ABSTRACT
Bone formation is an osteoblast-specific process characterized by high energy demands due to the secretion of matrix proteins and mineralization vesicles. While glucose has been reported as the principle fuel source for osteoblasts, recent evidence supports the tenet that osteoblasts can utilize fatty acids as well. Although the ability to accumulate lipid droplets has been demonstrated in many cell types, there has been little evidence that osteoblasts possess this characteristic. The current study provides evidence that osteoblastogenesis is associated with lipid droplet accumulation capable of supplying energy substrates (fatty acids) required for the differentiation process. Understanding the role of fatty acids in metabolic programming of the osteoblast may lead to novel approaches to increase bone formation and ultimately bone mass.
KEYWORDS: bone, fatty acids, lipolysis, lipophagy, marrow adipocytes
Introduction
The skeleton is an incredibly complex and dynamic tissue which is remodeled throughout the life span. During early childhood and adolescence, bone primarily undergoes a rapid phase of bone formation (i.e. bone modeling) until peak bone mass is attained.1,2 Following this phase of bone acquisition, bone remodeling maintains skeletal health and bone integrity. Three specified cell types are most commonly studied when discussing bone remodeling including bone forming osteoblasts, bone resorbing osteoclasts, and the mechanosensing osteocytes. Through the elegant communication of these cells with other bone remodeling components, mineral and bone matrix is resorbed and replaced by osteoid ultimately forming new bone. This continual remodeling is essential to maintain skeletal health and failure to do so results in debilitating clinical manifestations, such as osteoporosis and fractures. While each cell, the osteoblast, osteoclast, and osteocyte, is responsible for critical steps in the bone remodeling process, interest has heightened in regards to the ability of the osteoblast to reach the energetic demands of bone formation.
Secretion of matrix proteins and mineralization vesicles by the osteoblast has been viewed as an energy demanding process as early as the 1960s.3,4 While many initial studies focused on glucose utilization by bone, it wasn't until 1987 that Adamek and colleagues5 identified fatty acids as yet another substrate capable of supplying energy to bone tissue and isolated cells. Since this time bone has also been shown to take up exogenous, dietary lipids via chylomicron remnants6 and the chylomicron associated apolipoprotein, ApoE, is strongly induced upon osteoblast differentiation.7,8 More recently, studies by Frey et al.9 have elaborated on this relationship by demonstrating that Wnt-LRP5 signaling regulates fatty acid metabolism in osteoblasts, and is consequently responsible for the profound skeletal phenotypes associated in these transgenic mice (i.e., low bone mass in loss of function mutations; high bone mass in gain of function mutations). This study further confirmed the importance of fatty acid metabolism for osteoblast function, although, the authors did not elaborate on the origin nor source of these substrates. In other tissues and cells, fatty acid substrates can be acquired by (1) de novo synthesis from glucose substrates; (2) exogenous, dietary sources via chylomicron remnants; (3) endogenous mobilization from adipocytes; and/ or (4) intracellular lipolysis of stored lipid droplets. While all of these methods could supply the osteoblast with fatty acid substrates for energy generation, the recent identification of lipid droplets within osteo-progenitors is of particular interest.
Adipocytes have classically been identified as cells capable of storing lipids in the form of neutral lipid droplets predominantly composed of triacylglycerol (TAGs). In this regard, when cellular energy is in demand, either by the adipocyte or remote cells, these lipid droplets are broken down and mobilized via lipolysis.10 Free fatty acids are then metabolized via mitochondrial β-oxidation and subsequent oxidative phosphorylation, or mobilized to other tissues, while glycerol is liberated to the extracellular space. Although the storage, accumulation, and degradation of lipid droplets is relatively well defined in adipocytes, it has become evident that many different cell types, including osteoblasts and osteocytes, are capable of undergoing similar processes.
Lipid droplets were first observed in normal bone as early as 1965, specifically when Enlow and colleagues described them to occur in osteoblasts in the vicinity of the Haversian canal.11 Osteocytes have also been shown to accumulate lipid droplets during steroid treatment12 and alcoholism13,14 one author coined this phenomenon ‘bone steatosis’. While these studies primarily documented lipid droplets in osteocytes, others have noted lipid droplets in osteoblasts. The most recent work of Mcgee-Lawrence et al. demonstrated that conditional deletion of histone deacetylase (Hdac)-3 in Runx2 positive osteoblasts, significantly increased intracellular lipid droplets.15 Interestingly, aging and dexamethasone treatment, 2 scenarios in which bone mineral density (BMD) is known to be compromised, are also associated with decreased Hdac3 expression and increased lipid droplets in osteoblasts.15 Collectively, these studies provide evidence that osteoblasts have the ability to store lipid droplets during pathologic conditions; however, fundamental questions relative to the occurrence and function of these organelles remain to be explored. Therefore, the purpose of the current study was to determine whether osteo-progenitors and/ or osteoblasts possess intracellular lipid droplets, and glean preliminary insight as to how these organelles may impact osteoblast differentiation and function.
