ABSTRACT
Emerging evidence has demonstrated that microRNAs (miRNA) play a critical role in chemotherapy-induced epithelial-mesenchymal transition (EMT) in glioma. However, the underlying mechanism of chemotherapy-triggered EMT has not been fully understood. In the current study, we determined the role of miR-26b in regulation of EMT in stable temozolomide (TMZ)-resistant (TR) glioma cells, which have displayed mesenchymal features. Our results illustrated that miR-26b was significantly downregulated in TR cells. Moreover, ectopic expression of miR-26b by its mimics reversed the phenotype of EMT in TR cells. Furthermore, we found that miR-26b governed TR-mediate EMT partly due to governing its target Wee1. Notably, overexpression of miR-26b sensitized TR cells to TMZ. These findings suggest that upregulation of miR-26b or targeting Wee1 could serve as novel approaches to reverse chemotherapy resistance in glioma.
KEYWORDS: Glioma, growth, miR-26b, temozolomide, Wee1
Introduction
Glioblastoma (GBM) is the most prevalent and lethal primary intrinsic brain tumor among elderly patients with a poor prognosis.1 GBM often invades the surrounding brain but rarely metastasizes to other organs.2 Several chemotherapeutic agents have been reported to have modest efficacy in the treatment of high-grade glioma (HGG). However, blood-brain barrier impermeability leads to a major delivery obstacle.3 The current therapeutic strategy is maximal safe surgical resection followed by radiation therapy (RT) with concomitant temozolomide (TMZ). It has been reported that postoperative use of temozolomide concurrently and after radiotherapy improves the overall survival. Moreover, this treatment has become the current standard regimen for the treatment of glioma due to the advantage of wide applicability and minimal additional toxicity. However, it is unclear about acquired TMZ resistance, which is a serious impediment in the treatment of GBM.4 Thus, it is urgent to explore the molecular mechanism of chemotherapeutic drug resistance and find a novel strategy for enabling better therapeutic benefits of glioma patients.
Epithelial-mesenchymal transition (EMT) is a process in which epithelial cells lose their polarity and cell-cell adhesion signatures, and acquire the characteristics of mesenchymal cells. This process often includes spindle-cell shape, loss of polarity, intercellular separation, and pseudopodia formation.5 Specifically, during EMT process, the cobblestone appearance of epithelial cells will change to a spindle-like shape. In addition, epithelial biomarkers (such as E-cadherin) are lost, whereas mesenchymal markers (such as N-cadherin, vimentin, Slug and Snail) are acquired. EMT has been identified to be involved in metastasis and chemoresistance in human cancer cells.6 For example, inhibition of HR3 (histamine receptor 3) suppressed glioblastoma tumor growth, invasion, and EMT.7 High expression of RAB43 (Ras-related GTP-binding protein 43) predicted poor prognosis and was associated with EMT in gliomas.8 Additionally, KITENIN (KAI1 COOH-terminal interacting tetraspanin) enhanced glioma invasiveness and progression, associated with the induction of EMT and stemness markers.9 Consistently, drug resistant cancer cells often obtain the EMT features.
Recently, miRNAs have been implicated to regulate drug resistance. Drug resistance is a burden for cancer therapy and patients' outcome, which leads to more aggressive tumors and metastasize to distant organs.10 A number of studies validate the importance of miRNAs in drug metabolism via the regulation of drug-metabolizing enzymes, drug transporters, transcription factor or nuclear receptors.11 Moreover, miR-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R (insulin like growth factor 1 receptor) to regulate RAS/RAF/ERK (extracellular regulated kinase) signaling pathways.12 Furthermore, miR-634 restores drug sensitivity in resistant ovarian cancer cells by targeting the Ras-MAPK (mitogen activated protein kinase) pathway.13 These reports unraveled the crucial roles of miRNAs in drug resistance-mediated EMT.
Emerging evidences suggests that several miRNAs regulated TMZ resistance in human cancer cells. For example, miR-138 overexpression increased TMZ resistance in long-term glioblastoma cell lines and glioma initiating cell cultures.14 In the present study, we explored the role of miR-26b in controlling TR-mediated EMT in glioma cells. We further determine whether Wee1, one of miR-26b targets, was involved in TR-induced EMT. We identified that miR-26b was downregulated in TR cells and overexpression of miR-26b reversed the mesenchymal features in glioma cells. Notably, ectopic expression of miR-26b sensitized TR cells to TMZ. Our results revealed that re-expression of miR-26b could be potential therapeutic approaches for treating TR glioma.
Results
Creation of TR glioma cell lines
We created the TR glioma cells to explore the specific mechanism of drug resistance in glioma. SNB19 and T98G cells were exposed to increasing concentrations of TMZ for more than 6 months. As shown in Fig. 1A, 100 μM TMZ led to ∼45% and 60% cell growth inhibition in SNB19 and T98G cells, respectively. However, TR cells exhibited resistance to the growth inhibitory properties of 100 μM TMZ (Fig. 1A). The resistant cells were continuously maintained in DMEM medium containing 100 μM TMZ for the following study.
Figure 1.
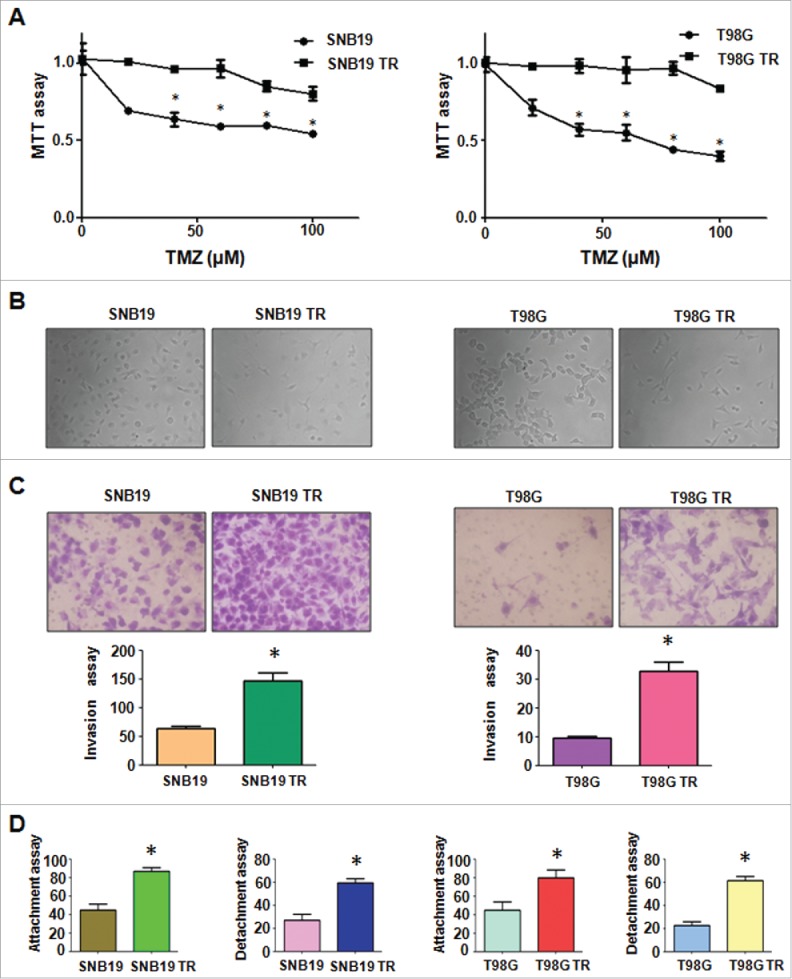
TR cells exhibited EMT phenotype. (A) MTT assay was conducted in parental and TR glioma cells. * P < 0.05 vs their parental cells. (B) Cell morphology was observed by microscope in parental and TR glioma cells. (C) Invasion assay was performed to measure the invasive capacity in parental and TR glioma cells. * P< 0.05 vs their parental cells. (D) Cell attachment and detachment assays were assessed in parental and TR glioma cells. * P < 0.05 vs their parental cells.
