Abstract
Purpose of review
Long-acting antiretroviral (ARV) agents are currently under development for the treatment of chronic HIV infection. This review focuses on data recently produced on injectable ARVs for patients living with HIV/AIDS and on the patients’ perspectives on the use of these agents.
Recent findings
Crystalline nanoparticle formulations of the nonnucleoside reverse transcriptase inhibitor rilpivirine (TMC278) and of the HIV-1 integrase strand transfer inhibitor cabotegravir (GSK1265744) have progressed into phase II clinical trials as injectable maintenance therapy for patients living with HIV/AIDS with an undetectable viral load.
Summary
Phase II studies evaluating the coadministration of rilpivirine and cabotegravir intramuscularly to HIV-infected individuals with an undetectable viral load are currently underway. Rilpivirine and cabotegravir are characterized by different mechanisms of action against HIV and a favorable drug interaction profile, providing a rationale for coadministration. The high potency and low daily dosing requirements of oral cabotegravir and rilpivirine facilitate long-acting formulation development. Intramuscular dosing is preceded by an oral lead-in phase to assess safety and tolerability in individual participants. In addition to assessing the safety of injectable therapies in ongoing studies, it will be important to evaluate whether differences in drug adherence between injectable and oral therapies lead to different virologic outcomes, including rates of virologic failure and the emergence of resistance. Long-acting formulations may be associated with challenges, such as the management of adverse effects with persistent drug concentrations and the risk of virologic resistance, as drug concentrations decline following discontinuation.
Keywords: cabotegravir, HIV-1, rilpivirine
INTRODUCTION
Since the introduction of combination antiretroviral therapy (cART), the lifespan of people living with HIV/AIDS (PLWH) has been remarkably prolonged and has been associated with significant reductions in HIV-related morbidity and mortality. Although available cART regimens do not provide a cure for HIV, it has transformed HIV infection into a chronic disease requiring lifelong daily oral treatment. More important, in patients taking oral medication for chronic diseases, poor adherence to medications is common, resulting in 50–75% nonadherence over time [1]. Adherence to cART is variable, with composite estimates of adherence as low as 70% over time [2]. When adherence to cART is suboptimal, drug exposure is insufficient to suppress HIV viral replication and increases the likelihood for emergence of resistance. At present, with the exception of the uncommonly prescribed enfuvirtide, all available antiretrovirals (ARVs) are administered orally. However, long-acting formulations are under development for both the prevention and treatment of HIV infection, which could potentially replace daily pill taking with routine injection visits. The aim of this review article is to discuss the data available on injectable ARVs under investigation for the treatment of HIV infection, with a focus on injectable rilpivirine and cabotegravir, which are being developed as a complete long-acting regimen.
Box 1.

no caption available
RILPIVIRINE LONG-ACTING
The nonnucleoside reverse transcriptase inhibitor rilpivirine is now commonly used for the treatment of HIV infection in combination with other ARVs at an oral dose of 25 mg once daily [3]. A parenteral long-acting formulation of rilpivirine is also being developed, which consists of a solid drug particle nanosuspension produced by milling of large fragments of drug until particles within the nanometer range are achieved [4]. In preclinical proof-of-concept studies, single injections of the nanoformulated drug led to sustained release of rilpivirine in plasma in dogs and mice [4]. Furthermore, rilpivirine plasma exposure was confirmed to be constant and dose-proportional for more than 2 months in rats and 6 months in dogs, with an absolute bioavailability of 100% [5].
Subsequently, the pharmacokinetics of rilpivirine long acting has been studied in humans following single and multiple doses [6]. In addition, a compartmental pharmacokinetics evaluation of rilpivirine long acting in HIV-negative volunteers for preexposure prophylaxis (PrEP) has been published [7▪▪].