Results
Undifferentiated bone marrow stromal cells (BMSCs; day 0) contained few lipid droplets, detected as green puncta by BODIPY493/503 staining (Fig. 1A), while 2 d under osteogenic conditions appeared to induce more lipid droplets (Fig. 1B). Interestingly, a pronounced detection of lipid droplets was observed after 7 d in osteogenic medium (Fig. 1C). While this method of osteogenic induction is readily used in the field of bone biology, the initiation and progression of osteoblast differentiation was confirmed by detecting osteoblast-specific genes including, Runx2, Sp7, and Alpl (Fig. 1D-F).
Figure 1.
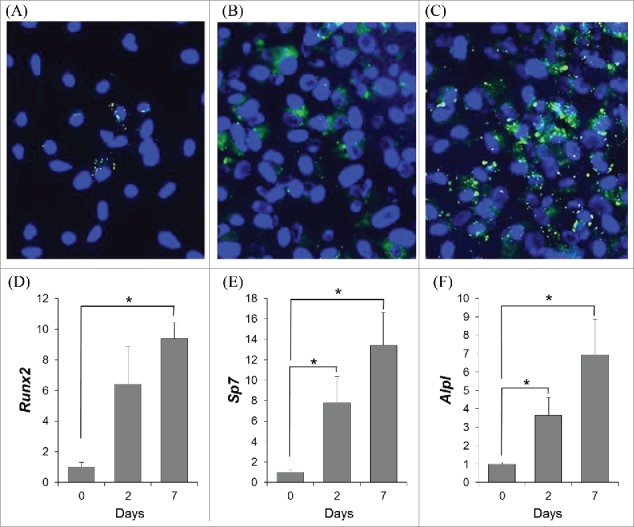
To determine whether lipid droplets were present during osteoblast differentiation, neutral lipids were selectively stained with BODIPY493/503 (green puncta), while nuclei were stained with Hoechst (blue) in bone marrow stromal cells (BMSCs) under osteogenic conditions for 0 (A), 2 (B), or 7 (C) days; representative images are shown. To confirm osteogenic induction, relative mRNA expression of osteoblast-related genes runt-related transcript factor (Runx2) (D), osterix (Sp7) (E), and alkaline phosphatase (Alpl) (F) were determined in BMSCs through osteogenic differentiation (0, 2, or 7 days). Data shown is presented as mean ± standard error. Symbol, *, denotes statistically significant differences (P < 0.05) between groups.
Once the presence of lipid droplets had been established during osteoblast differentiation, we sought to determine the relative gene expression of lipid droplet-associated proteins from the PAT family of proteins including perilipin or Plin1 (Plin1), adipose differentiation-related protein or ADRP (Plin2), tail-differentiation interacting protein of 47 kDa or TIP47 (Plin 3), S3–12 (Plin4), and lipid storage droplet protein 5 or LSDP5 (Plin5). While all of the perilipin genes were detected in BMSCs, osteoblasts, femur cortex, and bone marrow, Plin2 expression was highly expressed in all samples (Fig. 2A), with CQ values ranging from 20.6–24.3. Plin3 expression was the next highest, followed by low detection levels of Plin1, -4, and -5 (Fig. 2A). PLIN2 protein abundance was also confirmed in BMSCs differentiated under osteogenic conditions for 0, 2, or 7 days, with the highest abundance being detected at day 2 (Fig. 2B).
Figure 2.
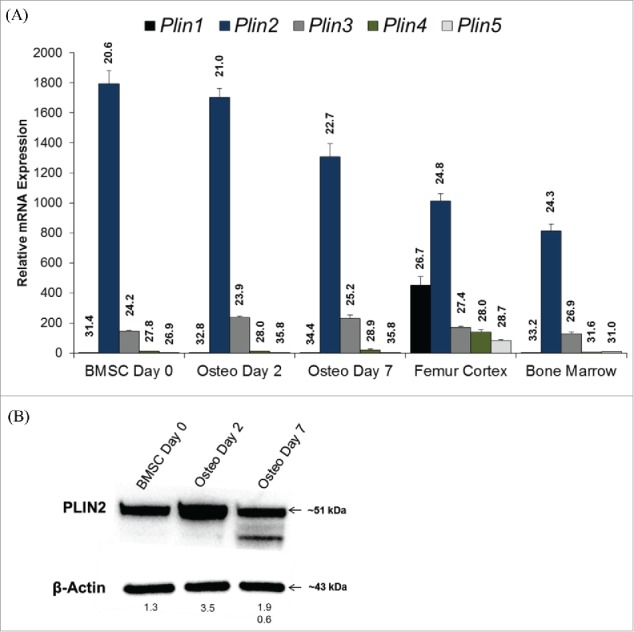
(A) Relative mRNA expression of lipid droplet-associated proteins from the PAT family of proteins including perilipin or Plin1 (Plin1), adipose differentiation-related protein or ADRP (Plin2), tail-differentiation interacting protein of 47 kDa or TIP47 (Plin 3), S3–12 (Plin4), and lipid storage droplet protein 5 or LSDP5 (Plin5) were detected in bone marrow stromal cells (BMSCs) under osteogenic conditions for 0, 2, or 7 days, femur cortex, and the bone marrow. All data are normalized to the housekeeping gene Hprt, and expressed relative to Plin1 expression in day 0 BMSCs. Data is represented as mean ± standard error. Uncorrected, mean CQ values are also indicated on graph for each target gene. (B) Plin2 protein abundance from BMSCs differentiated under osteogenic conditions for 0, 2, and 7 d. Mean protein abundance is expressed as density light units (DLUs x 103) relative to the loading control, β-actin.
