibrinogen solution (Sigma-Aldrich, #F8630) (60 µl, 3 mg/ml in 0.9% saline) was added, mixed gently and transferred to a well of a 96 well plate. Thrombin (Sigma-Aldrich, # T9549) solution (2.1 µl, 50 U/ml) was added and the suspension mixed by gentle pipetting. Clots formed over 20–30 minutes.
Cells were recovered from the clots after 5 d. First, the culture medium was removed from the top of the clots. Next, the matrices were rimmed with a 27 gauge needle and 100 µl of digestion mix comprised of 77 µl culture medium + 23 µl bovine plasminogen (Enzyme Research Laboratories, #BPg) (1 mg/ml) + 2 µl urokinase (Sigma-Aldrich, #U4010) (20 U/ml) was added for 30–40 minutes at 37°C. Cells were then pelleted at 300 g x 10 minutes and resuspended in MesenCult+SCSS medium and replated at 40,000 cells directly into one well of 96 well plate.
Mesenchymal differentiation
Cells recovered from fibrin and plated in MesenCult + SCSS medium were transferred to commercial medium designed to induce adipogenic (MesenCultBasal+Adipogenic Supplements, #05503), chondrogenic (StemPro Chondrogenesis Differentiation kit, Gibco, #A10071–01) and osteogenic (Osteogenesis Differentiation Kit, Gibco, #A10072–01) differentiation. Adipogenic and skeletal muscle differentiation occurred spontaneously, but adipogenesis was augmented by adipogenic agents. Analyses were performed using replicates, twice independently.
RT-PCR
PCR primers for RT-PCR were adiponectin forward 5′-TGG TGA GAA GGG TGA GAA-3′, adiponectin reverse 5′-AGA TCT TGG TAA AGC GAA TG-3′; perilipin-1 forward 5′-CTG CCG GTG GTG AGT GGC AC-3′, perilipin-1 reverse 5′-CAC AGA GGC CAC CAG GGG GT-3′; and GAPDH forward 5′- GGA GCG AGA TCC CTC CAA AAT-3′, GAPDH reverse 5′-GGC TGT TGT CAT ACT TCT CT GG-3′. Complimentary DNA (cDNA) was prepared from stromal cells using Cells-to-cDNA II reagents. PCR amplification was performed with 3 μl transcribed cDNA and 1 pmol of each primer for 35 cycles: hot-start at 94°C for 1 minute, denaturation at 94°C for 1 minute, annealing at appropriate temperature for 45 seconds, and elongation at 72°C for 2 minutes, followed by a final elongation for 8 minutes at 72°C. All samples were analyzed in triplicate.
siRNA Ablation of Integrin β1
Stromal myeloid cells were transfected with a pool of 3 Dicer substrate duplexes to integrin β1 or universal scrambled sequences using a kit from OriGene (#SR420242C) and 24-hours later underwent fibrin encapsulation. Cells were recovered from fibrin before plating on plastic wells for differentiation into adipocytes. Analysis was performed in triplicate and repeated twice independently.
In vivo adipogenesis in matrigel
Flow cytometry purified GFPDIM adipose stromal cells or post-fibrin adipose myeloid cells (approximately 2 × 106 cells) were gently resuspended in 200 µl of low growth factor Matrigel at 4°C. Female athymic, Crl:CD1-Foxn1nu, mice (Charles River, #086) (n = 3) were briefly anesthetized and the Matrigel/cell suspensions were injected subcutaneously into the abdomen anterior of the thigh. After 4 weeks the solid Matrigel plugs and adjacent skin and subcutaneous muscle were removed. The plugs were fixed overnight in 4% paraformaldehyde, and then sliced in half with a razor blade. The halves were oriented in paraffin blocks with the cut surfaces up for sectioning. Five μm sections were stained with hematoxylin and eosin for histological examination, or subjected to immunofluorescence staining for perilipin and GFP.