A phase I study of different doses of rilpivirine nanosuspension aimed at evaluating the pharmacokinetics and safety of gluteal or deltoid intramuscular injections or abdominal subcutaneous injections in 60 healthy HIV-negative volunteers showed consistent results with the preclinical experience: rilpivirine was slowly released from the injection site into plasma, with drug concentrations of more than 10 ng/ml for 12–26 weeks [6]. Dose proportionality and similar plasma concentration–time profiles were observed for both subcutaneous and gluteal intramuscular injections. A pharmacokinetics study investigating plasma and genital tract concentrations in 60 women and six men given intramuscular depot injections of three different doses of rilpivirine long-acting (300, 600, and 1200 mg) showed that rilpivirine maximum concentrations (Cmax) were 34, 82, and 160 ng/ml for the 300, 600, and 1200 mg doses, respectively [7▪▪]. Also observed from that study, concentration decay was slow during 3 months postdose: C28 were 19, 44, and 83 ng/ml for the 300 mg dose; C56 were 9, 23, and 45 ng/ml for the 600 mg dose; and C84 were 6, 16, and 30 ng/ml for the 1200 mg dose.
Intramuscular rilpivirine long acting was administered intragluteally through a 20-gauge, 1.5-inch needle. The nanosupension is formulated as 300 mg/ml, allowing for 1 or 2 ml injections of 300 and 600 mg doses, respectively. Twelve hundred milligrams of intramuscular injections were administered as two separate 2-ml injections. Both studies showed that injection site reactions (ISRs) consisted of mild-to-moderate redness, bruising, pain, and to a lesser extent, induration. The study drug was well tolerated with no high-grade adverse events or laboratory abnormalities. More important, no hypersensitivity reactions or ECG abnormalities were observed. During oral rilpivirine development, doses higher than that approved for treatment were shown to cause ECG QT-interval prolongation. However, the drug exposure achieved with the licensed oral dose of 25 mg once daily has not shown ECG QT prolongation. To date, the rilpivirine exposures achieved following single and multiple intramuscular injections of the nanosuspended formulations have not been associated with ECG alterations in humans and are predicted to achieve concentrations that remain below safety thresholds established with the oral product [6,7▪▪]. Further data on the safety of multiple doses of this agent will be generated in the near future by ongoing phase II studies.
CABOTEGRAVIR LONG-ACTING
Cabotegravir (GSK1265744) is an HIV-1 integrase strand transfer inhibitor under clinical development as both oral and long-acting injectable formulations. Cabotegravir has subnanomolar antiviral activity, with an in-vitro half-maximal inhibitory concentration (IC50) of 0.22 and 0.34 nmol/l against HIV-1 BAL and NL432 in peripheral blood mononuclear cells respectively. Cabotegravir is highly protein-bound to serum albumin, resulting in a protein-adjusted IC90 of 166 ng/ml with an oral plasma half-life of approximately 40 h, allowing for once-daily dosing. In a phase IIa proof-of-concept study, cabotegravir 5 or 30 mg once daily for 10 days exhibited potent antiviral activity with mean plasma HIV-1 RNA decreases of 2.2–2.3 log10 copies/ml, respectively [8▪].
The long-acting injectable nanosuspension of cabotegravir comprises crystalline active drug, milled to a median particle size of 200 nm, along with surfactant, polymer, mannitol, and water for injection. Cabotegravir long acting is formulated as a 200 mg/ml nanosuspension and has been evaluated via both subcutaneous and intramuscular routes of administration. The prolonged apparent half-life achieved following injection of cabotegravir can be explained by the slow absorption rate from poorly soluble nanoparticles in tissue, over time, rather than a change in plasma elimination half-life from dissolved drug. Cabotegravir long acting has been evaluated in single-dose and repeat-dose phase I studies and is currently in phase II development for both HIV treatment (in combination with rilpivirine) and as a single agent for PrEP.
The first human dosing of cabotegravir long acting occurred in a phase I, open-label, nine-cohort, parallel study that evaluated plasma and tissue pharmacokinetics following single-dose administration of cabotegravir in eight healthy participants (six active and two placebo). Cabotegravir was administered as a 200-mg/ml nanosuspension at doses of 100–800 mg intramuscular and 100–400 mg subcutaneous. Following single intramuscular or subcutaneous injection, plasma drug concentrations increased rapidly over the first week with sustained mean plasma concentrations above the protein-adjusted IC90 for approximately 24 weeks or longer for doses at least 200 mg, with a mean absorption-limited apparent terminal phase half-life ranging from 21 to 50 days, as shown in Fig. 1[9,10▪▪]. Additional descriptions from this study can be found in the article by Trezza et al. published concurrently.
FIGURE 1.
