ABSTRACT
The excessive expansion of white adipose tissue underlies the global obesity epidemic. However, not all fat is equal, and the impact of heterogeneity on the development and expansion of different adipose depots is becoming increasingly apparent. Two mechanisms are responsible for the growth of adipose tissue: hyperplasia (increasing adipocyte number) and hypertrophy (increasing adipocyte size). The former relies on the differentiation of adipocyte stem cells, which reside within the adipose stromal vascular fraction. Many differences in gene expression, adipogenesis, and the response to obesogenic stimuli have been described when comparing adipose stem cells from different depots. Considering that there is disparity in the pathogenicity of the depots, understanding this heterogeneity has clinically relevant implications. Here we review the current knowledge surrounding such differences, in the context of development, expansion and therapeutics. Moreover, given the importance of these differences, we suggest that careful consideration for the precise methodologies used, is essential if we are to truly understand the physiologically relevant consequences of this heterogeneity.
KEYWORDS: adipogenesis, adipose progenitor/stem cell, adipose heterogeneity, gene expression, white adipose tissue
Introduction
Owing to the current worldwide obesity epidemic, research into adipose tissue biology has increased substantially over the last few decades. Mammalian adipose tissue is generally divided into two types: white adipose tissue (WAT) and brown adipose tissue (BAT). Both function in the maintenance of energy balance, however, WAT stores energy in the form of triglycerides, whereas BAT utilizes lipids to burn energy through adaptive thermogenesis.1 Importantly, WAT is remarkably capable of significant expansion, and it is this property which can lead to the accumulation of excess adipose tissue, associated with obesity and related pathologies.2
Recently, a third form of adipose tissue has also been identified in rodents. “Beige” or “brite” adipocytes reside in WAT, but morphologically and functionally they more closely resemble brown adipocytes. Crucially, they express uncoupling protein-1 (UCP-1); the master regulator of thermogenesis.3-5 Since the beiging of WAT has been demonstrated to protect against obesity, this is an area of much interest.6 Recent excellent reviews have discussed, in detail, what is currently known about brown and beige adipose tissue, therefore this will not be the focus of this review.7-10
WAT is divided into two types: subcutaneous (SWAT), which is located beneath the skin, and visceral (VWAT) which is distributed within the body cavity, around the organs. Excess VWAT, or “bad fat”, is associated with metabolic disease and the pathologies linked to obesity, whilst SWAT, or “good fat”, is thought to be protective.11 Both SWAT and VWAT are further subdivided into distinct depots around the body. To some extent these depots, or fat pads, differ in location and size between humans and rodents, and are illustrated in Figure 1.
Figure 1.
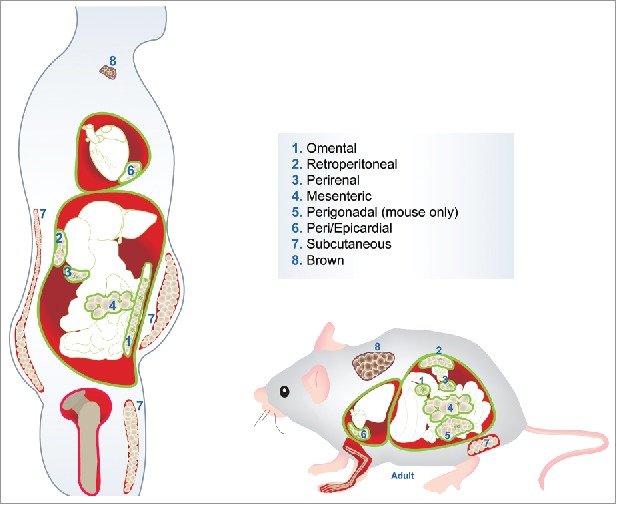
Location of human and murine adipose tissue depots. Depots 1-6 illustrate the visceral white adipose tissue (VWAT). Depot 7 indicates the subcutaneous white adipose tissue (SWAT): inguinal in mouse and abdominal, gluteal and femoral in human. Depot 8 shows the interscapular brown adipose tissue (BAT).
Increasing evidence demonstrates the vast heterogeneity that exists between different adipose depots, and more specifically, the adipose stem cells (ASCs) isolated from them.8 Moreover, our recent work has shown that ASCs isolated from a single VWAT depot, are not a homogeneous population.12 Given that the different depots contrast in their pathogenicity, understanding such differences is clinically relevant. The emphasis of this review shall be on what is currently known regarding inter-depot differences between WAT depots, with a specific focus on the ASCs.
Within the field of adipose tissue biology there are a multitude of terms that have been used to describe ASCs, which, when comparing various studies, can become confusing. Examples include “preadipocytes”, “adipocyte precursor cells” and “adipose mesenchymal stem cells”, amongst others.13 Considering the ever-increasing heterogeneity that is being uncovered between and within ASC populations, defining clearly the cell type being utilized will become increasingly important. In recent years there has been an effort to standardize terminology.13 Nevertheless, different terms are still used and with on-going research, cell surface marker profiles are ever changing and newly defined populations are being isolated. Throughout this review the terms ASC, preadipocyte and adipocyte progenitor will be used interchangeably, according to the study being described. Table 1 outlines the different studies that are discussed, including information on the species, gender, fat depot, health status of the subjects, and cell type utilized, as well as how the cells were isolated.
Table 1.
Summary of the studies discussed throughout the review. Including information on the species and specific fat depots used in each study, as well as the cell type utilised, how they were isolated from the fat (where appropriate), the sex of the subjects used and their metabolic/health status.