TR cells acquire EMT feature
It has been reported that drug-resistant cells exhibited EMT. In line with this, we found that SNB19 TR and T98G TR cells displayed the markedly morphologic changes, which were similar as EMT phenotype. Both SNB19 TR and T98G TR cells became longer and more fibrotic than SNB19 and T98G cells (Fig. 1B). Induction of EMT was correlated with aggressive characteristics including cell attachment, detachment, migration, and invasion. In keeping with this notion, we observed that TR cells exhibited significantly increased numbers of invaded cells through a Matrigel-coted membrane compared with their parental cells (Fig. 1C). Moreover, TR cells displayed enhanced capacity of attachment and detachment compared with their parental cells (Fig. 1D). Furthermore, our wound healing assay demonstrated that TR cells obtained enhanced motility activity (Fig. 2A). Taken together, our data suggest that TR cells were associated with EMT characteristics.
Figure 2.
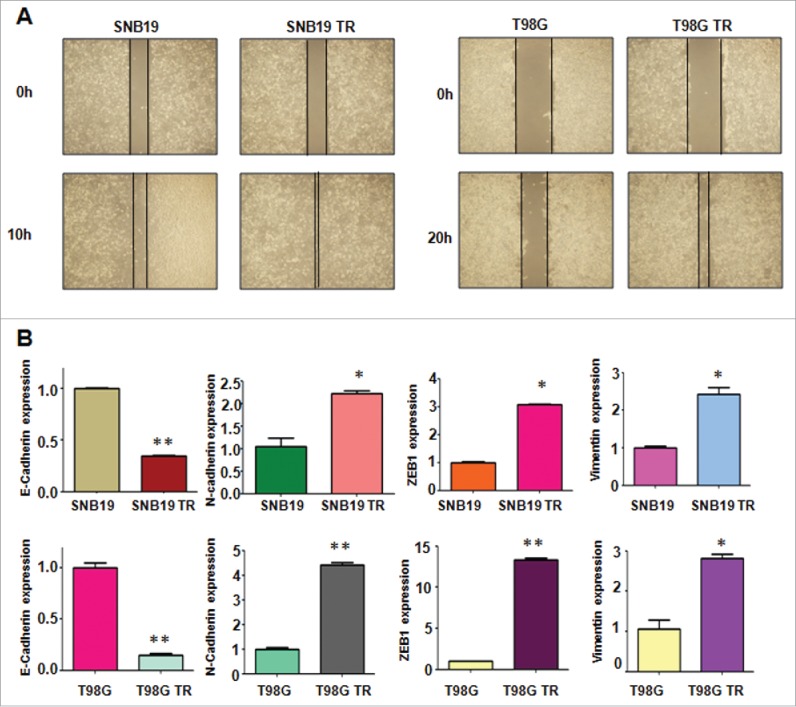
TR cells have enhanced motility activity. (A) Wound assays were performed to measure the motility activity in parental and TR glioma cells. (B) Real-time RT-PCR assay was conducted to detect the expression of EMT markers in parental and TR glioma cells. * P < 0.05; ** P < 0.01 vs their parental cells.
TR cells have EMT molecular marker changes
To explore whether TR cells have EMT molecular marker changes, we measured the expression of EMT markers in paired parental and resistant cell lines by RT-PCR and Western blotting analysis, respectively. In accordance with their EMT phenotype changes, we found that epithelial molecule E-cadherin was significantly decreased in TR cells, whereas the expression of mesenchymal markers such as Vimentin, ZEB1, N-cadherin, Nestin and CD133 was highly elevated in TR cells (Fig. 2B, Fig. 3A and B). These results validated that TR cells acquired a mesenchymal phenotype, which could be involved in TMZ resistance in glioma.
Figure 3.
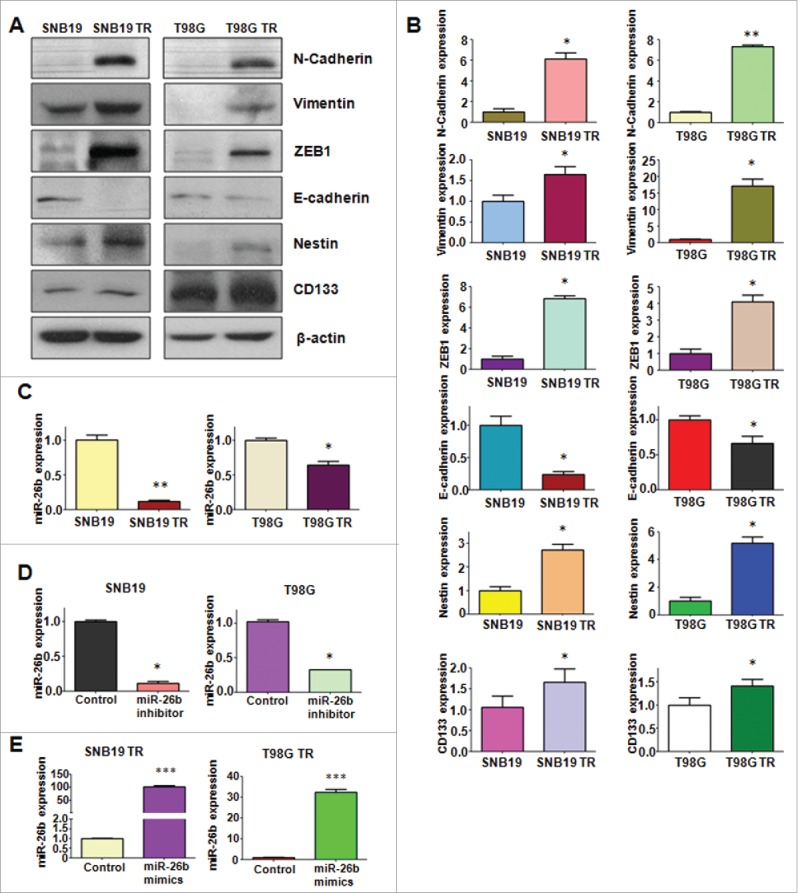
TR cells have EMT marker changes. (A) Western blotting analysis was used to detect the expression of E-cadherin, vimentin, ZEB1, N-cadherin, and Cancer Stem Cell marker, such as CD133 and Nestin in parental and TR glioma cells. (B) Quantitative results are illustrated for panel A. * P < 0.05; ** P < 0.01 vs their parental cells. (C) Real-time RT-PCR assay was conducted to detect the expression of miR-26b in parental and TR cells. * P < 0.05; ** P < 0.01 vs their parental cells. (D-E) Real-time RT-PCR was performed to detect the efficacy of miR-26b inhibitor and mimics transfection. * P < 0.05; *** P < 0.001 vs Control.