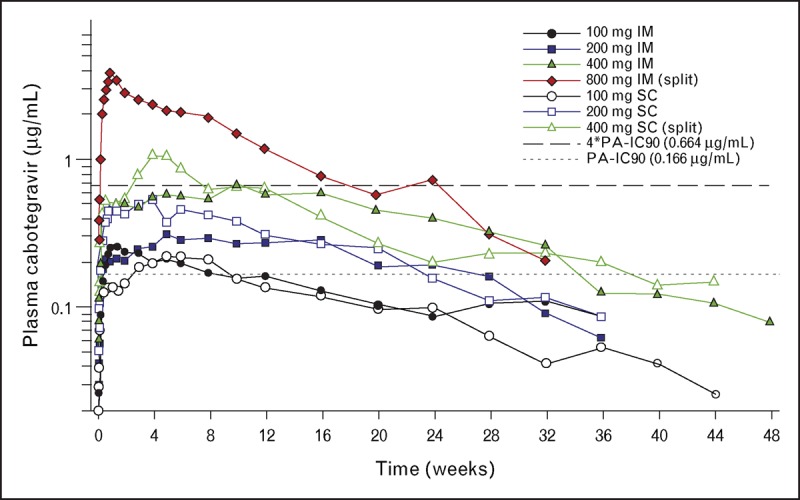
Mean plasma levels of cabotegravir over time following single-dose parenteral administration of cabotegravir long acting. Cabotegravir (200 mg/ml) was administered as a single intramuscular (gluteal) or subcutaneous (abdominal) injection or equally divided intramuscular or subcutaneous injection. Each cohort was composed of eight healthy adult participants (six active/two placebo). The dashed lines represent 1× or 4× the plasma protein-adjusted IC90 value for cabotegravir against wild-type HIV-1 of 0.166 and 0.664 μg/ml, respectively. Adapted with permission from [9,10▪▪].
In a repeat-dose phase I study, healthy adult participants received a 14-day lead-in of oral cabotegravir 30 mg/day to assess safety and tolerability before administration of the long-acting injection [11▪▪]. Participants were then randomized to four cohorts: cabotegravir 800 mg intramuscular (as 400 mg × 2) followed by 3 monthly doses of 200 mg subcutaneous, 200 mg intramuscular, 400 mg intramuscular, or after 3 months, a single further injection of 800 mg intramuscular. Cohorts 2 and 3 also received intramuscular doses of rilpivirine long acting at months 3 (1200 mg) and 4 (900 or 600 mg, respectively). Forty-seven participants enrolled and 40 participants received at least one long-acting injection; 38 completed all planned injections. Seven participants discontinued the oral lead-in phase, six for nondrug-related reasons and one for an adverse event (dizziness following the first 30 mg dose). All regimens achieved therapeutically relevant cabotegravir plasma concentrations within 3 days after injection, as defined by attaining mean plasma concentrations greater than four-fold the protein-adjusted IC90 for wild-type HIV (0.66 mg/ml), which persisted until the end of the dosing interval. Geometric mean pharmacokinetics parameters for cabotegravir long acting were similar between groups receiving intramuscular and subcutaneous doses of 200 mg. The Cmax and area under the curve (AUC)0–t values were generally linear between the 200 and 400 mg cabotegravir long-acting intramuscular doses [11▪▪]. Additional information from this study can be found in the article by Trezza et al. published concurrently.
CABOTEGRAVIR LONG-ACTING: SAFETY
Data from the first 98 participants (58 single dose, 40 repeat dose) to receive cabotegravir long acting were combined into a safety meta-analysis [12▪]. Intramuscular cabotegravir was administered intragluteally through a 25-gauge, 1.5-inch needle. The nanosuspension is formulated as 200 mg/ml, allowing for 1 or 2 ml injections of 200 and 400 mg, respectively. Eight hundred milligrams of intramuscular injections were administered as two separate 2 ml injections. Subcutaneous cabotegravir was administered via a 5/8-inch, 25-gauge needle in the abdomen. The most common ISRs for intramuscular and subcutaneous dosing, respectively, were pain (71 and 24%), erythema (9 and 23%), and nodules (7 and 23%). Median intramuscular ISR durations were ∼5 days for both pain and erythema and ∼22 days for nodules. The majority of ISRs were characterized as mild (93%), with no severe ISR symptoms reported, and no ISR-related withdrawals. On the basis of experience to date, the systemic tolerability of cabotegravir long acting appears similar to that seen with oral dosing. The most commonly occurring non-ISR drug-related adverse events were headache (6%) and abdominal pain (2%) [12▪]. Injection tolerability and overall safety will be further evaluated in ongoing and planned studies.