| REFERENCE | SPECIES | FAT DEPOTS USED | CELL TYPE ANALYSED/MARKERS USED FOR ISOLATION | SEX OF SUBJECTS | METABOLIC/HEALTH STATUS OF SUBJECTS |
|---|---|---|---|---|---|
| Cantile et al. (2003)15 | Human | Subcutaneous, visceral, dermal | Whole adipose tissue | Male & female | Healthy |
| Linder et al. (2004)16 | Human | Subcutaneous, omental | Whole adipose tissue | Male & female | Obese but otherwise healthy |
| Vohl et al. (2004)17 | Human | Subcutaneous, omental | Whole adipose tissue | Male | Obese but no diabetes and normal plasma lipid profile |
| Wu et al. (2008)18 | Mouse | Perigonadal, retroperitoneal, interscapular BAT | Adipocytes, whole SVF, preadipocytes isolated by adherence | Male | Not stated |
| Tchkonia et al. (2005)19 | Human | Subcutaneous, mesenteric, omental | Preadipocytes isolated by adherence | Male & female | Obese but otherwise healthy (fasting plasma glucose ≤110mg/dl) |
| Tchkonia et al. (2007)20 | Human | Subcutaneous, mesenteric, omental | Preadipocytes isolated by adherence | Male & female | Obese and lean subjects, otherwise healthy (fasting plasma glucose 106 ± 9mg/dl) |
| Cartwright et al. (2010)21 | Rat | Perigonadal, perirenal | Preadipocytes isolated by adherence | Male | Normal/not stated |
| Macotela et al. (2012)22 | Mouse | Subcutaneous, perigonadal | Adipocyte precursor cells: Ter119− CD45− CD31− Sca1+ CD34+ isolated by FACS | Male & female | Normal/not stated |
| Perrini et al. (2013)23 | Human | Subcutaneous, visceral | Adipose stem cells isolated by adherence | Male & female | Non-obese and healthy (fasting plasma glucose 85 ± 11mg/dl) |
| Kim et al. (2016)24 | Human | Subcutaneous, retroperitoneal | Adipose stem cells: CD31− CD45− isolated by MACS, then cultured to isolate adherent cells | Female | Lean, overweight and obese subjects used, no information on health status |
| Meissburger et al. (2016)25 | Mouse | Subcutaneous, perigonadal | Preadipocytes isolated by adherence | Male | Normal/not stated |
| Gesta et al. (2006)26 | Mouse & human | Mouse: subcutaneous, perigonadal. | Mouse: Adipocytes and whole SVF | Mouse: Male | Mouse: Normal/not stated |
| Human: Lean, overweight and obese subjects used - no information on healthy status | |||||
| Human: whole adipose tissue | Human: Male & female | ||||
| Human: subcutaneous, visceral | |||||
| Yamamoto et al. (2010)27 | Mouse | Inguinal subcutaneous, interscapular subcutaneous, perigonadal, mesenteric, perirenal, interscapular BAT | Whole adipose tissue | Male | Normal/not stated |
| Tchkonia et al. (2002)28 | Human | Subcutaneous, mesenteric, omental | Preadipocytes isolated by adherence | Male & female | Obese but otherwise healthy (fasting plasma glucose ≤120mg/dl) |
| Lysaght et al. (2011)32 | Human | Subcutaneous, omental | Whole adipose tissue | Male & female | 36% met the International Diabetes Federation definition for metabolic syndrome |
| Schlich et al. (2013)33 | Human | Subcutaneous, visceral | Whole adipose tissue | Female | Lean and moderately overweight, otherwise healthy |
| Baglioni et al. (2009)34 | Human | Subcutaneous, visceral (abdominal) | Adipose stem cells isolated by adherence | Male & female | Lean and overweight, otherwise healthy |
| Hutley et al. (2003)35 | Human | Subcutaneous, omental | Preadipocytes isolated by adherence | Male & female | Lean, overweight and obese, but otherwise healthy |
| Tchkonia et al. (2006)36 | Human | Subcutaneous, omental | Preadipocytes isolated by adherence | Male & female | Obese but otherwise healthy (fasting plasma glucose ≤120mg/dl) |
| Toyoda et al. (2009)37 | Human | Subcutaneous, omental | Adipose stem cells isolated by adherence | Male & female | Lean, no information on health status |
| Entenmann & Hauner (1996)41 | Human | Subcutaneous | Adipocyte precursor cells isolated by adherence | Female | Lean and moderately overweight, otherwise healthy |
| Shahparaki et al. (2002)44 | Human | Subcutaneous, omental | Preadipocytes isolated by filtration of the SVF | Male & female | Stable weight, healthy |
| Van Harmelen et al. (2004)45 | Human | Subcutaneous, omental | Preadipocytes isolated by filtration of the SVF | Male & female | Obese but otherwise healthy |
| Rodeheffer et al. (2008)47 | Mouse | Subcutaneous, perigonadal | Adipocyte progenitors: CD45− CD31− Ter119−CD29+ CD34+ Sca1+ CD24+Preadipocytes: CD45− CD31− Ter119− CD29+ CD34+ Sca1+ CD24−isolated by FACS | Female | Normal/not stated |
| Joe et al. (2009)48 | Mouse | Subcutaneous, perigonadal | Adipocyte progenitors: CD45− CD31− αintegrin− Sca1+ CD34+ isolated by FACS | Male & female | Normal/not stated |
| Djian et al. (1983)49 | Rat | Perigonadal, perirenal | Adipocyte precursors isolated by adherence | Male | Normal/not stated |
| Djian et al. (1985)50 | Rat | Perigonadal, perirenal | Adipocyte precursors isolated by adherence | Male | Normal/not stated |
| Wang et al. (1989)51 | Rat | Perigonadal, perirenal | Adipocyte precursors isolated by adherence | Male | Normal/not stated |
| Kirkland et al. (1990)52 | Rat | Perigonadal, perirenal | Adipocyte precursors isolated by adherence | Male | Normal/not stated |
| Grandl et al. (2016)56 | Mouse | Subcutaneous, perigonadal, interscapular BAT | Adipocyte precursors: Lin− PDGFRα+ isolated by FACS | Not stated | Normal/not stated |
Another major area of research in the ASC field focuses on investigating their potential in regenerative medicine. There is a growing body of work which aims to compare subcutaneous ASCs with bone marrow stem cells (BMSCs) to understand their suitability for therapeutics, which is an area we shall also discuss. Bone marrow also contains marrow adipose tissue (MAT) which, unlike visceral and subcutaneous depots, consists of scattered adipocytes, the progenitors of which are different from those of WAT/BAT.14 However, the BMSCs that we henceforth refer to are the total mesenchymal stem cell (MSC) population as isolated from bone marrow aspirate.