We then asked whether impairing lipid droplet formation with triacsin C (TriC) would impact osteogenic differentiation. Indeed, TriC treatment caused a marked decrease in osteoblast differentiation as detected by lower alkaline phosphate (ALP) and Von Kossa staining (Fig. 3A-D). This decrease in osteoblastogenesis did not appear to be attributed to cell death following the 24 hour TriC treatment (Fig. 3E-H), but rather was related to a bona fide reduction of osteoblast differentiation.
Figure 3.
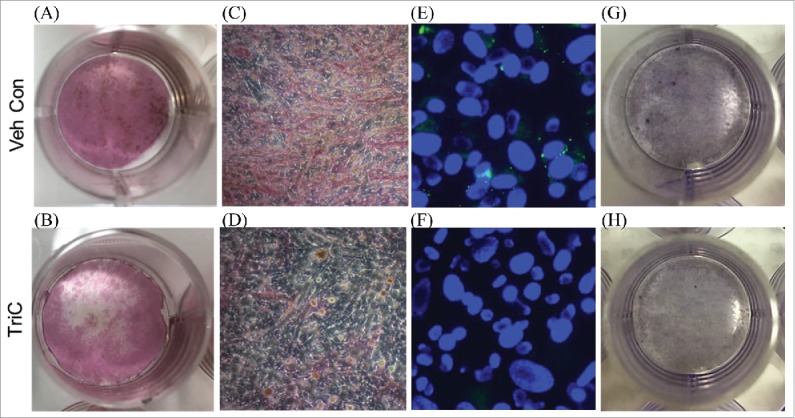
Primary bone marrow stromal cells (BMSCs) were treated with vehicle (A, C) or TriC (B, D) during the first 24 hours of osteogenic differentiation; treatments were then removed and cells were allowed to differentiate an additional 7 days, and stained for alkaline phosphatase (ALP) and Von Kossa. To confirm TriC's ability to blunt lipid droplet formation, BMSCs were stained with BODIPY493/503 and Hoechst immediately following the initial 24 hour treatment period of vehicle (E) or TriC (F). Additionally, vehicle (G) or TriC (H) treated BMSCs were stained with hematoxylin to confirm cell viability following the initial 24 hours of treatment.
After establishing that osteoblasts contain lipid droplets and that these lipid droplets support osteoblastogenesis, we next sought to determine whether these organelles were being used as a source of fatty acids capable of providing the cell with adenosine triphosphate (ATP). In fact, blocking mitochondrial fatty acid uptake with the carnitine palmitoyl-transferase 1 (CPT1) inhibitor, etomoxir (Eto), resulted in a marked reduction of oxygen consumption rates (OCR) compared with controls at day 0 and 2 of BMSCs osteogenic differentiation (Fig. 4A, B). Conversely, mature osteoblasts (BMSCs day 7) did not appear to utilize fatty acids to the same extent, as the addition of Eto resulted in only a modest decrease in OCR (Fig. 4C). Extracellular acidification rates (ECAR), a surrogate measure of glycolysis, was not altered in these experiments (data not shown).
Figure 4.