CABOTEGRAVIR PLUS RILPIVIRINE
Cabotegravir, in combination with rilpivirine, is being evaluated as a two-drug regimen for the maintenance of HIV suppression, following suppression to an HIV-1 RNA less than 50 copies/ml with oral cART. The ongoing phase IIb LATTE (Long Acting Antiretroviral Treatment Enabling) study was designed to provide efficacy and safety data with the oral combination of cabotegravir + rilpivirine in HIV-1-infected participants. Two hundred forty-three HIV-1-infected participants were randomized to cabotegravir 10, 30, or 60 mg/day, or to a control arm of efavirenz, with all groups receiving an investigator-selected backbone regimen of abacavir/lamivudine or tenofovir/emtricitabine. Following 24 weeks of triple-drug therapy, participants randomized to cabotegravir who achieved a plasma HIV-1 RNA level of less than 50 copies/ml entered the maintenance phase and simplified their regimen to oral cabotegravir and oral rilpivirine 25 mg/day for an additional 72 weeks. The primary endpoint for this study is the proportion of participants with HIV-1 RNA less than 50 copies/ml at Week 48 based on the intent to treat-exposed population using the United States Food and Drug Administration Snapshot algorithm. At Week 48, 82% of participants across the three cabotegravir + rilpivirine arms achieved the primary endpoint, with similar response rates across cabotegravir doses, relative to a 71% response rate on the efavirenz control arm (Fig. 2) [13▪▪]. Three participants met the criteria for protocol-defined virologic failure through Week 48 (cabotegravir 10 mg, cabotegravir 30 mg, efavirenz), including one subject with persistently low cabotegravir and rilpivirine drug levels (∼50% of predicted levels at multiple time points) who developed treatment-emergent integrase inhibitor (Q148R) and nonnucleoside reverse transcriptase inhibitor (E138Q) mutations, representing a fold change relative to wild-type virus of 3 (cabotegravir) and 2 (rilpivirine). The participant was withdrawn from the study and placed on guideline approved highly active antiretroviral therapy, although follow-up viral load data are not available. Cabotegravir + rilpivirine was generally well tolerated with no drug-related serious adverse events and few adverse event-related withdrawals [13▪▪]. The most common drug-related adverse events through Week 48 were nausea (17%), headache (15%), and diarrhea (10%) for participants receiving cabotegravir and rilpivirine and were dizziness (23%), abnormal dreams (21%), nausea (15%), and insomnia (15%) for participants receiving efavirenz.
FIGURE 2.
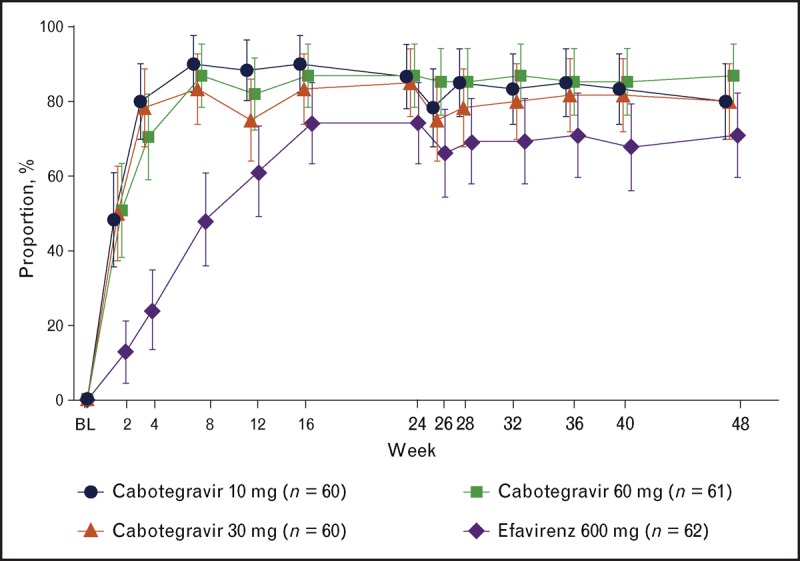
Proportion [95% confidence interval (CI)] of participants with plasma HIV-1 RNA less than 50 copies/ml by visit by snapshot [missing, switch, or discontinuation equals failure (MSDF)] analysis. 95% CIs are normal approximation MSDF.