Variation in gene expression profiles between white adipose depots
Numerous studies, both human and rodent, have described differences in gene expression between subcutaneous and visceral adipose tissue depots when looking at total fat.15-18 It is hypothesized that differences between depots influence adipose development as well as response to environmental cues, such as diet, and are therefore important in understanding obesity.8 Typically, such studies in rodents compare inguinal SWAT with perigonadal VWAT, and in humans, abdominal SWAT and omental or mesenteric VWAT are compared (Fig. 1). ASCs reside in the stromal vascular fraction (SVF) of each depot and in addition to investigating differences between whole adipose tissue depots, many groups have specifically examined the isolated SVF, thus excluding the mature adipocytes from their analysis.19-25
Differential expression of genes implicated in developmental processes
Gesta et al (2006) compared the gene expression profiles of the SVF from murine inguinal SWAT and perigonadal VWAT adipose tissues and showed an enrichment of genes implicated in developmental processes. When the SVF was cultured for six days, this differential gene expression was retained, indicating that the differences were inherent to the preadipocytes, and not an effect of the microenvironment in which they exist in vivo. Moreover, when analyzing differences in gene expression between human abdominal subcutaneous and visceral adipose tissue, they demonstrated that many of the differences observed in the mice were recapitulated in the human study, and that for certain genes there was a correlation with obesity.26
Much attention has been paid to differences that exist between subcutaneous and visceral WAT, however, emerging evidence shows that there is significant variation between different visceral depots. One of the first studies to compare gene expression profiles of ASCs from different human visceral depots revealed that, in addition to the expected differences between subcutaneous and visceral preadipocytes, distinct differences were observed between mesenteric and omental depots (which make up the majority of the visceral fat in humans).20 As with studies that have focused on subcutaneous versus visceral fat, one major class of genes identified in this study were those involved in developmental processes, revealing that surprisingly, mesenteric preadipocytes clustered with subcutaneous, rather than omental, with regards to developmental gene expression.20 Similarly, when gene expression was compared between rat perirenal and perigonadal preadipocytes, developmental genes were one of the main categories identified.21
There is clear evidence that distinct differences in the expression profiles of developmental genes exist between all WAT depots in humans and rodents.15,17,20-23,26 Yamamoto et al (2010) performed a comprehensive study investigating differences in the expression of key developmental genes between total fat from six murine adipose depots. Having compared the expression of Tbx15, Shox2, En1, Hoxc9, Hoxc5 and Hoxa5, across inguinal and interscapular SWAT, as well as perigonadal, perirenal and mesenteric VWAT and interscapular BAT, they showed that each depot is distinct, with each one harboring a unique pattern of developmental gene expression.27 For example, whilst Shox2 was shown to be highly expressed in the inguinal SWAT, and not in any of the VWAT, En1 and Tbx15 were highly expressed in both subcutaneous fat pads, and additionally in the perirenal VWAT, but not in the perigonadal or mesenteric. Therefore, this study provides supporting evidence for the discovery that major differences in expression exist between all depots, thus advocating the idea that adipose depots exist as individual “mini-organs”.19-21
Differential expression of genes implicated in adipogenesis, metabolism, inflammation and angiogenesis
Whilst developmental genes are one of the most commonly occurring groups identified through differential expression analysis, other interesting categories have also been highlighted. Genes implicated in adipogenesis and lipid metabolism have been shown to be differentially expressed among human subcutaneous, mesenteric, omental and retroperitoneal preadipocytes.20,24,28 For example, it has been shown that when induced to differentiate in vitro, human subcutaneous preadipocytes express higher levels of key adipogenic transcription factors such as PPAR-γ and C/EBP-α than omental and mesenteric preadipocytes.28 Moreover, gene ontology analysis of microarray data obtained from human subcutaneous and retroperitoneal ASCs has revealed that genes involved in cholesterol biosynthesis, lipid metabolism and negative regulation of MAPK activity (which regulates ASC differentiation) were overexpressed in the retroperitoneal ASCs.24
Furthermore, genes involved in cellular processes such as replication and apoptosis, as well as those implicated in transcription, angiogenesis and inflammation have been highlighted in studies on rodents and humans.21,24 For example, in rats, proinflammatory genes were found to be more highly expressed in perirenal preadipocytes than in perigonadal preadipocytes, a difference which is also more obvious with age.21 This finding is in line with other evidence suggesting that aging leads to detrimental changes in the function of adipose tissue.29 Moreover, in terms of secreted adipokines, VWAT is generally considered to secrete more inflammatory cytokines than SWAT.30 However, differences also exist between different visceral depots, for example, the mesenteric depot has been observed to express higher levels of TNF-α than the omental in insulin resistant individuals.31
In humans, isolated VWAT and SWAT progenitor cells have also been shown to exhibit different expression profiles for genes involved in angiogenesis, with retroperitoneal VWAT progenitors expressing higher levels of angiogenic genes than abdominal SWAT progenitors.24 Similarly, VWAT isolated from obese individuals has been shown to secrete higher levels of VEGF compared to SWAT from the same subjects, although these studies examined the whole adipose tissue, rather than the isolated progenitor cells.32,33 Conversely, in a study utilizing isolated adipose progenitors from human omental and subcutaneous depots, no significant differences were observed in the expression of pro-angiogenic or pro-inflammatory genes such as VEGF, TNF-α and IL-6.34
Comparing differentiation potential in vitro
Evidently, results from gene expression studies indicate that ASCs from different WAT depots are inherently different. As discussed in this section, many groups have used in vitro methods to assess differences in ASC behavior, predominantly proliferation and adipogenic differentiation. We shall discuss whether using the same culture conditions for ASCs isolated from different depots is appropriate, for example; perhaps conditions that are optimal for subcutaneous cells are sub-optimal for visceral cells, due to their innate differences.
Evidence from human studies
Numerous studies have shown that human SWAT preadipocytes replicate faster and differentiate into adipocytes more efficiently than VWAT preadipocytes isolated from the same subjects.24,28,34-37 As discussed in the previous section, SWAT preadipocytes express higher levels of adipogenic transcription factors, during differentiation in vitro, than omental and mesenteric.28 A difference has also been observed between omental and mesenteric depots, with a higher percentage of replicating and differentiating cells in the mesenteric preadipocyte cultures.19,28 Therefore, differences exist between VWAT depots as well as between SWAT and VWAT.
The key question is why do such differences occur? From what we have heard so far, an obvious explanation is that the cells from different depots are inherently different. In addition to this, several other possible reasons have been addressed. When VWAT and SWAT preadipocytes were co-cultured using transwell inserts, the replication of the SWAT cells was not slowed by the presence of the VWAT, indicating that the difference is not due to the secretion of anti-replicative factors.19 In addition to preadipocytes, the SVF contains multiple non-adipogenic cell types, the percentages of which differ between depots.38 Nevertheless, the differentiation capability of colonies derived from single adherent SVF cells still differs between depots, therefore this disparate behavior cannot be explained by differences in the proportion of non-adipogenic cells.28 However, it has been noted that results from studies that analyze colonies derived from adherent cells should be interpreted with caution. This method naturally enriches for cells of a particular immunophenotype, and so is not truly representative of the heterogeneous population, thus highlighting the point that analyzing the properties of adherent SVF cells in vitro is not overly reflective of the situation in vivo.7,39
As discussed, various studies show that not only do human SWAT preadipocytes differentiate better than VWAT, they are also more replicative.24,36 Rodent preadipocytes and pre-adipose cells lines, such as 3T3-L1 cells, should be confluent before they can effectively differentiate, due to the requirement for growth arrest.40,41 Therefore, it is logical that more replicative cells will become confluent more easily and thus differentiate more efficiently. However, this does not appear to be the case for human preadipocytes, as it has been shown that they will differentiate without requiring confluency.41 Consequently, although differences in in vitro replication and differentiation exist between cells from different human depots, the later cannot be fully attributed to the former. Therefore, the differences in differentiation cannot be fully explained by the differences in replication.
Contrasting results from human studies
Not all studies performed using human SWAT and VWAT preadipocytes show the same trend. For example, work performed by Shahparaki et al (2002), in which they measured cytosolic glycerol phosphate dehydrogenase (GPDH) activity as a determinant of in vitro terminal adipocyte differentiation,42,43 did not show a difference in differentiation between cultured omental and subcutaneous preadipocytes.44 Similarly, another study which assessed the differentiation of omental and subcutaneous preadipocytes, by measuring GPDH activity and counting the number of lipid-filled cells, also failed to show a difference in differentiation between the two depots.45 However, proliferation was also assessed, revealing that subcutaneous cells proliferated at a higher rate than the omental cells.45
There are several plausible explanations as to why such inter-experiment differences exist. For example, the subjects from which the adipose tissue was taken vary considerably in age, weight, disease status, gender; all factors which could feasibly influence the condition of the isolated preadipocytes. Additionally, different groups use different methods to isolate the preadipocytes. Traditionally, the entire SVF is plated and non-adherent cells are removed through washing and subsequent passaging, however as has already been alluded to, there are several problems with this method ultimately meaning that the cells used in assays do not well reflect the heterogeneous nature of the in vivo precursor cell population.39 Moreover, there is much variation between cells used at different passages, which may account for differences observed between studies.39 More recent studies use cell sorting techniques to remove non-adipogenic cells, rather than relying on adherence/non-adherence;24 however a panel of suitable markers to permit further enrichment of ASCs from human adipose tissue has not been established.7 Additionally, there is disparity in how the cells are differentiated, with different groups using variations of an adipogenic induction cocktail. Finally, various methods of determining differentiation are used in different experiments, including Oil Red O staining, measuring GPDH activity, quantification of lipid droplet accumulation and measuring the expression of adipogenic genes. With this inter-experiment variation in mind, it is unsurprising that different studies report different results, and importantly highlights the requirement for more standardized procedures and methods for the isolation, growth and differentiation of human ASCs.