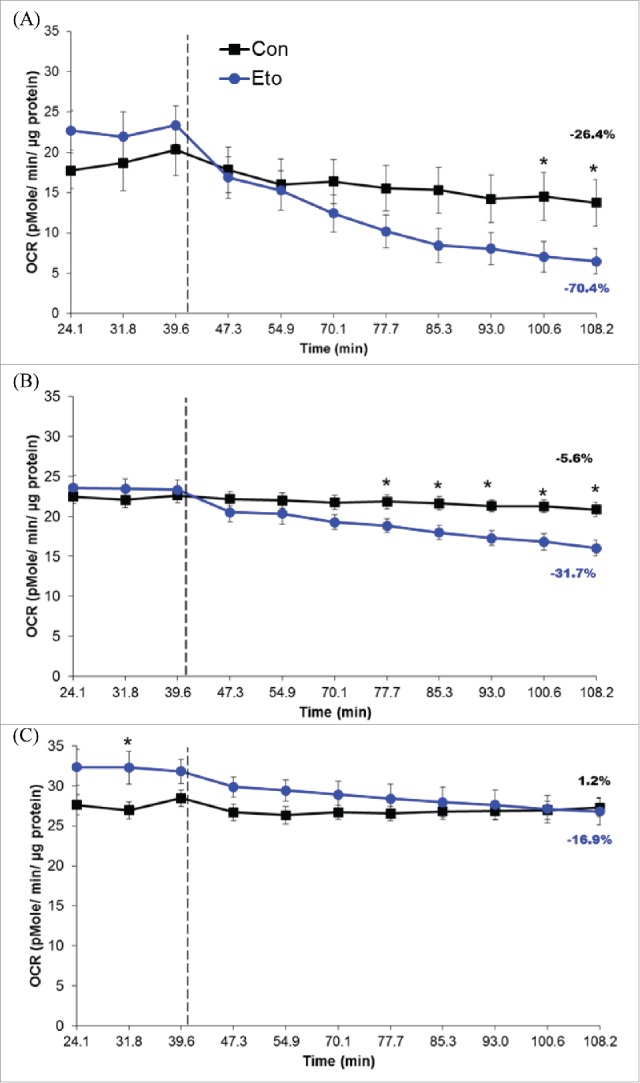
Oxygen consumption rates (OCR) from bone marrow stromal cells (BMSCs) cultured under osteogenic conditions for 0 (A), 2 (B), or 7 (C) days. Hashed lines represent time at which XF Base medium (control, Con) or 50 µM etomoxir (Eto) were injected. Percent change in OCR, from baseline to final reading, is also represented on graphs. Data shown is presented as mean ± standard error. Symbol, *, denotes statistically significant differences (P < 0.05) between groups at a given time point.
Discussion
In the current study, we provide strong in vitro evidence that lipid droplets are detected throughout osteoblastogenesis, and that these organelles support osteoblast differentiation by providing a readily available fuel source. Previously described examples of lipid droplets in osteoblasts and/ or osteocytes (steroid treatment, alcoholism, and aging) implied that intra-osteoblastic lipid droplets were pathological and corresponded to scenarios of low bone mass with increased bone marrow adiposity.12-16 Our data would argue that lipid droplets and their degradation and/or utilization are essential components of the osteoblast differentiation scheme.
Interestingly, lipid droplets weren't readily visualized in osteoblasts until they reached a mature stage (day 7), whereas less lipid droplets were observed in the stromal cell population (day 0) and committed osteo-progenitor (day 2) cells. These data correspond closely to the bioenergetic studies from these cells, suggesting that lipid droplets did not accumulate at earlier time points due to their rapid break-down and utilization. In contrast, the mature osteoblasts did not appear to be using fatty acids to a similar extent, resulting in their subsequent accumulation and visualization, a process referred to as lipid droplet flux. While the mechanism of lipid droplet break-down in osteoblasts remains to be elucidated, in other tissues it has been described to occur via 2 distinct mechanisms: (1) lipid droplet-associated cytoplasmic lipases, and/ or (2) autophagosome sequestration of lipids and subsequent degradation by lysosomal acid lipase, termed lipophagy.17,18 Both processes result in the liberation of free fatty acids which can enter into the mitochondria through the carnitine shuttle mediated by CPT1, undergo β-oxidation, and enter into the TCA cycle to generate ATP by means of oxidative phosphorylation. While our data are the first to describe the capacity of osteoblasts to utilize intracellular lipid droplets as a substrate source for energy generation, it has previously been described during the activation of T-cells19,20 as well as the trans-differentiation of quiescent cells including hepatocytes21,22 myocytes23 and macrophages.24 Additionally, blocking lipid droplet formation, and thus any future utilization, early during the osteoblast differentiation process resulted in a striking reduction in osteoblastogenesis. Taken together, these data suggest that osteoblast progenitor cells and committed osteo-progenitor cells utilize lipid droplets as an endogenous source of fatty acids capable of providing the cell with ATP.