Down-regulation of miR-26b is observed in TR cells
It has been reported that miR-26b is critically involved in regulating EMT in human cancer.15,16 To dissect whether miR-26b plays a pivotal role in TR-induced EMT in glioma cells, we detected the expression of miR-26b in TR cells and there parental cells. Our results demonstrated that miR-26b was significantly downregulated in TR cells (Fig. 3C). This finding indicated that downregulation of miR-26b could contribute to TR-induced EMT.
Depletion of miR-26b promotes cells motility and invasion in TR cells
To determine the function of miR-26b in regulation of EMT, miR-26b mimics or miR-26b inhibitor were transfected into the TR cells. The efficiency of these transfections was detected by using Q-PCR. We found that miR-26b inhibitors decreased the miR-26b level, whereas its mimics increased the miR-26b expression (Fig. 3D and E). Notably, parental cells with miR-26b inhibitor exhibited fiber cell-like morphology (Figs. 4A and 5A). To further validate the role of miR-26b in parental glioma cells, we detected the cell motility and invasion capacities in parental cells after miR-26b inhibitor transfection. Our results showed that depletion of miR-26b could promote glioma parental cell migration and invasion (Figs. 4A and 5B).
Figure 4.
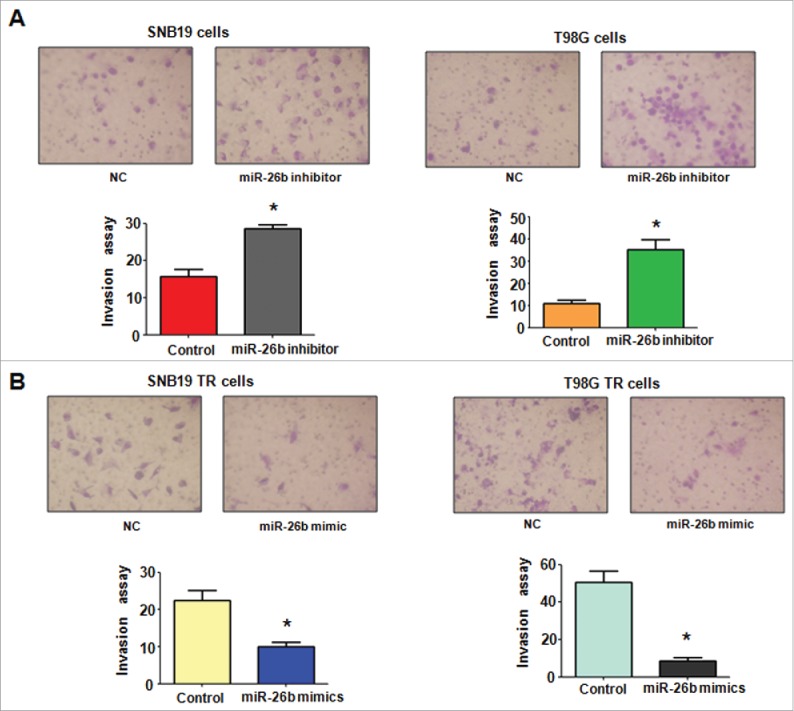
miR-26b mimics inhibited cell invasion in TR glioma cells. (A) Top panel: Invasion assays were conducted in glioma cells transfected with miR-26b inhibitor. Bottom panel: Quantitative results are illustrated for top panel. * P < 0.05 vs Control. (B) Top panel: Invasion assays were performed in TR glioma cells transfected with miR-26b mimics. Bottom panel: Quantitative results are illustrated for top panel. * P < N0.05 vs Control.
Figure 5.
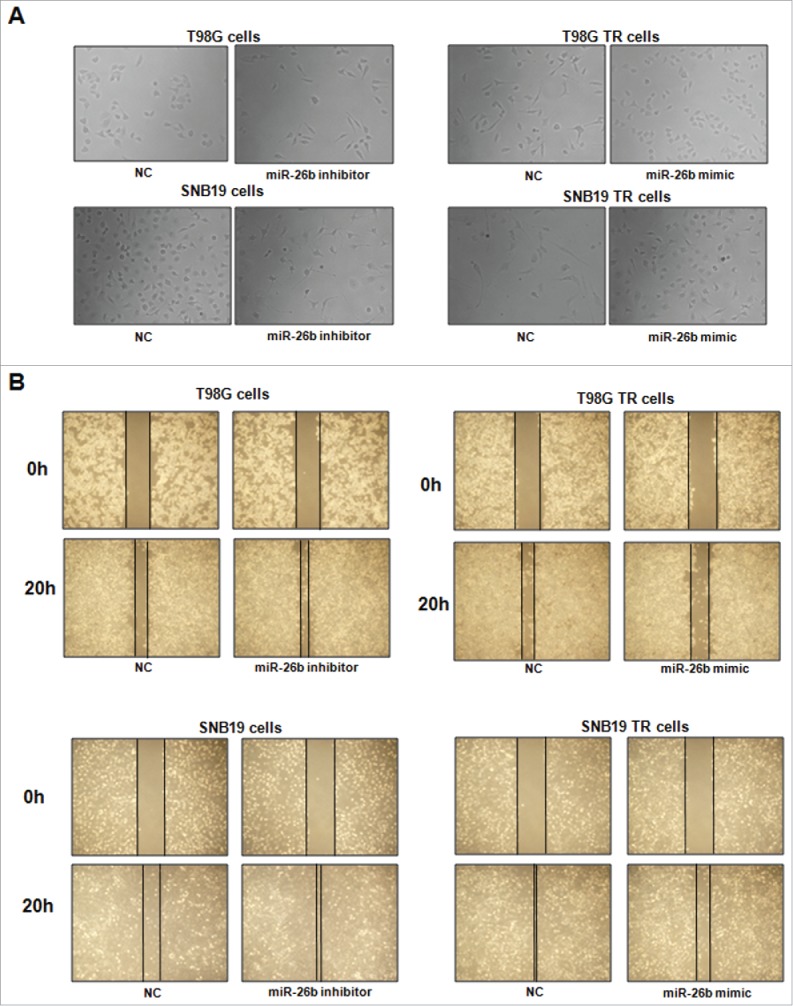
miR-26b inhibitor enhanced motility and invasion in glioma cells.(A) Cell morphology was taken by microscopy in parental cells treated with miR-26b inhibitor or miR-26b mimics. (B) Wound healing assays were used to detect the motility in SNB19 and T98G cells transfected with miR-26b inhibitor or miR-26b mimics.