Evidence from rodent studies
For many years, rodent cell cultures have also been used to examine differences between SWAT and VWAT, and between the different visceral depots. In contrast to the current situation in the human ASC field, panels of markers that permit the isolation of cells harboring the properties of adipocyte progenitors and preadipocytes from the SVF of mice have been identified.46,47 CD45−;CD31−;Ter119−;CD29+;CD34+;Sca-1+;CD24+ SVF cells, or adipocyte progenitor cells, can form functional fat depots when transplanted into lypodystrophic mice, as well as being adipogenic in vitro. CD45−;CD31−;Ter119−;CD29+;CD34+;Sca-1+;CD24− SVF cells (preadipocytes) are derived from the CD24+ progenitor cells and are committed to the adipocyte lineage. Therefore they are capable of adipogenesis in vitro, but are unable to form WAT depots in vivo.46,47 Many groups now use these markers, or a subset of them, to isolate adipocyte progenitor cells and preadipocytes from mice using flow cytometry. Nevertheless, there is still variability within the field in terms of experimental protocol.
Many studies have shown that, as with human cells, murine subcutaneous preadipocytes replicate faster and have greater adipogenic potential than visceral.22,25,48 Additionally, it has been demonstrated that rat perirenal adipocyte precursor cell cultures have a higher frequency of replicating and differentiating cells than perigonadal, therefore highlighting differences between visceral depots.49-52 Moreover, there is evidence to support that these differences in the behavior of the precursor cells in vitro is reflected in the mature adipocytes in vivo, with the perirenal depot exhibiting a greater increase in fat cell number over 70 days than the perigonadal depot.51
A recent study that utilized murine subcutaneous and perigonadal adipocyte precursor cells (isolated based on the cell-surface marker profile: CD31−;CD45−;Ter119−;CD34+;Sca-1+) revealed that the addition of BMP4 affects subcutaneous and perigonadal cultures differently.22 As described previously, the subcutaneous adipocyte precursor cells differentiated very efficiently, with greater than 90% of the cells forming large lipid droplets, compared to less than 20% of the perigonadal cells. However, when the cells were pre-treated with BMP4 before the addition of the differentiation media, approximately 90% of the perigonadal cells formed lipid droplets, but no further differentiation in the subcutaneous cultures was observed. A similar result was seen when BMP2 was used. Moreover, the expression of genes associated with differentiated adipocytes, such as Fabp4 and AdipoQ, increased to levels similar to those recorded for the differentiated subcutaneous cells.22 It is well documented that BMPs can promote the differentiation of mesenchymal stem cells into osteoblasts, chondrocytes and adipocytes.53-55 Further gene expression analysis revealed that, in the visceral precursor cells, the block in differentiation occurs because standard differentiation media is unable to induce the expression of the key adipogenesis gene: Ppar-γ, but that induction of BMP signaling releases this block. Interestingly, microarray analysis showed that BMP2 and 4 are expressed at higher levels in the subcutaneous precursor cells, compared with the perigonadal, possibly helping to explain why the subcutaneous cultures did not require the addition of BMP2/4.22 This study provides an excellent example of how differences in gene expression between adipocyte precursor cells from different depots can influence their ability to differentiate into mature adipocytes, and thus how heterogeneity between the depots can influence function. Moreover, this relates to our suggestion that perhaps the “one-size fits all” approach to culturing ASCs in vitro is not the best approach, given the inherent differences between cells from different depots.
Recent work performed by Grandl et al (2016) indicates that the extracellular matrix (ECM) of the adipose precursor cells influences their differentiation potential. Having decellularized mouse subcutaneous and perigonadal SVF cultures, and re-seeded them with freshly isolated adipocyte precursor cells, they found that when perigonadal precursor cells were cultured in the decellularized ECM from the subcutaneous cells, they differentiated better than when cultured in the ECM from the perigonadal SVF.56 Moreover, it has also been demonstrated that several proteins which are preferentially secreted by the perigonadal SVF are capable of inhibiting adipogenesis, and thus may help to explain why perigonadal adipocyte precursor cells have a lower capacity to differentiate than subcutaneous.25 Both studies therefore show strong support for the hypothesis that extracellular factors impact the differentiation potential of adipocyte precursor cells. The results from the later study contradict what has been reported previously, whereby co-culturing human omental and subcutaneous preadipocytes had no detrimental effect on the replication of the subcutaneous cells.19 Of course, there are many differences between the two studies, not least that one used murine cells, and the other, human. Furthermore, the co-culture experiment looked only at whether replication was affected, not differentiation.
Expansion of adipose tissue in vivo
Many studies that aim to investigate differences in adipogenic potential between adipocyte precursor cells from different depots, and the cellular and molecular mechanisms underlying these differences, have been carried out in vitro. Nevertheless, the benefits of understanding such differences in vivo are clear, and recent work has begun to shift in this direction. In vivo, WAT can expand through two mechanisms; hypertrophy (an increase in adipocyte size) and hyperplasia (an increase in adipocyte number), and it is thought that the mechanism of expansion could influence the depot's role in the occurrence and progression of metabolic disease.48 Recent reviews have described much of the in vivo work in some detail, including the contrasting results from several studies that investigated the contributions of hypertrophy and hyperplasia to the expansion of WAT.7,8 For this reason, we shall not discuss this area in too much detail, however it is evident that multiple factors influence the relative contributions of these two mechanisms to WAT expansion in different depots.