In addition to the visualization of lipid droplets during osteoblast differentiation, we also report relative gene expression of the lipid droplet-associated proteins, perilipin (Plin) 1–5. The perilipin proteins are the major LD-associated proteins that regulate lipid droplet size, stability, and metabolism in a tissue specific manner.25 For example, while PLIN1 and -4s major site of expression is the mature adipocyte26,27 PLIN2 and -3 can be found ubiquitously28,29 with PLIN2 highly expressed in the liver.30 Conversely, PLIN5 appears to mainly be found in cardiac and skeletal muscle.31,32 In this regard, our data revealed that while Plin1–5 could be detected in BMSCs, osteoblasts, femur cortex, and bone marrow, Plin2 was the most highly expressed. Particularly noteworthy, Plin2 gene expression was highest in stromal cells and osteo-progenitor cells. In addition to Plin2 gene expression, we further confirmed PLIN2 protein abundance during osteoblast differentiation demonstrating PLIN2 is highest in the committed osteo-progenitor cell (day 2). Surprisingly, we consistently detected a cleaved PLIN2 protein product in mature osteoblasts. While PLIN2 has recently been described to be targeted for lysosomal degradation via chaperon mediated autophagy (CMA), thus initiating lipolysis, far less in known about PLIN2 cleavage.33,34 In addition to PLIN2s role in regulating lipophagy, cytosolic PLIN2 has been shown to be targeted for proteasomal degradation35 which provides another potential explanation for the lower molecular weight PLIN2 detected. While these data are provocative, further characterization of PLIN2 protein expression as well as lipid droplet abundance and localization are currently underway to better understand the role of PLIN2 in osteoblast differentiation and maturation. Another interesting finding was the relatively high abundance of Plin1 in the femur cortex, despite its classic restriction to large lipid droplets36 typically noted in adipose tissue depots.37 Given the cellular composition of the femur cortex, this data suggests that osteocytes may in fact express Plin1. Moreover, the presence of particularly large lipid droplets in osteocytes following glucocorticoid12 or alcohol13,14 treatments, suggest PLIN1s presence in osteocytes. To our knowledge, no other studies have published data relative to Plin expression in osteocytes, and therefore these conclusions remain speculative. Additionally, while the femur cortex was flushed of the marrow, we cannot rule out the presence of other contaminating cells.
In conclusion, the current study demonstrates that osteo-progenitors and osteoblasts synthesize, mobilize, and utilize intracellular lipid droplets as a fuel source. While these novel data establish a basis for lipid biology in the bone field, future studies are warranted to further delineate why lipid droplet accumulation and utilization differs at various stages of osteoblast maturation. Moreover, looming questions remain relative to the role of the lipid droplets in the mature osteoblast as these organelles do not appear to be used for fuel generation at this stage. Additionally, the process by which these organelles are mobilized via cytoplasmic lipases or lipophagy is of interest and could offer novel insights into conditions associated with excess lipid accumulation and bone abnormalities. Continued exploration of these basic intracellular processes greatly contributes to the fundamental knowledge of bone biology, and is an essential component to target many clinical scenarios.
Materials and methods
Primary cell culture
BMSCs were harvested and plated from male C57BL/6J mice, 8–10 weeks of age. Briefly, femora, tibiae, and iliac crest were cleaned of all soft-adherent tissue, bones were cut at both the distal and proximal ends, and bone marrow was isolated via centrifugation at 12,000 x g for 10 seconds at 4°C. Total bone marrow was then pooled and plated in complete αMEM (Gibco, Cat#12571) containing 10% fetal bovine serum (FBS) (SeraDigm, Cat#97068–085), 100 U/L penicillin G and 100 mg/L streptomycin (Gibco, Cat# 15140–122), and maintained at 37°C in a humidified atmosphere of 5% CO2 for 48 hours. Following 48 hours in co-culture, the non-adherent haematopoietic cell population was aspirated off, and the adherent, BMSCs was isolated via trypsinization (Gibco, Cat#25200–056). BMSCs were then counted and plated accordingly for experimental procedures. For all osteoblast differentiation experiments osteogenic medium contained complete αMEM supplemented with 5 mM β-glycerophosphate (Sigma Aldrich, Cat#G6152) and 50 μg/ mL ascorbic acid (Sigma Aldrich, Cat#A4544).
To assess intracellular lipid droplets, BMSCs were plated in 96-well clear, black walled plates at a density of 6.25 × 104 cells/ cm2 and cultured as described previously under osteogenic conditions for 0, 2, or 7 d. At a given time point, cells were fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature, and then incubated with 0.5 µg/ mL 4,4-difluoro-1,3,5,7,8-pentamethyl-4-bora-3a,4a-diaza-s-indacene (BODIPY®) 493/503 (ThermoFisher Scientific, Cat#D3922) for 45 min protected from light. For morphological purposes, nuclei were also stained with Hoechst (ThermoFisher Scientific, Cat#62249).