Immunohistochemistry
Five µm sections of paraformaldehyde-fixed, paraffin-embedded adipose tissue were deparaffinized with CitriSolv (Fisher Scientific, Pittsburgh, PA), and rehydrated in a graded ethanol/water series. Sections, in triplicate, were subjected to antigen retrieval in citrate buffer in an electric pressure cooker for 20 minutes. Sections were blocked with PBS containing 5% goat serum for 30 minutes at room temperature. The sections were incubated overnight in PBS + 5% goat serum at 4°C with primary antibodies to GFP (Abcam, #ab290), perilipin (Abcam, #ab3526), desmin (Abcam, #ab32362) or actinin (Abcam, #ab90776). The sections were then washed and incubated with Alexa Fluor 555 (Invitrogen, #A21428) or Alexa Fluor 488 (Invitrogen, #A11008)-conjugated secondary antibodies for 1 hour at room temperature.
Statistical analysis
qPCR data was analyzed by one-way ANOVA followed by Tukey's HSD post-hoc analysis. Data are presented as ± standard error from the mean ( ± SE).
Supplementary Material
Disclosure of potential conflicts of interest
No conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr. Carey Lumeng (Univ. Michigan Medical School) for his advice in developing the flow cytometry sorting strategy to isolate adipose tissue stromal myeloid subpopulations.
Funding
This research was funded by NIH R01 DK078966 and DK109547 (to D.J.K.), R21 DK092718 and P50 HD073063 (to W.M.K.) and K01 DK109053 (to K.M.G.). Additional support was provided by a Veterans' Administration GRECC Pilot Project 1 I21 CX001341–01 (to D.J.K.). This research was also supported in part by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082 and NIH/NCI CCDG P30 CA046934.
References
- [1].Bays H, Ballantyne C. Adiposopathy: why do adiposity and obesity cause metabolic disease. Future Lipidol 2006; 1:389-420; http://dx.doi.org/ 10.2217/17460875.1.4.389 [DOI] [Google Scholar]
- [2].Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbiditites related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009; 9:88-108; PMID:19320986; http://dx.doi.org/ 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med 1998; 38:52-63; PMID:16448989; http://dx.doi.org/ 10.1080/07853890500383895 [DOI] [PubMed] [Google Scholar]
- [4].Wajchenberg BL, Giannella-Neto D, da Silva MER, Santos RF. Depot-specific hormonal characterization of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res 2002; 34:616-21; http://dx.doi.org/ 10.1055/s-2002-38256 [DOI] [PubMed] [Google Scholar]
- [5].Bluher M. The distinction of metabolically ‘health’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 2010; 21:38-43; PMID:19915462; http://dx.doi.org/ 10.1097/MOL.0b013e3283346ccc [DOI] [PubMed] [Google Scholar]
- [6].Huang-Doran I, Sleigh A, Rochford JJ, O'Rahilly S, Savage DB. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol 2010; 207:245-55; PMID:20870709; http://dx.doi.org/ 10.1677/JOE-10-0272 [DOI] [PubMed] [Google Scholar]
- [7].Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular rosk factors with chronic heart failure. J Am Acad Cardiol 2004; 43:1439-44; PMID:15093881; http://dx.doi.org/ 10.1016/j.jacc.2003.11.039 [DOI] [PubMed] [Google Scholar]
- [8].Schmidt DS, Salahudeen AK. Cardiovascular and survival paradoxes in dialysis patients: Obesity-survival paradox-still a controversy. Semin Dialysis 2007; 20:486-92; http://dx.doi.org/ 10.1111/j.1525-139X.2007.00349.x [DOI] [PubMed] [Google Scholar]
- [9].Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, Arbab-Zadeh A, Mukherjee D, Lazar JM. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol 2015; 115:1428-34; PMID:25772740; http://dx.doi.org/ 10.1016/j.amjcard.2015.02.024 [DOI] [PubMed] [Google Scholar]