Overexpression of miR-26b inhibits cells motility in TR cells
The TR cells after miR-26b transfection were photographed under inverted microscope. We observed that TR cells with miR-26b mimics exhibited round cell-like morphology (Fig. 5A). Moreover, we detected the cell migration and invasion in TR cells after miR-26b mimics treatment by wound healing assay and invasion assay, respectively. Our results showed that miR-26b mimic inhibited the cell migration both in SNB19 TR and T98G TR cells (Fig. 5B). Our results indicate that miR-26b could play a critical role in governing TR-mediate EMT.
Overexpression of miR-26b reverses EMT markers in TR cells
To further determine whether ectopic expression of miR-26b could reverse the mesenchymal characteristics in TR cells, we measured the expression of EMT markers in TR cells by Western blotting assay. We found that miR-26b transfection caused the higher expression of E-cadherin and lower expression of mesenchymal markers including Vimentin, ZEB1, N-cadherin and CD133. Our results revealed that reduction of miR-26b could be responsible for TR-induced EMT in glioma(Fig. 6A–B).
Figure 6.
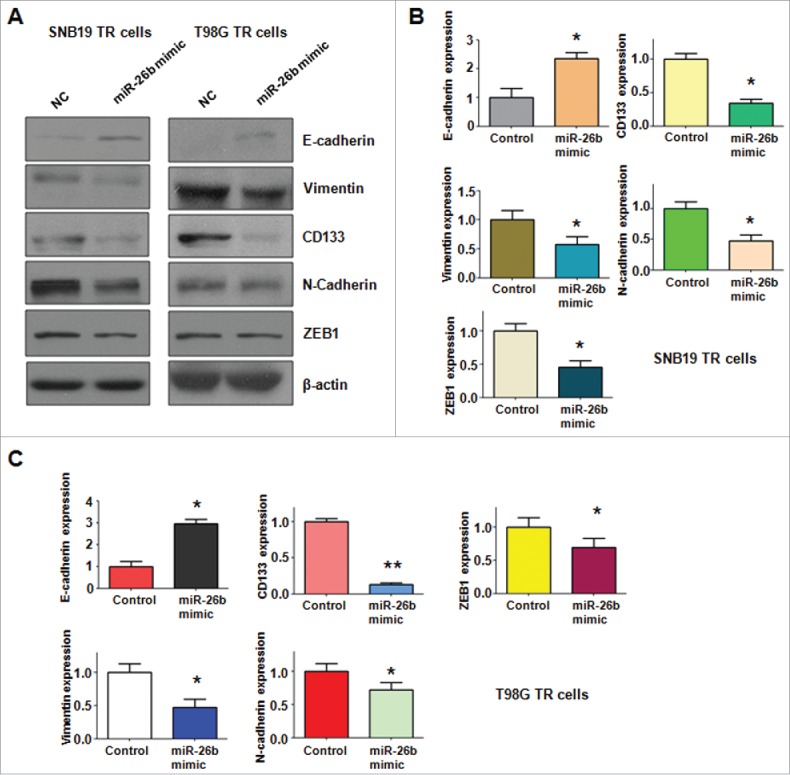
miR-26b mimic regulated the expression of EMT markers in TR glioma cells. (A) Western blotting analysis was performed to detect the expression of EMT markers in TR glioma cells. (B-C) Quantitative results are illustrated for panel A. * P < 0.05; ** P < 0.01 vs Control.
miR-26b regulates EMT by targeting Wee1 in TR cells
It has been well known that miRNA exerts its function via binding to 3′-URT (untranslated region) of target genes through partial sequence homology. Therefore, to further investigate the role of miR-26b in controlling TR-mediated EMT, we used 2 prediction programs. TargetScan and miRanda, to screen the potential targets of miR-26b. Our analysis predicted that Wee1 could be a potential miR-26b target (Fig. 7A). To confirm this notion, we measured the expression of Wee1 using Western blotting assay in both SNB19 TR and T98G TR cells (Fig. 7B). We observed that miR-26b mimic transfection could down-regulate the expression of Wee1, whereas miR-26b inhibitor transfection could up-regulate the expression of Wee1 (Fig. 7C). Finally, we measured the effect of miR-26b on the cell viability by MTT assay. Our MTT results showed that the miR-26b mimic inhibited TR cell growth. On the other hand, miR-26b inhibitor promoted the parental glioma cell growth (Fig. 7D).
Figure 7.
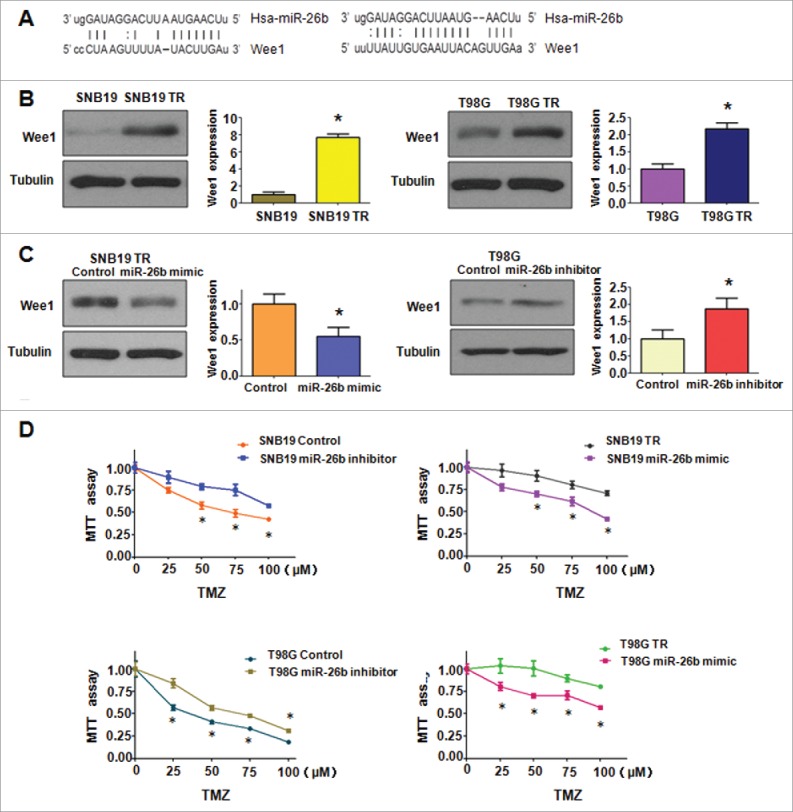
miR-26b targeted Wee1 expression. (A) Sequences of target sites for miR-26b in Wee1 are shown. (B) Western blotting analysis was conducted to measure the expression of Wee1 in TR and their parental cells. (C) Western blotting analysis was performed to detect the expression of Wee1 in cells treated with miR-26b inhibitor or mimics. (D) The effect of miR-26b inhibitor and mimics on the cell viability by MTT assay. * P < 0.05 vs Control.