Osteoblast differentiation and mineralization was determined by plating 13.0 × 104 cells/cm2 BMSCs. Control cells were treated as described previously under osteogenic conditions for 7 d. To block lipid droplet formation, BMSCs were treated with 5.4 µM of TriC (Cayman Chemical, Cat#76896–80–5) during the initial 24 hr of osteogenic induction, and then removed as cells were cultured under normal osteogenic conditions for the remaining 6 d. Following 7 d in culture, cells were stained for ALP using a commercially available kit (Sigma-Aldrich, Cat#86R-1KT) as well as Von Kossa stain to determine calcium salts. To confirm that TriC was in fact blocking lipid droplet formation and not inducing cell death within the treatment time frame (24 hr), BMSCs were cultured and treated for 24 hours with vehicle (DMSO) or 5.4 µM TriC immediately following the addition of osteogenic differentiation medium, and then stained with BODIPY493/503 and Hoechst (see above) or hematoxylin.
RNA isolation and qPCR analyses
Total RNA was extracted using TriZol Reagent (Invitrogen, Cat#1556026), following the manufacturers protocol. cDNA was synthesized using 2 μg of total RNA pre-treated with DNase I and subjected to reverse-transcription (Invitrogen, Cat #4368813). Each qPCR reaction was performed in duplicate using SYBR green chemistry (BioRad, Cat#1708882) on the BioRad CFX384Real-Time System. All qPCR results were evaluated by the comparative cycle number at threshold (CQ) method (User Manual #2, Applied Biosystems), using hypoxanthine phosphoribosyltransferase 1 (Hprt1) as the invariant control, FWD 5′-GCC TAA GAT GAG CGC AAG TTG; REV 5′-TAC TAG GCA GAT GGC CAC AGG. Target gene primer sequences are as follows: Runx2 FWD 5′-CGA CAG TCC CAA CTT CCT GT, REV 5′-CGG TAA CCA CAG TCC CAT CT; Sp7 FWD 5′-GAA GTT CAC CTG CCT GCT CTG T, REV 5′-CGT GGG TGC GCT GAT GT; Alpl FWD 5′-GGT ATG GGC GTC TCC ACA GT, REV 5′-GCC CGT GTT GTG GTG TAG CT; Plin1 FWD 5′-AGG CTG TCT CCT CTA CCA AA, REV 5′-CCA CAG TGT CTA CCA CGT TAT C; Plin2 FWD 5′-AAG AGC CAG GAG ACC ATT TC, REV 5′-CCA CGA GAC ATA GAG CTT ATC C; Plin3 FWD 5′-CTG GAC AGA CTG CAG GAA AG, REV 5′-CAC TGT AGA TGA CAC CAG TTC C; Plin4 5′-GCT GTT GGT AGC CT GAG AT, REV 5′-CTG GTT ACT AGG CTG CTG TAA G; Plin5 5′-GTG TGT AGT GTG ACT TGT G, REV 5′-GCA GCT CTT CCA ATT TGT C.
Isolation of protein and immunoblot
BMSCs were cultured as detailed previously, and total protein was isolated using RIPA buffer (Cell Signaling Technologies, Cat#9806) supplemented with 1 mM PMSF. Cells were scraped, briefly sonicated, and centrifuged for 10 min at 14,000 x g at 4°C, supernatant was then removed and total protein was quantified. For immunoblotting, primary antibodies included PLIN2/ ADFP (Novus Biologicals, Cat#NB110–40877) and β-Actin (Santa Cruz, Cat#sc-47778).
Agilent seahorse XF24 flux analyzer
Bioenergetic capacity was determined using the Agilent Seahorse XF24. Primary BMSCs were harvested and isolated as detailed above and 9.375 × 104 cells/ cm2 were plated. BMSCs were then differentiated for 0, 2, or 7 d and subjected to Seahorse XF24 analysis using the standard protocol supplied by the manufacturer. Cells were assayed in XF Base medium (Agilent, Cat#102353–100) which lack exogenous substrates (glutamine, glucose, etc.). This allowed us to specifically report OCR relative to endogenous substrates. Cartridge plates were also loaded in such a manner that injection port A would introduce either XF Base medium (Con) or 50 µM Eto (Sigma, Cat#E1905). Following the completion of Seahorse XF analysis, total protein was isolated, quantified and each well was normalized to total µg protein.