During normal homeostasis there is not much requirement for hyperplasia or hypertrophy, as the adipose tissue is not expanding, therefore the majority of studies are performed under obesogenic conditions. A number of studies have demonstrated that hyperplasia contributes to obesity in rodents,22,48,57,58 and recent work in humans has revealed that obese individuals have more adipocytes than lean individuals, which are not lost following substantial weight loss, indicating that hyperplasia must play a role in WAT expansion during obesity.59,60 Regarding differences between adipose depots recently it has been demonstrated that, following high-fat feeding, mice exhibit a significant increase in the formation of new adipocytes in perigonadal VWAT, but not in SWAT.61,62 This indicates that the visceral depot expands by hyperplasia in the high-fat diet state, whilst the subcutaneous depot does not. Moreover, this result was coupled with data that showed an increase in proliferation of the visceral adipocyte precursor cells in vivo (but not subcutaneous), within the first week of high-fat feeding, thus rapidly expanding the pool of precursor cells available for differentiation into adipocytes. Additionally, AKT2 signaling in the adipocyte precursor cells was demonstrated to be required for their activation in response to the high-fat diet.62 Importantly, it was shown that these newly formed adipocyte precursor cells in the VWAT do go on to differentiate into adipocytes after prolonged high-fat feeding.62 It has also recently been shown that hyperplasia, rather than hypertrophy, is the major contributor to the expansion of omental VWAT in human obesity.60 This data does somewhat contrast to that from other studies, which have indicated that upon high-fat feeding, hyperplasia contributes to the expansion of SWAT more so than to the expansion of VWAT,48 and the number of adipocyte precursor cells is increased in both visceral and subcutaneous depots.22 There are many potential reasons as to why these studies obtained contrasting results given that multiple factors can impact on adipose tissue expansion, such as the age, strain and sex of the mice, as well as the methods used to assess adipogenesis (the differentiation of adipocyte precursor cells into adipocytes). One major difference between these studies is the length of HFD feeding, with one focusing on the initial short-term response, and the other on the long-term response.48,62 Nevertheless, there is a growing body of evidence that reveals differences in in vivo adipogenesis between different depots and in different states.
Further work has shown that differences in adipogenesis between SWAT and VWAT depots in vivo, depend on sex.63 Whilst in male mice it appears that adipogenesis is increased in VWAT in high-fat diet states, but not in SWAT, this is not the case in female mice, which exhibit increased adipogenesis in both the SWAT and VWAT depots upon high-fat feeding. This result is thought to be due to the influence of sex-hormones on adipogenesis, which aligns with the fact that in humans, men commonly gain visceral adipose tissue, giving the typical “apple-shaped” figure and premenopausal women gain subcutaneous adipose tissue, giving the “pear-shaped” figure, whilst in postmenopausal women there is a transition to a more male-like adipose distribution.63-65 Moreover, another study which focused on human SWAT showed that, in women, the contribution of hyperplasia and hypertrophy to abdominal adipose tissue gain is dependent on the size of the existing adipocytes.66 If the existing adipocytes were smaller than average, hypertrophy was the predominant mechanism of expansion, whilst if the existing adipocytes were of average size, new adipocytes were recruited. However, this was not the case in men, for whom hypertrophy was the prevailing method of expansion in abdominal SWAT.66
It has also been shown, perhaps surprisingly, that the proliferation of murine adipocyte precursor cells in response to high-fat feeding is controlled by cell-extrinsic factors present in the microenvironment.63 When tdTomato-labelled adipocyte precursor cells isolated from male SWAT were transplanted into the VWAT of male mice, both the subcutaneous and visceral cells were shown to proliferate upon high-fat feeding. Whilst in the converse experiment, where male VWAT precursor cells were transplanted into male SWAT, no proliferation was observed for either cell population upon high-fat stimulation.63 Therefore, in both situations the adipocyte precursor cells took on the behavior typical of the depot into which they had been transplanted. Understanding the regulatory signals governing the activation of the adipocyte precursor cells in obesity is an essential next step, with evidence already suggesting that sex-hormones may play a role.63 To some extent this result conflicts with that from an earlier study, which showed that the transplantation of SWAT into the VWAT region resulted in decreased weight and fat mass as well as improved glucose metabolism. Therefore indicating that inherent differences between the depots, rather than their location, were responsible for such improvements.67 Obviously these two studies differ significantly in their approach, with one transplanting just the precursor cells, and the other transplanting the total fat tissue. Moreover, the focus of the precursor cell study was on the proliferative response to HFD feeding, whereas the total fat transplantation study assessed the consequences for metabolism in mice fed normal chow.63,67 Nevertheless, both studies are a powerful reminder of the necessity for in vivo studies.
Regenerative potential of subcutaneous ASCs
The use of mesenchymal stem cells (MSCs) in tissue repair and immune disorder therapy has increased in recent years, with many clinical trials currently taking place.68 Some of these therapies show promise; for instance, one MSC based drug, Prochymal, has already been approved in Canada for the treatment of graft-versus-host disease and is currently in FDA-approved Phase III trials for the treatment of Crohn's disease.69
Historically, the most widely investigated MSCs have been those isolated from the bone marrow (BMSCs). However, like BMSCs, subcutaneous ASCs have the potential to differentiate into many cell types and thus are also being investigated for use in regenerative therapies. Moreover, subcutaneous ASCs are more readily available as they can be isolated after lipoaspiration, a procedure which is both less painful than bone marrow aspiration (which involves extraction from the hip) and more efficient in terms of the quantity of stem cells obtained. Stem cells make up approximately 3% of the adipose tissue SVF in humans, whereas this figure is just 0.002% for the bone marrow.70 Multiple studies have compared the differentiation potential of subcutaneous ASCs with that of BMSCs, in order to assess their suitability for specific therapies. In this section BMSCs refers to the total MSC population as isolated from bone marrow aspirate.
Comparing the differentiation potential of BMSCs and subcutaneous ASCs in a regenerative setting
Much of the work in this area has concentrated on the potential of these cells to differentiate into lineages appropriate for application in a regenerative setting. Although several studies show that subcutaneous ASCs and BMSCs have similar differentiation potentials, some differences are noted. Contrasting results have been obtained from a number of studies that have investigated the ability of the two populations to differentiate into the traditional mesenchymal lineages (chondrocytes, adipocytes, osteoblasts).71 Such disparity in the results could be explained by several factors, including differences in the sex and age of the patients/model animals and variation in the methods used by different groups.
The microenvironment influences the ability of BMSCs and ASCs to differentiate, and in some cases the two cell types respond differently to the same conditions. The osteogenic potential of BMSCs has been investigated to assess their suitability for bone-fracture repair. Platelet-derived growth factor BB (PDGF-BB) is over-secreted in fracture microenvironments and has previously been found to inhibit mineralization in BMSC cultures. Surprisingly, a recent study has shown that PDGF-BB has the opposite effect in subcutaneous ASC cultures, causing them to produce more calcium.72 Changes in gene expression in response to the administration of PDGF-BB were also found to differ between the two cell types, with the subcutaneous ASCs upregulating the expression of osteogenic genes, whilst in the BMSCs these were downregulated.72 Therefore these results suggest that, in combination with PDGF-BB, subcutaneous ASCs may be a more suitable cell type for bone regeneration than BMSCs.