Discussion
TMZ is often used for the treatment of glioblastoma patients. However, therapeutic benefits of TMZ were compromised due to drug resistance. Several genes and signaling pathways have been characterized to regulate the TMZ resistance. For example, the ZEB1 pathway has been found to link glioblastoma chemoresistnace of TMZ.17 ZEB1 knockdown significantly increased TMZ sensitivity in vitro and survival time of shZEB1 animals.17 Additionally, RRAD (RAS associated with diabetes) promoted EGFR (epidermal growth factor receptor)-mediated STAT3 (signal transducer and activator of transcription 3) activation and induced TMZ resistance of malignant glioblastoma.18 Moreover, BMP7 (bone morphogenetic protein 7) sensitized glioblastoma stem cells to clinically relevant dose of TMZ.19 Furthermore, SRPX2 (sushi repeat-containing protein, X-linked 2) enhanced the EMT and TMZ resistance via MAPK pathway in glioblastoma cells.20 Yu et al. found that SPOCK1 (SPARC/osteonectin, cwcv and kazal-like domains proteoglycan 1) mediated TMZ resistance via the Akt signaling pathway in glioblastoma multiforma.21 A small molecule inhibitor of STAT3 dimerization, STX-0119, was reported to overcome TMZ resistance in recurrent glioma tumor.18 These reports decoded the molecular mechanism of TMZ resistance in glioma cells. In the current study, we found that miR-26b governed the TMZ resistance in glioma cells.
Emerging evidence demonstrated that miR-26b was critically involved in tumorigenesis including glioma. One study has shown that the miR-26b expression level was downregulated in human glioma samples, which was negatively correlated with the increased malignancy of glioma.22 Moreover, miR-26a/b has been shown to block the G1/S transition of the cell cycle by targeting cyclin D2, Cyclin E1/2, CDK6 (cyclin dependent kinase 6), and EZH2 (enhancer of zeste 2 polycomb repressive complex 2 subunit) in hepatocellular carcinoma (HCC) and nasopharyngeal carcinoma.23–25 Notably, miR-26a/b induced cell apoptosis by targeting metadherin (MTDH), EZH2 and SLC7A11 (solute carrier family 7 member 11) in breast cancer cells and targeting SODD (silencer of death domain) in melanoma cells.26–28 Down-regulation of miR-26b predicted poor disease-free survival and overall survival in epithelial ovarian carcinoma patients.29 KPNA2 (karyopherin subunit α 2) was validated as a direct target of miR-26b. Knockdown of KPNA2 or ectopic expression of miR-26b could downregulate OCT4 (organic cation/carnitine transporter 4), vimentin and upregulate E-cadherin.29 Importantly, miR-26b inhibits cell proliferation, migration, and invasion in most of human cancers, such as hepatocellular, non-small cell lung cancer osteosarcoma and tongue squamous.30–33 Our study identified that miR-26b exerts its biologic function via targeting Wee1 in glioma cells.
Increasing evidence has suggested that miRNAs are involved in EMT process in human cancers. Zhang et al. reported that miR-361–5p inhibited EMT through targeting Twist1 in glioma cells.34 Moreover, miR-203 downregulation is responsible for chemoresistance through EMT via Snail2 in human GBM cells.35 Consistently, miR-200b-3p inhibited EMT and tumor growth via downregulation of ERK5 in glioma.36 One study showed that miR-26b dramatically suppressed Twist1-induced EMT and the invasion ability in hepatocellular carcinoma cells via inhibition of SMAD1 (mothers against decapentaplegic homolog 1) expression.15 Moreover, miR-26b was reported to inhibit the proliferation, migration, and even the epithelial-mesenchymal transition of lens epithelial cells.37 Similarly, it has been reported that miR-26b inhibited EMT by targeting USP9X (ubiquitin specific peptidase 9, X-linked) in hepatocellular carcinoma, which in turn affects EMT through Smad4 and the TGF-β (transforming growth factor-β) signaling pathway.16 In line with these reports, our study demonstrated that miR-26b suppressed TMZ-resistant induced EMT in glioma cells. These findings revealed that miR-26b is important for governing EMT in human cancer cells. Multiple studies have validated that miR-26b controlled drug resistance in human cancers. Zhao et al found that miR-26b suppressed the NF-κB (nuclear factor-kappa B) signaling and enhanced the chemosensitivity via targeting TAK1 (TGF-β activated kinase 1) and TAB3 (TGF-β activated kinase-binding protein 3) in hepatocellular carcinoma cells.38 Interestingly, downregulation of miR-26b modulated chemoresistance and migration via the association of PTEN (phosphatase and tensin homolog) in non-small cell lung cancer cells.31 Jin et al reported that miR-26 enhanced chemosensitivity and promoted apoptosis through inhibition of autophagy in hepatocellular carcinoma cells.39 In keeping with this, our results showed that overexpression of miR-26b enhanced TR cells to TMZ treatment.
Wee1 kinase has been described as a major gate keeper at the G2 cell cycle checkpoint and to be involved in tumor progression in different malignant tumors.40 It has been demonstrated that Wee1 played a crucial role in regulating EMT and drug resistance. Wee1 inhibition alleviates resistance to immune episode of tumor cells undergoing EMT.41 Moreover, Wee1 negatively regulated the multidrug resistance potential of human hepatic cancer cells by modulating the expression of relevant drug resistance genes and the activity of the MEK/ERK pathway.42 AZD1775, one of Wee1 inhibitors, has been reported to maintain synergy with cisplatin and show reduced single agent cytotoxicity in medulloblastoma cells.43 In addition, the combination of AZD1775 and platinum agents was potentiated by PAXIP1 (PAX interacting protein 1) in lung cancer.44 Since Wee1 is one of miR-26b targets, targeting miR-26b could be a novel strategy for targeting human glioma cancer. In conclusion, activation of miR-26b or inactivation of Wee1 may be a useful strategy to reverse chemotherapy resistance in glioma.