Statistical analyses
Statistical analyses were performed using Statistical Analysis Software (SAS) version 9.3. Student's paired t-test was used to determine the differences between groups indicated. Data are presented as mean ± standard error and a P < 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
Funding
This work was supported by the NIH under NIDDK DK092759–06 (CJR); and NIAMS AR 066120–0 (CJR), AR068095 (ARG)
References
- [1].Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, Andon MB, Smith KT, Heaney RP. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93(2):799-08. https://doi.org/ 10.1172/JCI117034; PMID:8113412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: An estimation of peak bone mass. J Bone Miner Res. 2011;26(8):1729-39. https://doi.org/ 10.1002/jbmr.412; PMID:21520276 [DOI] [PubMed] [Google Scholar]
- [3].Borle AB, Nichols N, Nichols G Jr. Metabolic studies of bone in vitro. II. The metabolic patterns of accretion and resorption. J Biol Chem. 1960;235:2351211-1214. PMID:13802862 [PubMed] [Google Scholar]
- [4].Borle AB, Nichols N, Nichols G Jr. Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem. 1960;235:2351206-1210. PMID:13802861 [PubMed] [Google Scholar]
- [5].Adamek G, Felix R, Guenther HL, Fleisch H. Fatty acid oxidation in bone tissue and bone cells in culture. Characterization and hormonal influences. Biochem J. 11-15-1987;248(1):129-37. https://doi.org/ 10.1042/bj2480129; PMID:3325035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, Rensen PC, Heeren J. Uptake of postprandial lipoproteins into bone in vivo: Impact on osteoblast function. Bone. 2008;43(2):230-37. https://doi.org/ 10.1016/j.bone.2008.03.022; PMID:18538644 [DOI] [PubMed] [Google Scholar]
- [7].Schilling AF, Schinke T, Munch C, Gebauer M, Niemeier A, Priemel M, Streichert T, Rueger JM, Amling M. Increased bone formation in mice lacking apolipoprotein E. J Bone Miner Res. 2005;20(2):274-82. https://doi.org/ 10.1359/JBMR.041101; PMID:15647822 [DOI] [PubMed] [Google Scholar]
- [8].Niemeier A, Kassem M, Toedter K, Wendt D, Ruether W, Beisiegel U, Heeren J. Expression of LRP1 by human osteoblasts: A mechanism for the delivery of lipoproteins and vitamin K1 to bone. J Bone Miner Res. 2005;20(2):283-93. https://doi.org/ 10.1359/JBMR.041102; PMID:15647823 [DOI] [PubMed] [Google Scholar]
- [9].Frey JL, Li Z, Ellis JM, Zhang Q, Farber CR, Aja S, Wolfgang MJ, Clemens TL, Riddle RC. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. June-1-2015;35(11):1979-91. https://doi.org/ 10.1128/MCB.01343-14; PMID:25802278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50(1):14-27. https://doi.org/ 10.1016/j.plipres.2010.10.004; PMID:21087632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Enlow DH, Conklin JL, Bang S. Observations on the occurrence and the distribution of lipids in compact bone. Clin Orthop Relat Res. 1965;38 38157-169. PMID:4172974 [DOI] [PubMed] [Google Scholar]
- [12].Kawai K, Tamaki A, Hirohata K. Steroid-induced accumulation of lipid in the osteocytes of the rabbit femoral head. A histochemical and electron microscopic study. J Bone Joint Surg Am. 1985;67(5):755-63. https://doi.org/ 10.2106/00004623-198567050-00010; PMID:3997928 [DOI] [PubMed] [Google Scholar]
- [13].Wang Y, Li Y, Mao K, Li J, Cui Q, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: A possible mechanism for osteonecrosis. Clin Orthop Relat Res. 2003;(410):213-24. https://doi.org/ 10.1097/01.blo.0000063602.67412.83; PMID:12771833 [DOI] [PubMed] [Google Scholar]
- [14].Maurel DB, Pallu S, Jaffre C, Fazzalari NL, Boisseau N, Uzbekov R, Benhamou CL, Rochefort GY. Osteocyte apoptosis and lipid infiltration as mechanisms of alcohol-induced bone loss. Alcohol Alcohol. 2012;47(4):413-22. https://doi.org/ 10.1093/alcalc/ags057; PMID:22596044 [DOI] [PubMed] [Google Scholar]
- [15].McGee-Lawrence ME, Carpio LR, Schulze RJ, Pierce JL, McNiven MA, Farr JN, Khosla S, Oursler MJ, Westendorf JJ. Hdac3 deficiency increases marrow adiposity and induces lipid storage and glucocorticoid metabolism in osteochondroprogenitor cells. J Bone Miner Res. 2016;31(1):116-28. https://doi.org/ 10.1002/jbmr.2602; PMID:26211746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maddalozzo GF, Turner RT, Edwards CH, Howe KS, Widrick JJ, Rosen CJ, Iwaniec UT. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporos Int. 2009;20(9):1529-38. https://doi.org/ 10.1007/s00198-009-0836-y; PMID:19238309 [DOI] [PubMed] [Google Scholar]
- [17].Dugail I. Lysosome/lipid droplet interplay in metabolic diseases. Biochimie. 2014;96:102-105. https://doi.org/ 10.1016/j.biochi.2013.07.008; PMID:23880642 [DOI] [PubMed] [Google Scholar]