Moreover, subcutaneous ASCs appear to increase their replicative and osteogenic potential when cultured on bone marrow-derived extracellular matrix produced by bone marrow stromal cells.73 Additionally, the two cell types exhibit contrasting differentiation capabilities when cultured on 3D bioactive glass-based scaffolds. Whilst subcutaneous ASCs showed a propensity towards osteo-differentiation even in the absence of osteogenic medium, BMSCs failed to differentiate down the osteogenic lineage in the absence of osteo-inducing medium.74 Conversely, ASCs cultured in the presence of adipose tissue extracellular matrix tend to undergo adipogenesis but also do so when no particular cues are present, suggesting that the intrinsic properties of the cells are important for lineage commitment.75
Some studies have also focused on differentiation into other therapeutically relevant lineages. For example, human subcutaneous ASCs and BMSCs have been compared in terms of their potential to differentiate into pancreatic islet cells, for the purpose of autologous transplants in the treatment of diabetes. In this case, BMSCs showed an increased level of insulin secretion, suggesting that they might be better suited for the treatment of diabetes.76 On the contrary, when compared for their muscle regeneration potential in an in vivo rat abdominal wall reconstruction model, ASCs showed better proliferation capacity, better angiogenic potential, as well as good viability even in hypoxic conditions.77 Differences in the secretory phenotype of the two cell populations have also attracted much attention. For example, subcutaneous ASCs have been found to be more suitable than BMSCs for improving left ventricular function in a mouse myocardial infarction model, through their paracrine secretion and angiogenic potential.78 On the other hand, human BMSCs were found to be better than subcutaneous ASCs at maintaining neuronal function after cerebral ischemia through the secretion of brain-derived neurotrophic factor (BDNF).79 Clearly, these two populations of stem cells differ in their suitability for different therapies, and to a large extent this is likely due to their intrinsic differences. Nevertheless, multiple studies suggest that subcutaneous ASCs offer a promising alternative to BMSCs in a therapeutic setting.
Conclusions
Evidently, WAT depots and the ASCs derived from them are remarkably heterogeneous. Whilst classically the differences between SWAT and VWAT have been studied, there is mounting evidence to show that individual VWAT depots are quite different. As we have described, gene expression profiles differ significantly between the ASCs from different depots, as well as their differentiation capabilities both in vitro and in vivo.19-21,27,28,49-52 Understanding this heterogeneity and its consequences in a physiological setting is an essential step in understanding why different adipose depots respond differently to external cues, such as diet.
Much progress has been made over the past decade in this field, however, it is clear that our knowledge still only touches the surface of the complexities of this heterogeneity. As has been made obvious in this review, there are many studies that contrast in their results regarding differences in proliferation, differentiation and interaction with the microenvironment. It is likely that these differences are at least partially due to disparity in experimental protocols and origin of the tissue. We believe that standardization of protocols, where possible, is necessary if we are to gain an accurate understanding of the true heterogeneity and the consequences of it. Moreover, with recent work highlighting that heterogeneity also exists within the ASC population of a single adipose depot, and that this may have functional consequences for the adipocytes derived from the ASCs, the situation is only getting more complex.12
Another major field in which ASCs have been investigated is in regenerative medicine. SWAT is an attractive source of MSCs, due to their abundance and ease of isolation. Many studies have compared subcutaneous ASCs to BMSCs, which is the traditional source of MSCs for therapeutics. As discussed, there are contrasting results in terms of differentiation potential and therapeutic suitability of ASCs and BMSCs, depending on the required lineage. Factors such as the influence of the microenvironment, and the addition of growth factors affect the behaviors of the two cell types.72-75,80 Here too, results may be affected by differences in the isolation, culture and analysis protocols used by different groups, so standardizing the procedures employed may help in reaching a consensus.
The reasons for further studying and characterizing the different WAT depots, and specifically the ASCs, are thus twofold. Firstly, the properties of various progenitor populations may give insight into the pathological aspects of obesity and obesity-associated diseases. Secondly, the same properties might prove useful in the treatment of other unrelated conditions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank C. Nicol for graphics assistance.
Funding
This work is funded by the Medical Research Council HGU core grant and a University of Edinburgh and British Heart Foundation Fellowship.
References
- [1].Billon N, Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev Reports. 2012;8:55-66. doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- [2].Tang QQ, Lane MD. Adipogenesis : from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715-36. doi: 10.1146/annurev-biochem-052110-115718. PMID:22463691 [DOI] [PubMed] [Google Scholar]
- [3].Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153-64. doi: 10.1074/jbc.M109.053942. PMID:20028987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012;302:E19-31. doi: 10.1152/ajpendo.00249.2011. PMID:21828341 [DOI] [PubMed] [Google Scholar]
- [5].Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al.. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366-76. doi: 10.1016/j.cell.2012.05.016. PMID:22796012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96-105. doi: 10.1172/JCI44271. PMID:21123942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab. 2014;19:8-20. doi: 10.1016/j.cmet.2013.10.003. PMID:24239569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kwok KHM, Lam KSL, Xu A. Heterogeneity of white adipose tissue : molecular basis and clinical implications. Exp Mol Med. 2016;48:e215-12. doi: 10.1038/emm.2016.5. PMID:26964831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016;17:691-702. doi: 10.1038/nrm.2016.96. PMID:27552974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang S, Yang X. Inter-organ regulation of adipose tissue browning. Cell Mol Life Sci. 2017;74:1765-76. doi: 10.1007/s00018-016-2420-x. PMID:27866221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wajchenburg BL. Subcutaneous and visceral adipose tissue : their relation to the metabolic syndrome. Endocr Rev. 2014;21:697-738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- [12].Chau YY, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, et al.. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367-75. doi: 10.1038/ncb2922. PMID:24609269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baer PC. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J Stem Cells. 2014;6:256-65. doi: 10.4252/wjsc.v6.i3.256. PMID:25126376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow adipose tissue: Trimming the fat. Trends Endocrinol Metab. 2016;27:392-403. doi: 10.1016/j.tem.2016.03.016. PMID:27094502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cantile M, Procino A, D'Armiento M, Cindolo L, Cillo C. HOX gene network is involved in the transcriptional regulation of in vivo human adipogenesis. J Cell Physiol. 2003;194:225-36. doi: 10.1002/jcp.10210. PMID:12494461 [DOI] [PubMed] [Google Scholar]