- [10].Loprinzi P, Smit E, Lee H, Crespo C, Andersen R, Blair SN. The “fit but fat” paradigm addressed using accelerometer-determined physical activity data. N Am J Med Sci 2014; 6:295-301; PMID:25077076; http://dx.doi.org/ 10.4103/1947-2714.136901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Crossno JT Jr, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes differentiation of bone marrow-derived circulating progenitor cells to multilocular adipocytes in adipose tissue. J Clin Invest 2006; 116:3220-8; PMID:17143331; http://dx.doi.org/ 10.1172/JCI28510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Majka SM, Fox KE, Psilas JC, Helm KM, Childs CR, Acosta AS, Janssen RC, Friedman JE, Woessner BT, Shade TR, et al.. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc Natl Acad Sci U S A 2010; 107:14781-6; PMID:20679227; http://dx.doi.org/ 10.1073/pnas.1003512107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Majka SM, Miller HL, Sullivan T, Erickson PF, Kong R, Weiser-Evans M, Nemenoff R, Moldovan R, Morandi SA, Davis JA, et al.. Adipose lineage specification of bone marrow-derived myeloid cells. Adipocyte 2012; 1:215-29; PMID:23700536; http://dx.doi.org/ 10.4161/adip.21496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ryden M, Uzunel M, Hard JL, Borgstrom E, Mold JE, Arner E, Mejhert N, Andersson DP, Widlund Y, Hassan M, et al.. Transplanted bone marrow-derived cells contribute to human adipogenesis. Cell Metab 2015; 22:408-17; PMID:26190649; http://dx.doi.org/ 10.1016/j.cmet.2015.06.011 [DOI] [PubMed] [Google Scholar]
- [15].Gavin KM, Gutman JA, Kohrt WM, Wei Q, Shea KL, Miller HL, Sullivan TM, Erickson PF, Helm KM, Acosta AS, et al.. De novo generation of adipocytes from circulating progenitor cells in mouse and human adipose tissue. FASEB J 2016; 30:1096-108; PMID:26581599; http://dx.doi.org/ 10.1096/fj.15-278994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Camargo FD, Green R, Capetenaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med 2003; 9:1520-7; PMID:14625546; http://dx.doi.org/ 10.1038/nm963 [DOI] [PubMed] [Google Scholar]
- [17].Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci U S A 2006; 103:13156-61; PMID:16920790; http://dx.doi.org/ 10.1073/pnas.0604203103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, Finegold M, Fleming WH, Grompe M. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med 2004; 10:744-8; PMID:15195088; http://dx.doi.org/ 10.1038/nm1062 [DOI] [PubMed] [Google Scholar]
- [19].Majka SM, Miller HL, Helm KM, Acosta AS, Childs CR, Kong R, Klemm DJ. Analysis and isolation of adipocytes by flow cytometry : MacDougald OA, ed. Methods in Adipose Tissue Biology: Elsevior Press, 2014:281-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bartsch K, Al-Ali H, Reinhardt A, Franke C, Hudecek M, Kamprad M, Tschiedel S, Cross M, Niederwieser D, Gentilini C. Mesenchymal stem cells remain host-derived independent of the source of the stem-cell graft and conditioning regimen used. Transplantation 2009; 87:217-21; PMID:19155975; http://dx.doi.org/ 10.1097/TP.0b013e3181938998 [DOI] [PubMed] [Google Scholar]
- [21].Dickhut A, Schwerdtfeger R, Kuklick L, Ritter M, Thiede C, Neubauer A, Brendel C. Mesenchymal stem cells obtained after bone marrow transplantation or peripheral blood stem cell transplantation originate from host tissue. Ann Hematol 2005; 84:722-7; PMID:16132912; http://dx.doi.org/ 10.1007/s00277-005-1067-8 [DOI] [PubMed] [Google Scholar]
- [22].Garcia-Castro J, Balas A, Ramirez M, Perez-Martinez A, Madero L, Gonzalez-Vicent M, Díaz MA. Mesenchymal stem cells are of recipient origin in pediatric transplantations using umbilical cord blood, peripheral blood, or bone marrow. J Pediatr Hematol Oncol 2007; 29:388-92; PMID:17551400; http://dx.doi.org/ 10.1097/MPH.0b013e3180645186 [DOI] [PubMed] [Google Scholar]
- [23].Hoogduijn MJ, Verstegen M, Engela AU, Korevaar SS, Roemeling-van Rhijn M, Merino A, Franquesa M, de Jonge J, Ijzermans JN, Weimar W, et al.. No evidence for circulating mesenchymal stem cells in patients with organ injury. Stem Cells Dev 2014; 23(19):2328-35; PMID:25105211; http://dx.doi.org/ 10.1089/scd.2014.0269 [DOI] [PubMed] [Google Scholar]