- [18].Lettieri Barbato D, Tatulli G, Aquilano K, Ciriolo MR. FoxO1 controls lysosomal acid lipase in adipocytes: Implication of lipophagy during nutrient restriction and metformin treatment. Cell Death Dis. 2013;4:e861. https://doi.org/ 10.1038/cddis.2013.404. PMID:24136225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hubbard VM, Valdor R, Patel B, Singh R, Cuervo AM, Macian F. Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol. December-15-2010;185(12):7349-57. https://doi.org/ 10.4049/jimmunol.1000576; PMID:21059894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bronietzki AW, Schuster M, Schmitz I. Autophagy in T-cell development, activation and differentiation. Immunol Cell Biol. 2015;93(1):25-34. https://doi.org/ 10.1038/icb.2014.81; PMID:25287445 [DOI] [PubMed] [Google Scholar]
- [21].Thoen LF, Guimaraes EL, Dolle L, Mannaerts I, Najimi M, Sokal E, van Grunsven LA. A role for autophagy during hepatic stellate cell activation. J Hepatol. 2011;55(6):1353-60. https://doi.org/ 10.1016/j.jhep.2011.07.010; PMID:21803012 [DOI] [PubMed] [Google Scholar]
- [22].Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, Czaja MJ, Friedman SL. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142(4):938-46. https://doi.org/ 10.1053/j.gastro.2011.12.044; PMID:22240484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tang AH, Rando TA. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J. December-1-2014;33(23):2782-97. https://doi.org/ 10.15252/embj.201488278; PMID:25316028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang Y, Morgan MJ, Chen K, Choksi S, Liu ZG. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood. March-22-2012;119(12):2895-05. https://doi.org/ 10.1182/blood-2011-08-372383; PMID:22223827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Itabe H, Yamaguchi T, Nimura S, Sasabe N. Perilipins: A diversity of intracellular lipid droplet proteins. Lipids Health Dis. April-28-2017;16(1):83. https://doi.org/ 10.1186/s12944-017-0473-y. PMID:28454542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res. 1995;36(6):1211-26. https://doi.org/ 10.1074/jbc.M304025200; PMID:7665999 [DOI] [PubMed] [Google Scholar]
- [27].Wolins NE, Skinner JR, Schoenfish MJ, Tzekov A, Bensch KG, Bickel PE. Adipocyte protein S3-12 coats nascent lipid droplets. J Biol Chem. September-26-2003;278(39):37713-721. PMID:12840023 [DOI] [PubMed] [Google Scholar]
- [28].Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38(11):2249-63. https://doi.org/ 10.1007/s00335-01-2055-5; PMID:9392423 [DOI] [PubMed] [Google Scholar]
- [29].Lu X, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C, Kimmel AR. The murine perilipin gene: The lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome. 2001;12(9):741-49. PMID:11641724 [DOI] [PubMed] [Google Scholar]
- [30].Motomura W, Inoue M, Ohtake T, Takahashi N, Nagamine M, Tanno S, Kohgo Y, Okumura T. Up-regulation of ADRP in fatty liver in human and liver steatosis in mice fed with high fat diet. Biochem Biophys Res Commun. February-24-2006;340(4):1111-18. https://doi.org/ 10.1016/j.bbrc.2005.12.121; PMID:16403437 [DOI] [PubMed] [Google Scholar]
- [31].Yamaguchi T, Matsushita S, Motojima K, Hirose F, Osumi T. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem. May-19-2006;281(20):14232-240. https://doi.org/ 10.1074/jbc.M601682200; PMID:16571721 [DOI] [PubMed] [Google Scholar]
- [32].Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, et al.. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem. July-6-2012;287(28):23852-863. https://doi.org/ 10.1074/jbc.M111.328708; PMID:22532565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kaushik S, Cuervo AM. AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy. 2016;12(2):432-38. https://doi.org/ 10.1080/15548627.2015.1124226; PMID:26902588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17(6):759-70. https://doi.org/ 10.1038/ncb3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Takahashi Y, Shinoda A, Kamada H, Shimizu M, Inoue J, Sato R. Perilipin2 plays a positive role in adipocytes during lipolysis by escaping proteasomal degradation. Sci Rep. 2016;6:20975. https://doi.org/ 10.1038/srep20975. PMID:26876687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brasaemle DL, Barber T, Kimmel AR, Londos C. Post-translational regulation of perilipin expression. Stabilization by stored intracellular neutral lipids. J Biol Chem. April-4-1997;272(14):9378-87. https://doi.org/ 10.1074/jbc.272.14.9378; PMID:9083075 [DOI] [PubMed] [Google Scholar]
- [37].Kimmel AR, Sztalryd C. The Perilipins: Major Cytosolic Lipid Droplet-Associated Proteins and Their Roles in Cellular Lipid Storage, Mobilization, and Systemic Homeostasis. Annu Rev Nutr. July-17-2016;36:471-09. https://doi.org/ 10.1146/annurev-nutr-071813-105410; PMID:27431369 [DOI] [PubMed] [Google Scholar]