Materials and methods
Cell culture
Human glioma cancer cell lines SNB19, SNB19 TR, T98G and T98G TR were cultured in 5% CO2 at 37°C in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum. The resistant cells were maintained in culture medium with 50 μM TMZ.
Reagents and antibodies
MTT3-[(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide] was purchased from Sigma (St. Louis, Mo). Tumor Invasion Assay Kit was brought from BD Biosciences (Bedford, MA, USA). The primary antibodies against N-cadherin, Vimentin, ZEB1, E-cadherin, Nestin, CD133, β-actin, Tubulin and the second antibodies were purchased from Cell Signaling Technology.
MTT assay
Cells were seeded in 6 replicates in 96-well plates at a density of 6 × 103 cells per well for overnight and treated with different concentrations of TMZ for 72 hours. Then the cells treated with 10 μl of the MTT (5 mg/ml) solution and incubated at 37°C. After 4 hours, the supernatant was absorbed and 100 μl DMSO was added to dissolve the MTT-formazan crystals. The absorption was measured by the microplate at 490 nm.
Wound healing assay
The cells were seeded in 6-well plate until the cells grew to 90–95% confluency. The scratch wound was generated in the surface of the plates with a 20 μl pipette tip. The scratch area was photographed by a microscope at 0 hour and 20 hours.
Cell attachment and detachment assay
Cell attachment and detachment assays were performed as described previously.45 Briefly, for attachment assay, 5 × 104 cells per well were seeded in 24-well plates. After 1 h, unattached cells were removed. The attached cells were counted after trypsinization. The data were presented as a percentage of the attached cells compared with total cells. For cell detachment assay, the cells were incubated with 0.05% trypsin for 3 minutes to detach the cells after 24 hours incubation. Then, the detached cells were collected. The remaining cells were counted after detached with 0.25% trypsin. The data were presented as a percentage of the detached cells to total cells.
Transwell invasion assays
The invasive capacity of cells was performed using Transwell inserts with Matrigel (BD Biosciences). The cells were seeded in a Matrigel-coated chamber. The upper chamber has serum-free media, whereas bottom chamber has complete growth media. After 16 hours of incubation, the upper surfaces of the Transwell chambers were scraped with cotton swabs, and the invaded cells were fixed and stained with Giemsa solution. The stained cells were photographed under a light microscope.46
RNA extraction and real time RT-PCR (RT-qPCR)
The total RNA from cells was isolated with Trizol (Invitrogen, Carlsbad, CA) and reversed-transcribed into cDNA by RevertAid First Strand cDNA Synthesis Kit. PCR were performed using Power SYBR Green PCR Master Mix and the results were calculated by 2-lrtri; lrtri;Ct method). The primers used in the PCR reaction are GAPDH, forward primer 5′- ACC CAG AAG ACT GTG GAT GG -3′; and reverse primer 5′- CAG TGA GCT TCC CGT TCA G- 3′; E-cadherin, forward primer 5′-GAA GTG TCC GAG GAC TTT GG- 3′ and reverse primer 5′-CAG TGT CTC TCC AAA TCC GAT A-3′; N-cadherin, forward primer 5′-CCT GCG CGT GAA GGT TTG CC- 3′ and reverse primer 5′-CCA AGC CCC GCA CCC ACA AT-3′;ZEB1, 5′-GCA CAA CCA AGT GCA GAA GA- 3′; and reverse primer 5′-GCC TGG TTC AGG AGA AGA TG- 3′; Vimentin, 5′-TGT CCA AAT CGA TGT GGA TGT TTC- 3′ and reverse primer 5′-TTG TAC CAT TCT TCT GCC TCC TG- 3′.
miR-26b mimic transfection
The cells were seeded in 6-well plates and transfected with miR-26b mimic or the nonspecific control (GenePharma, Shanghai, China) using lipofectamine RNAiMAX reagent (Invitrogen) following the manufacture's protocol.46 MiR-26b mimic: Sense 5′-UUC AAG UAA UUC AGG AUA GGU-3′; antisense 5′-CUA UCC UGA AUU ACU UGA AUU -3′. The cells were subjected to further analysis as presented under the results section.
miR-26b inhibitor transfection
The cells were seeded in 24-well plates and transfected with miR-26b inhibitor (GenePharma, Shanghai, China) or the nonspecific control by DharmaFect Transfection Reagent (Dharmacon, CO) following the manufacture's protocol.47
Western blotting analysis
The harvested cells were washed by PBS and lysed with protein lysis buffer. The concentrations of the proteins were tested by BCA Protein Assay kit (Thermo Scientific, MA). Same amount of protein samples were separated by electrophoresis in Sodium Dodecyl Sulfonate (SDS)-polyacrylamide gel and then transferred onto a Polyvinylidene Fluoride (PVDF) membrane, and then incubated with primary antibody at 4°C overnight. After washed with TBST for 3 times and incubated with second antibody at room temperature for one hour. Then the expression of protein was detected by electrochemiluminescence (ECL) assay.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism 5.0 (Graph Pad Software, La Jolla, CA). Student's t-test was performed to evaluate statistical significance. Results were presented as means ± SD. P < 0.05 was considered as statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant from National Natural Science Foundation of China (NSFC number 81572936) and the priority academic program development of Jiangsu higher education institutions.