- [16].Linder K, Arner P, Flores-Morales A, Tollet-Egnell P, Norstedt G. Differentially expressed genes in visceral or subcutaneous adipose tissue of obese men and women. J Lipid Res. 2004;45:148-54. doi: 10.1194/jlr.M300256-JLR200. PMID:14563828 [DOI] [PubMed] [Google Scholar]
- [17].Vohl M, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D, Hudson TJ, Sladek ROB. A survey of Genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12:1217-22. doi: 10.1038/oby.2004.153. PMID:15340102 [DOI] [PubMed] [Google Scholar]
- [18].Wu Y, Kim JY, Zhou S, Smas CM. Differential screening identifies transcripts with depot-dependent expression in white adipose tissues. BMC Genomics. 2008;9:397. doi: 10.1186/1471-2164-9-397. PMID:18721461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, Koo A, Stevenson M, Chinnappan D, Cartwright A, et al.. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267-77. doi: 10.1152/ajpendo.00265.2004. PMID:15383371 [DOI] [PubMed] [Google Scholar]
- [20].Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, et al.. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298-307. doi: 10.1152/ajpendo.00202.2006. PMID:16985259 [DOI] [PubMed] [Google Scholar]
- [21].Cartwright MJ, Schlauch K, Lenburg ME, Tchkonia T, Pirtskhalava T, Cartwright A, Thomou T, Kirkland JL. Aging, depot origin, and preadipocyte gene expression. J Gerontol - Ser A Biol Sci Med Sci. 2010;65:242-51. doi: 10.1093/gerona/glp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61:1691-9. doi: 10.2337/db11-1753. PMID:22596050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Perrini S, Ficarella R, Picardi E, Cignarelli A, Barbaro M, Nigro P, Peschechera A, Palumbo O, Carella M, De Fazio M, et al.. Differences in gene expression and cytokine release profiles highlight the heterogeneity of distinct subsets of adipose tissue-derived stem cells in the subcutaneous and visceral adipose tissue in humans. PLoS One. 2013;8:e57892. doi: 10.1371/journal.pone.0057892. PMID:23526958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim B, Lee B, Kyung M, Seung K, Gong P, Hyun N, Hyun P, Seung H, Jae K, No H, et al.. Gene expression profiles of human subcutaneous and visceral adipose ‐ derived stem cells. Cell Biochem Funct. 2016;34:563-71. doi: 10.1002/cbf.3228. PMID:27859461 [DOI] [PubMed] [Google Scholar]
- [25].Meissburger B, Perdikari A, Moest H, Müller S, Geiger M, Wolfrum C. Regulation of adipogenesis by paracrine factors from adipose stromal-vascular fraction - a link to fat depot-specific differences. Biochim Biophys Acta - Mol Cell Biol Lipids. 2016;1861:1121-31. doi: 10.1016/j.bbalip.2016.06.010. [DOI] [PubMed] [Google Scholar]
- [26].Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676-81. doi: 10.1073/pnas.0601752103. PMID:16617105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yamamoto Y, Gesta S, Lee KY, Tran TT, Saadatirad P, Kahn CR. Adipose depots possess unique developmental gene signatures. Obesity (Silver Spring). 2010;18:872-8. doi: 10.1038/oby.2009.512. PMID:20111017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, Deponte M, Stevenson M, Guo W, Han J, et al.. Fat depot origin affects adipogenesis in primary cultured and clone human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286-96. doi: 10.1152/ajpregu.00653.2001. PMID:11959668 [DOI] [PubMed] [Google Scholar]
- [29].Palmer AK, Kirkland JL. Aging and adipose tissue: Potential interventions for diabetes and regenerative medicine. Exp Gerontol. 2016;86:97-105. doi: 10.1016/j.exger.2016.02.013. PMID:26924669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17:644-56. doi: 10.1016/j.cmet.2013.03.008. PMID:23583168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xu XJ, Gauthier M-S, Hess DT, Apovian CM, Cacicedo JM, Gokce N, Farb M, Valentine RJ, Ruderman NB. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res. 2012;53:792-801. doi: 10.1194/jlr.P022905. PMID:22323564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lysaght J, van der Stok EP, Allott EH, Casey R, Donohoe CL, Howard JM, McGarrigle SA, Ravi N, Reynolds JV, Pidgeon GP. Pro-inflammatory and tumour proliferative properties of excess visceral adipose tissue. Cancer Lett. 2011;312:62-72. doi: 10.1016/j.canlet.2011.07.034. PMID:21890265 [DOI] [PubMed] [Google Scholar]
- [33].Schlich R, Willems M, Greulich S, Ruppe F, Knoefel WT, Ouwens DM, Maxhera B, Lichtenberg A, Eckel J, Sell H. VEGF in the crosstalk between human adipocytes and smooth muscle cells: Depot-specific release from visceral and perivascular adipose tissue. Mediators Inflamm. 2013;2013:982458. doi: 10.1155/2013/982458. PMID:23935253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, Angeli R, Gelmini S, Guasti D, Benvenuti S, Annunziato F, et al.. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 2009;23:3494-505. doi: 10.1096/fj.08-126946. PMID:19584303 [DOI] [PubMed] [Google Scholar]
- [35].Hutley LJ, Newell FM, Joyner JM, Suchting SJ, Herington AC, Cameron DP, Prins JB. Effects of rosiglitazone and linoleic acid on human preadipocyte differentiation. Eur J Clin Invest. 2003;33:574-81. doi: 10.1046/j.1365-2362.2003.01178.x. PMID:12814394 [DOI] [PubMed] [Google Scholar]
- [36].Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, Von Zglinicki T, et al.. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571-8. doi: 10.2337/db06-0540. PMID:16936206 [DOI] [PubMed] [Google Scholar]
- [37].Toyoda M, Matsubara Y, Lin K, Sugimachi K, Furue M. Characterization and comparison of adipose tissue-derived cells from human subcutaneous and omental adipose tissues. Cell Biochem Funct. 2009;27:440-7. doi: 10.1002/cbf.1591. PMID:19691084 [DOI] [PubMed] [Google Scholar]
- [38].Church CD, Berry R, Rodeheffer MS. Isolation and study of adipocyte precursors In: Macdougald OA, editor Methods in Enzymology. San Diego, CA: Elsevier Inc.; 2014. p:31-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, et al.. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376-85. doi: 10.1634/stemcells.2005-0234. PMID:16322640 [DOI] [PubMed] [Google Scholar]
- [40].Ailhaud G, Dani C, Amri EZ, Djian P, Vannier C, Doglio A, Forest C, Gaillard D, Negrel R, Grimaldi P. Coupling growth arrest and adipocyte differentiation. Environ Health Perspect. 1989;80:17-23. doi: 10.1289/ehp.898017. PMID:2647477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Entenmann G, Hauner H. Relationship between replication and differentiation in cultured human adipocyte precursor cells. Am J Physiol. 1996;270:C1011-6. PMID:8928727 [DOI] [PubMed] [Google Scholar]
- [42].Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979;254:273-5. PMID:762059 [PubMed] [Google Scholar]
- [43].Bell A, Grunder L, Sorisky A. Rapamycin inhibits human adipocyte differentiation in primary culture. Obes Res. 2000;8:249-54. doi: 10.1038/oby.2000.29. PMID:10832768 [DOI] [PubMed] [Google Scholar]
- [44].Shahparaki A, Grunder L, Sorisky A. Comparison of human abdominal subcutaneous versus omental preadipocyte differentiation in primary culture. Metabolism. 2002;51:1211-5. doi: 10.1053/meta.2002.34037. PMID:12200769 [DOI] [PubMed] [Google Scholar]
- [45].Van Harmelen V, Röhrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism. 2004;53:632-7. doi: 10.1016/j.metabol.2003.11.012. PMID:15131769 [DOI] [PubMed] [Google Scholar]