- [24].Wang J, Huang XJ, Xu LP, Liu DH, Chen H, Chen YH, Lai YY, Hou RQ, Qin XY, Liu KY. Monitoring the source of mesenchymal stem cells in patients after transplantation of mismatched-sex hematopoietic stem cells plus third-party cells. Chin Med J (Engl) 2013; 126:4254-9; PMID:24238507 [PubMed] [Google Scholar]
- [25].Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, Geletka L, Meyer KA, O'Rourke RW, Lumeng CN. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. J Immunol 2016; 197:3650-61; PMID:27683748; http://dx.doi.org/ 10.4049/jimmunol.1600820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bin L, Pozzi A, Young PP. TNFalpha accelerates monocyte to endothelial transdifferentiation in tumors by the induction of integrin alpha5 expression and adhesion to fibronectin. Mol Cancer Res 2011; 9:702-11; PMID:21536688; http://dx.doi.org/ 10.1158/1541-7786.MCR-10-0484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen Q, Shou P, Zhang L, Xu C, Zheng C, Han Y, Li W, Huang Y, Zhang X, Shao C, et al.. An osteopontin-integrin interaction plays a critical rol in directing adipogenesis and osteogenesis by mesenchymal stem cells. Stem Cells 2014; 32:327-37; http://dx.doi.org/ 10.1002/stem.1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol 2015; 185:2596-606; PMID:26118749; http://dx.doi.org/ 10.1016/j.ajpath.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Catacchio I, Berardi S, Reale A, De Luisi A, Racanelli V, Vacca A, Ria R. Evidence for bone marrow adult stem cell plasticity: properties, molecular mechanisms, negative aspects, and clinical applications of hematopoietic and mesenchymal stem cells transdifferentiation. Stem Cells Int 2013; 2013:589139; PMID:23606860; http://dx.doi.org/ 10.1155/2013/589139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol 2004; 4:432-44; PMID:15173832; http://dx.doi.org/ 10.1038/nri1375 [DOI] [PubMed] [Google Scholar]
- [31].Gerard C, Blouin K, Tchernof A, Doillon CJ. Adipogenesis in nonadherent and adherent bone marrow stem cells grown in fibrin gel in the presence of adult plasma. Cells Tissues Organs 2008; 187:186-98; PMID:18042972; http://dx.doi.org/ 10.1159/000111804 [DOI] [PubMed] [Google Scholar]
- [32].Chazenbalk G, Bertolotto C, Heneidi S, Jumabay M, Trivax B, Aronowitz J, Yoshimura K, Simmons CF, Dumesic DA, Azziz R. Novel pathway of adipogenesis through cross-talk between adipose tissue macrophages, adipose stem cells and adipocytes: evidence of cell plasticity. PLoS One 2011; 6:e17834; PMID:21483855; http://dx.doi.org/ 10.1371/journal.pone.0017834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Eto H, Ishimine H, Kinoshita K, Watanabe-Susaki K, Kato H, Doi K, Kuno S, Kurisaki A, Yoshimura K. Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency. Stem Cells Dev 2013; 22:985-97; PMID:23137270; http://dx.doi.org/ 10.1089/scd.2012.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, Kawakami Y, Ikeda Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol 2003; 74:833-45; PMID:12960274; http://dx.doi.org/ 10.1189/jlb.0403170 [DOI] [PubMed] [Google Scholar]
- [35].Hong KM, Buridick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J 2005; 19:2029-31; PMID:16188961 [DOI] [PubMed] [Google Scholar]
- [36].Bellik L, Musilli C, Vinci MC, Ledda F, Parenti A. Human mature endothelial cells modulate peripheral blood mononuclear cell differentiation toward an endothelial phenotype. Exp Cell Res 2008; 314:2965-74; PMID:18692498; http://dx.doi.org/ 10.1016/j.yexcr.2008.07.016 [DOI] [PubMed] [Google Scholar]