References
- [1].Jordan JT, Gerstner ER, Batchelor TT, Cahill DP, Plotkin SR. Glioblastoma care in the elderly. Cancer. 2016;122:189-97. doi: 10.1002/cncr.29742. PMID:26618888 [DOI] [PubMed] [Google Scholar]
- [2].Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203-17. doi: 10.1101/gad.261982.115. PMID:26109046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wait SD, Prabhu RS, Burri SH, Atkins TG, Asher AL. Polymeric drug delivery for the treatment of glioblastoma. Neuro-Oncol. 2015;17 Suppl 2:ii9-ii23. doi: 10.1093/neuonc/nou360. PMID:25746091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Han J, Chen Q. MiR-16 modulate temozolomide resistance by regulating BCL-2 in human glioma cells. Int J Clin Exp Pathol. 2015;8:12698-707. PMID:26722459 [PMC free article] [PubMed] [Google Scholar]
- [5].Huang D, Duan H, Huang H, Tong X, Han Y, Ru G, Qu L, Shou C, Zhao Z. Cisplatin resistance in gastric cancer cells is associated with HER2 upregulation-induced epithelial-mesenchymal transition. Scientific Reports. 2016;6:20502. doi: 10.1038/srep20502. PMID:26846307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu Y, Du F, Zhao Q, Jin J, Ma X, Li H. Acquisition of 5-fluorouracil resistance induces epithelial-mesenchymal transitions through the Hedgehog signaling pathway in HCT-8 colon cancer cells. Oncol Letters. 2015;9:2675-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin JJ, Zhao TZ, Cai WK, Yang YX, Sun C, Zhang Z, Xu YQ, Chang T, Li ZY. Inhibition of histamine receptor 3 suppresses glioblastoma tumor growth, invasion, and epithelial-to-mesenchymal transition. Oncotarget. 2015;6:17107-20. doi: 10.18632/oncotarget.3672. PMID:25940798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Han MZ, Huang B, Chen AJ, Zhang X, Xu R, Wang J, Li XG. High expression of RAB43 predicts poor prognosis and is associated with epithelial-mesenchymal transition in gliomas. Oncol Reports. 2017;37:903-12 [DOI] [PubMed] [Google Scholar]
- [9].Lee KH, Ahn EJ, Oh SJ, Kim O, Joo YE, Bae JA, Yoon S, Ryu HH, Jung S, Kim KK, et al.. KITENIN promotes glioma invasiveness and progression, associated with the induction of EMT and stemness markers. Oncotarget. 2015;6:3240-53. doi: 10.18632/oncotarget.3087. PMID:25605251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gomes BC, Rueff J, Rodrigues AS. MicroRNAs and cancer drug resistance. Methods Mol Biol. 2016;1395:137-62. doi: 10.1007/978-1-4939-3347-1_9. PMID:26910073 [DOI] [PubMed] [Google Scholar]
- [11].Riquelme I, Letelier P, Riffo-Campos AL, Brebi P, Roa JC. Emerging Role of miRNAs in the drug resistance of gastric cancer. Int J Mol Sci. 2016;17:424. doi: 10.3390/ijms17030424. PMID:27011182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu Y, Huang J, Ma L, Shan J, Shen J, Yang Z, Liu L, Luo Y, Yao C, Qian C. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Letters. 2016;371:171-81. doi: 10.1016/j.canlet.2015.11.034. PMID:26655273 [DOI] [PubMed] [Google Scholar]
- [13].van Jaarsveld MT, van Kuijk PF, Boersma AW, Helleman J, van IWF, Mathijssen RH, Pothof J, Berns EM, Verweij J, Wiemer EA. miR-634 restores drug sensitivity in resistant ovarian cancer cells by targeting the Ras-MAPK pathway. Mol Cancer. 2015;14:196. doi: 10.1186/s12943-015-0464-4. PMID:26576679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stojcheva N, Schechtmann G, Sass S, Roth P, Florea AM, Stefanski A, Stuhler K, Wolter M, Muller NS, Theis FJ, et al.. MicroRNA-138 promotes acquired alkylator resistance in glioblastoma by targeting the Bcl-2-interacting mediator BIM. Oncotarget. 2016;7:12937-50. doi: 10.18632/oncotarget.7346. PMID:26887050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Y, Sun B, Zhao X, Zhao N, Sun R, Zhu D, Zhang Y, Li Y, Gu Q, Dong X, et al.. Twist1-related miR-26b-5p suppresses epithelial-mesenchymal transition, migration and invasion by targeting SMAD1 in hepatocellular carcinoma. Oncotarget. 2016;7:24383-401. doi: 10.18632/oncotarget.8328. PMID:27027434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shen G, Lin Y, Yang X, Zhang J, Xu Z, Jia H. MicroRNA-26b inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting USP9X. BMC Cancer. 2014;14:393. doi: 10.1186/1471-2407-14-393. PMID:24890815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Siebzehnrubl FA, Silver DJ, Tugertimur B, Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT, Kupper MD, Neal D, et al.. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol Med. 2013;5:1196-212. doi: 10.1002/emmm.201302827. PMID:23818228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ashizawa T, Akiyama Y, Miyata H, Iizuka A, Komiyama M, Kume A, Omiya M, Sugino T, Asai A, Hayashi N, et al.. Effect of the STAT3 inhibitor STX-0119 on the proliferation of a temozolomide-resistant glioblastoma cell line. Int J Oncol. 2014;45:411-8. PMID:24820265 [DOI] [PubMed] [Google Scholar]
- [19].Tso JL, Yang S, Menjivar JC, Yamada K, Zhang Y, Hong I, Bui Y, Stream A, McBride WH, Liau LM, et al.. Bone morphogenetic protein 7 sensitizes O6-methylguanine methyltransferase expressing-glioblastoma stem cells to clinically relevant dose of temozolomide. Mol Cancer. 2015;14:189. doi: 10.1186/s12943-015-0459-1. PMID:26546412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tang H, Zhao J, Zhang L, Zhuang Y, Liang P. SRPX2 enhances the Epithelial-Mesenchymal transition and temozolomide resistance in glioblastoma cells. Cell Mol Neurobiol. 2016;36:1067-76. doi: 10.1007/s10571-015-0300-9. PMID:26643178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yu F, Li G, Gao J, Sun Y, Liu P, Gao H, Li P, Lei T, Chen Y, Cheng Y, et al.. SPOCK1 is upregulated in recurrent glioblastoma and contributes to metastasis and Temozolomide resistance. Cell Prolif. 2016;49:195-206. doi: 10.1111/cpr.12241. PMID:26923184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu N, Zhao X, Liu M, Liu H, Yao W, Zhang Y, Cao S, Lin X. Role of microRNA-26b in glioma development and its mediated regulation on EphA2. PloS One. 2011;6:e16264. doi: 10.1371/journal.pone.0016264. PMID:21264258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu Y, Lu Y, Zhang Q, Liu JJ, Li TJ, Yang JR, Zeng C, Zhuang SM. MicroRNA-26a/b and their host genes cooperate to inhibit the G1/S transition by activating the pRb protein. Nucleic Acids Res. 2012;40:4615-25. doi: 10.1093/nar/gkr1278. PMID:22210897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q, Chen YC, Peng Y, Yao KT, Kung HF, et al.. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225-33. doi: 10.1158/0008-5472.CAN-10-1850. PMID:21199804 [DOI] [PubMed] [Google Scholar]