- [46].Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302-8. doi: 10.1038/ncb2696. PMID:23434825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240-9. doi: 10.1016/j.cell.2008.09.036. PMID:18835024 [DOI] [PubMed] [Google Scholar]
- [48].Joe AWB, Lin Y, Even Y, Vogl AW, Rossi FM V. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563-70. doi: 10.1002/stem.190. PMID:19658193 [DOI] [PubMed] [Google Scholar]
- [49].Djian P, Roncari DAK, Hollenberg CH. Influence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in culture. J Clin Invest. 1983;72:1200-8. doi: 10.1172/JCI111075. PMID:6630508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Djian P, Roncari DAK, Hollenberg CH. Adipocyte precursor clones vary in capacity for differentiation. Metabolism. 1985;34:880-3. doi: 10.1016/0026-0495(85)90114-3. PMID:4033429 [DOI] [PubMed] [Google Scholar]
- [51].Wang H, Kirkland JL, Hollenberg CH. Varying capacities for replication of rat adipocyte precursor clones and adipose tissue growth. J Clin Invest. 1989;83:1741-6. doi: 10.1172/JCI114075. PMID:2708530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kirkland JL, Hollenberg CH, Gillon WS. Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am J Physiol. 1990;258:C206-10. PMID:2305864 [DOI] [PubMed] [Google Scholar]
- [53].Ahrens M, Ankenbauer T, Schröder D, Hollnagel A, Mayer H, Gross G. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12:871-80. PMID:8274220 [DOI] [PubMed] [Google Scholar]
- [54].Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle. 2007;6:385-9. doi: 10.4161/cc.6.4.3804. PMID:17314508 [DOI] [PubMed] [Google Scholar]
- [55].Schulz TJ, Tseng Y. Emerging role of bone morphogenetic proteins in angiogenesis and energy metabolism. Cytokine Growth Factor Rev. 2009;20:523-31. doi: 10.1016/j.cytogfr.2009.10.019. PMID:19896888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Grandl G, Müller S, Moest H, Moser C, Wollscheid B, Wolfrum C. Depot specific differences in the adipogenic potential of precursors are mediated by collagenous extracellular matrix and Flotillin 2 dependent signaling. Mol Metab. 2016;5:937-47. doi: 10.1016/j.molmet.2016.07.008. PMID:27689006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Faust IM, Johnson PR, Stern JS, Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978;235:E279-86. PMID:696822 [DOI] [PubMed] [Google Scholar]
- [58].Lemonnier D. Effect of age, sex, and sites on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J Clin Invest. 1972;51:2907-15. doi: 10.1172/JCI107115. PMID:5080416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, et al.. Dynamics of fat cell turnover in humans. Nature. 2008;453:783-7. doi: 10.1038/nature06902. PMID:18454136 [DOI] [PubMed] [Google Scholar]
- [60].Arner P, Andersson DP, Thörne A, Wirén M, Hoffstedt J, Näslund E, Thorell A, Rydén M. Variations in the size of the major omentum are primarily determined by fat cell number. J Clin Endocrinol Metab. 2013;98:897-901. doi: 10.1210/jc.2012-4106. [DOI] [PubMed] [Google Scholar]
- [61].Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338-44. doi: 10.1038/nm.3324. PMID:23995282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17:376-85. doi: 10.1038/ncb3122. PMID:25730471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Peña R, Church CD, Colman L, Berry R, Rodeheffer MS. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 2016;24:1-9. doi: 10.1016/j.cmet.2016.05.012. PMID:27411001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242-56. doi: 10.1016/j.cell.2007.10.004. PMID:17956727 [DOI] [PubMed] [Google Scholar]
- [65].Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. PMID:22651247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107:18226-31. doi: 10.1073/pnas.1005259107. PMID:20921416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410-20. doi: 10.1016/j.cmet.2008.04.004. PMID:18460332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wei X, Yang X, Han Z, Qu F, Shao L, Shi Y. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747-54. doi: 10.1038/aps.2013.50. PMID:23736003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mannon PJ. Remestemcel-L: human mesenchymal stem cells as an emerging therapy for Crohn's disease. Expert Opin Biol Ther. 2011;11:1249-56. doi: 10.1517/14712598.2011.602967. PMID:21787241 [DOI] [PubMed] [Google Scholar]
- [70].Fraser JK, Zhu M, Wulur I, Alfonso Z. Adipose-derived stem cells In: Prockop DJ, Bunnell BA, Phinney DG, editors. Mesenchymal Stem Cells: Methods and Protocols. Totowa, NJ: Humana Press; 2008. p:59-67. [DOI] [PubMed] [Google Scholar]
- [71].Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724-52. doi: 10.1089/scd.2011.0722. PMID:22468918 [DOI] [PubMed] [Google Scholar]
- [72].Hung BP, Hutton DL, Kozielski KL, Bishop CJ, Naved B, Green JJ, Caplan AI, Gimble JM, Dorafshar AH, Grayson WL. Platelet-derived growth factor BB enhances osteogenesis of adipose-derived but not bone marrow-derived mesenchymal stromal/stem cells. Stem Cells. 2015;33:2773-84. doi: 10.1002/stem.2060. PMID:26013357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhang Z, Luo X, Xu H, Wang L, Jin X, Chen R, Ren X, Lu Y, Fu M, Huang Y, et al.. Bone marrow stromal cell-derived extracellular matrix promotes osteogenesis of adipose-derived stem cells. Cell Biol Int. 2015;39:291-9. doi: 10.1002/cbin.10385. PMID:25264269 [DOI] [PubMed] [Google Scholar]
- [74].Rath SN, Nooeaid P, Arkudas A, Beier JP, Strobel LA, Brandl A, Roether JA, Horch RE, Boccaccini AR, Kneser U. Adipose- and bone marrow-derived mesenchymal stem cells display different osteogenic differentiation patterns in 3D bioactive glass-based scaffolds. J Tissue Eng Regen Med. 2016;10:E497-509. doi: 10.1002/term.1849. PMID:24357645 [DOI] [PubMed] [Google Scholar]
- [75].Guneta V, Tan NS, Chan SKJ, Tanavde V, Lim TC, Wong TCM, Choong C. Comparative study of adipose-derived stem cells and bone marrow-derived stem cells in similar microenvironmental conditions. Exp Cell Res. 2016;348:155-64. doi: 10.1016/j.yexcr.2016.09.012. PMID:27658569 [DOI] [PubMed] [Google Scholar]
- [76].Marappagounder D, Somasundaram I, Dorairaj S, Sankaran RJ. Differentiation of mesenchymal stem cells derived from human bone marrow and subcutaneous adipose tissue into pancreatic islet-like clusters in vitro. Cell Mol Biol Lett. 2013;18:75-88. doi: 10.2478/s11658-012-0040-5. PMID:23271432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].van Steenberghe M, Schubert T, Guiot Y, Goebbels RM, Gianello P. Improvement of mesh recolonization in abdominal wall reconstruction with adipose vs. bone marrow mesenchymal stem cells in a rodent model. J Pediatr Surg. 2017;52:1355-62. [DOI] [PubMed] [Google Scholar]
- [78].Rasmussen JG, Frøbert O, Holst-hansen C, Kastrup J, Baandrup U, Zachar V, Fink T, Simonsen U. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transplant. 2014;23:195-206. doi: 10.3727/096368912X659871. PMID:23211469 [DOI] [PubMed] [Google Scholar]
- [79].Razavi S, Razavi MR, Zarkesh Esfahani H, Kazemi M, Mostafavi FS. Comparing brain-derived neurotrophic factor and ciliary neurotrophic factor secretion of induced neurotrophic factor secreting cells from human adipose and bone marrow-derived stem cells. Dev Growth Differ. 2013;55:648-55. doi: 10.1111/dgd.12072. PMID:23944834 [DOI] [PubMed] [Google Scholar]
- [80].Li C, Wu X, Tong J, Yang X, Zhao J, Zheng Q, Zhao G, Ma Z. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6:55. doi: 10.1186/s13287-015-0066-5. PMID:25884704 [DOI] [PMC free article] [PubMed] [Google Scholar]