- [37].Jackson KA, Majka SM, Wang H, Pocius J, Hartely CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001; 107:1395-402; PMID:11390421; http://dx.doi.org/ 10.1172/JCI12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hofmann TJ, Otsuru S, Marino R, Rasini V, Veronesi E, Murgia A, Lahti J, Boyd K, Dominici M, Horwitz EM. Transplanted murine long-term repopulating hematopoietic cells can differentiate to osteoblasts in the marrow stem cell niche. Mol Ther 2013; 21:1224-31; PMID:23587920; http://dx.doi.org/ 10.1038/mt.2013.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Miyata E, Masuya S, Nakamura S, Kato K, Sugimoto Y, Shibasaki T, Yamamura K, Ohishi K, Nishii K, Ishikawa F, et al.. Hematopoietic origin of hepatic stellate cells in the adult liver. Blood 2008; 111:2427-35; PMID:18042797; http://dx.doi.org/ 10.1182/blood-2007-07-101261 [DOI] [PubMed] [Google Scholar]
- [40].Dalakas E, Newsome PN, Boyle S, Brown R, Pryde A, McCall S, Hayes PC, Bickmore WA, Harrison DJ, Plevris JN. Bone marrow stem cells contribute to alcohol liver fibrosis in humans. Stem Cells Dev 2010; 19:1417-25; PMID:20025456; http://dx.doi.org/ 10.1089/scd.2009.0387 [DOI] [PubMed] [Google Scholar]
- [41].Kisseleva T, Brenner DA. The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis. J Hepatol 2012; 56:965-27; PMID:22173163; http://dx.doi.org/ 10.1016/j.jhep.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112:1796-808; PMID:14679176; http://dx.doi.org/ 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bonig H, Papayannopoulou T. Mobilization of hematopoietic stem/progenitor cells: general principles and molecular mechanisms. Methods Mol Biol 2012; 904:1-14; PMID:22890918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Borowicz S, Van Scoyk M, Avasarala S, Karuppusamy Rathinam MK, Tauler J, Bikkavili RK, Tauler J, Bikkavilli RK, Winn RA. The soft agar colony formation assay. J Vis Exp 2014; 92:e51998; PMID:25408172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Frith JE, Mills RJ, Hudon JE, Cooper-White JJ. Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev 2012; 21:2442-56; http://dx.doi.org/ 10.1089/scd.2011.0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu J, DeYoung SM, Zhang M, Zhang M, Cheng A, Saltiel AR. Changes in integrin expression during adipocyte differentiation. Cell Metab 2005; 2:165-77; PMID:16154099; http://dx.doi.org/ 10.1016/j.cmet.2005.08.006 [DOI] [PubMed] [Google Scholar]
- [47].Volloch V, Olsen BR. Why cellular stress suppresses adipogenesis in skeletal tissue, but is ineffective in adipose tissue: Control of mesenchymal cell differentiation via integrin binding sites in extracellular matrices. Matrix Biol 2013; 32:365-71; http://dx.doi.org/ 10.1016/j.matbio.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature 1996; 380:171-5; PMID:8600394; http://dx.doi.org/ 10.1038/380171a0 [DOI] [PubMed] [Google Scholar]
- [49].Poitz DM, Stolzel F, Arabanian L, Friedrichs J, Docheva D, Schieker M, Fierro FA, Platzbecker U, Ordemann R, Werner C, et al.. MiR-134-mediated beta1 integrin expression and function in mesenchymal stem cells. Biochim Biophys Acta 2013; 1833:3396-404; PMID:24135056; http://dx.doi.org/ 10.1016/j.bbamcr.2013.10.003 [DOI] [PubMed] [Google Scholar]
- [50].Fierro FA, Taubenberger A, Puech PH, Ehninger G, Bornhauser M, Muller DJ, Illmer T. BCR/ABL expression of myeloid progenitors increases beta1-integrin mediated adhesion to stromal cells. J Mol Biol 2008; 377:1082-93; PMID:18313694; http://dx.doi.org/ 10.1016/j.jmb.2008.01.085 [DOI] [PubMed] [Google Scholar]
- [51].Li B, Pozzi A, Young PP. TNFalpha accelerates monocyte to endothelial transdifferentiation in tumors by the induction of integrin alpha5 expression and adhesion to fibronectin. Mol Cancer Res 2011; 9:702-11; PMID:21536688; http://dx.doi.org/ 10.1158/1541-7786.MCR-10-0484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zuk A, Hay ED. Expression of beta 1 integrins changes during transformation of avian lens epithelium to mesenchyme in collagen gels. Dev Dyn 1994; 201:378-93; PMID:7534501; http://dx.doi.org/ 10.1002/aja.1002010409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.