- [25].Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, et al.. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005-17. doi: 10.1016/j.cell.2009.04.021. PMID:19524505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang B, Liu XX, He JR, Zhou CX, Guo M, He M, Li MF, Chen GQ, Zhao Q. Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis. 2011;32:2-9. doi: 10.1093/carcin/bgq209. PMID:20952513 [DOI] [PubMed] [Google Scholar]
- [27].Reuland SN, Smith SM, Bemis LT, Goldstein NB, Almeida AR, Partyka KA, Marquez VE, Zhang Q, Norris DA, Shellman YG. MicroRNA-26a is strongly downregulated in melanoma and induces cell death through repression of silencer of death domains (SODD). J Invest Dermatol. 2013;133:1286-93. doi: 10.1038/jid.2012.400. PMID:23190898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Letters. 2011;585:1363-7. doi: 10.1016/j.febslet.2011.04.018. PMID:21510944 [DOI] [PubMed] [Google Scholar]
- [29].Lin J, Zhang L, Huang H, Huang Y, Huang L, Wang J, Huang S, He L, Zhou Y, Jia W, et al.. MiR-26b/KPNA2 axis inhibits epithelial ovarian carcinoma proliferation and metastasis through downregulating OCT4. Oncotarget. 2015;6:23793-806. doi: 10.18632/oncotarget.4363. PMID:26204489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li H, Sun Q, Han B, Yu X, Hu B, Hu S. MiR-26b inhibits hepatocellular carcinoma cell proliferation, migration, and invasion by targeting EphA2. Int J Clin Exp Pathol. 2015;8:4782-90. PMID:26191168 [PMC free article] [PubMed] [Google Scholar]
- [31].Liang N, Zhou X, Zhao M, Zhao D, Zhu Z, Li S, Yang H. Down-regulation of microRNA-26b modulates non-small cell lung cancer cells chemoresistance and migration through the association of PTEN. Acta Biochim Et Biophys Sinica. 2015;47:530-8. doi: 10.1093/abbs/gmv046 [DOI] [PubMed] [Google Scholar]
- [32].Du JY, Wang LF, Wang Q, Yu LD. miR-26b inhibits proliferation, migration, invasion and apoptosis induction via the downregulation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 driven glycolysis in osteosarcoma cells. Oncol Reports. 2015;33:1890-8 [DOI] [PubMed] [Google Scholar]
- [33].Cao J, Guo T, Dong Q, Zhang J, Li Y. miR-26b is downregulated in human tongue squamous cell carcinoma and regulates cell proliferation and metastasis through a COX-2-dependent mechanism. Oncol Reports. 2015;33:974-80 [DOI] [PubMed] [Google Scholar]
- [34].Zhang X, Wei C, Li J, Liu J, Qu J. MicroRNA-361-5p inhibits epithelial-to-mesenchymal transition of glioma cells through targeting Twist1. Oncol Reports. 2017;37:1849-56 [DOI] [PubMed] [Google Scholar]
- [35].Liao H, Bai Y, Qiu S, Zheng L, Huang L, Liu T, Wang X, Liu Y, Xu N, Yan X, et al.. MiR-203 downregulation is responsible for chemoresistance in human glioblastoma by promoting epithelial-mesenchymal transition via SNAI2. Oncotarget. 2015;6:8914-28. doi: 10.18632/oncotarget.3563. PMID:25871397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu J, Cui H, Zhu Z, Wang L. MicroRNA-200b-3p suppresses epithelial-mesenchymal transition and inhibits tumor growth of glioma through down-regulation of ERK5. Biochem Biophys Res Commun. 2016;478:1158-64. doi: 10.1016/j.bbrc.2016.08.085. PMID:27545608 [DOI] [PubMed] [Google Scholar]
- [37].Dong N, Xu B, Benya SR, Tang X. MiRNA-26b inhibits the proliferation, migration, and epithelial-mesenchymal transition of lens epithelial cells. Mol Cell Biochem. 2014;396:229-38. doi: 10.1007/s11010-014-2158-4. PMID:25063219 [DOI] [PubMed] [Google Scholar]
- [38].Zhao N, Wang R, Zhou L, Zhu Y, Gong J, Zhuang SM. MicroRNA-26b suppresses the NF-kappaB signaling and enhances the chemosensitivity of hepatocellular carcinoma cells by targeting TAK1 and TAB3. Mol Cancer. 2014;13:35. doi: 10.1186/1476-4598-13-35. PMID:24565101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jin F, Wang Y, Li M, Zhu Y, Liang H, Wang C, Wang F, Zhang CY, Zen K, Li L. MiR-26 enhances chemosensitivity and promotes apoptosis of hepatocellular carcinoma cells through inhibiting autophagy. Cell Death Dis. 2017;8:e2540. doi: 10.1038/cddis.2016.461. PMID:28079894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bhattacharya A, Schmitz U, Wolkenhauer O, Schonherr M, Raatz Y, Kunz M. Regulation of cell cycle checkpoint kinase WEE1 by miR-195 in malignant melanoma. Oncogene. 2013;32:3175-83. doi: 10.1038/onc.2012.324. PMID:22847610 [DOI] [PubMed] [Google Scholar]
- [41].Hamilton DH, Huang B, Fernando RI, Tsang KY, Palena C. WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition. Cancer Res. 2014;74:2510-9. doi: 10.1158/0008-5472.CAN-13-1894. PMID:24626094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhao W, Liu S, Dou Q, Li C, Du J, Ren W. The role and mechanism of WEE1 on the cisplatin resistance reversal of the HepG2/DDP human hepatic cancer cell line. Oncol Letters. 2015;10:3081-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Matheson CJ, Venkataraman S, Amani V, Harris PS, Backos DS, Donson AM, Wempe MF, Foreman NK, Vibhakar R, Reigan P. A WEE1 inhibitor analog of AZD1775 maintains synergy with cisplatin and demonstrates reduced single-agent cytotoxicity in medulloblastoma cells. ACS Chem Biol. 2016;11:921-30. doi: 10.1021/acschembio.5b00725. PMID:26745241 [DOI] [PubMed] [Google Scholar]
- [44].Jhuraney A, Woods NT, Wright G, Rix L, Kinose F, Kroeger JL, Remily-Wood E, Cress WD, Koomen JM, Brantley SG, et al.. PAXIP1 potentiates the combination of WEE1 inhibitor AZD1775 and platinum agents in lung cancer. Mol Cancer Ther. 2016;15:1669-81. doi: 10.1158/1535-7163.MCT-15-0182. PMID:27196765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang L, Hou Y, Yin X, Su J, Zhao Z, Ye X, Zhou X, Zhou L, Wang Z. Rottlerin inhibits cell growth and invasion via down-regulation of Cdc20 in glioma cells. Oncotarget. 2016;7:69770-82. PMID:27626499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget. 2015;6:18027-37. doi: 10.18632/oncotarget.4090. PMID:26046466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng L, Shi Y, Wang H, Yin B, Xia J, et al.. Down-regulation of miR-223 reverses epithelial-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Oncotarget. 2015;6:1740-9. doi: 10.18632/oncotarget.2714. PMID:25638153 [DOI] [PMC free article] [PubMed] [Google Scholar]