Population pharmacokinetics modeling and simulation, in combination with the data generated from the LATTE study, were utilized to select the long-acting regimens of cabotegravir and rilpivirine to progress to phase IIb trials ([14], data on file). Cabotegravir regimens were selected based upon their ability to quickly achieve therapeutic plasma concentrations and maintain concentrations above the mean trough concentration obtained with oral cabotegravir 10 mg (1.35 μg/ml; ∼8× protein-adjusted IC90). Achieving these factors while balancing the desire to construct regimens that are clinically viable and amenable to ‘real-world’ administration (e.g., allowing for some delayed doses) led to the selection of the following two long-acting regimens for evaluation in phase IIb: cabotegravir 800 mg intramuscular loading dose on Day 1, followed by 400 mg intramuscular every 4 weeks beginning on Week 4, in combination with rilpivirine long-acting 600 mg intramuscular every 4 weeks, and cabotegravir 800 mg intramuscular loading dose on Day 1, cabotegravir 600 mg intramuscular on Week 4, followed by cabotegravir 600 mg intramuscular every 8 weeks beginning on Week 8, in combination with rilpivirine 900 mg intramuscular every 8 weeks.
The LATTE-2 study evaluating the combination of injectable cabotegravir + rilpivirine is currently ongoing and is the first study to evaluate the safety and efficacy of an entirely long acting injectable regimen in HIV-1-infected patients. The study enrolled 310 ARV therapy-naive patients, who will receive 20 weeks of oral suppressive therapy with cabotegravir 30 mg + nucleoside reverse transcriptase inhibitors followed by an injectable regimen of cabotegravir + rilpivirine long acting, administered as a gluteal intramuscular injection every 4 or 8 weeks (Fig. 3). The overall objective of this study is to select an intramuscular dosing regimen of cabotegravir long acting plus rilpivirine long-acting based on a comparison of the Week 32 antiviral activity, tolerability, and safety of two intramuscular dosing regimens, relative to cabotegravir 30 mg plus ABC/3TC orally once daily. The primary endpoint is the proportion of participants with an HIV-1 RNA less than 50 c/ml at Week 32 based on the FDA ‘Snapshot’ algorithm, in addition to an evaluation of virologic failures and the incidence of adverse events and laboratory abnormalities over time. Secondary endpoints include an evaluation of cabotegravir and rilpivirine long-acting pharmacokinetics, virologic resistance, and treatment satisfaction. Primary endpoint data from LATTE-2 is estimated to be available in the fourth quarter of 2105, and will inform follow-up phase III pivotal studies with cabotegravir long acting + rilpivirine long acting as maintenance therapy in virologically suppressed participants.
FIGURE 3.
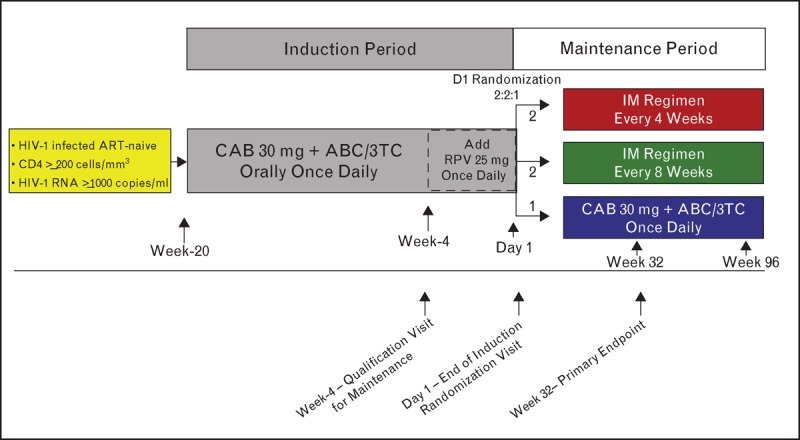
LATTE-2 study design schematic. ABC/3TC, abacavir/lamivudine; ART, antiretroviral therapy; CAB, cabotegravir; RPV, rilpivirine.
OTHER LONG-ACTING ANTIRETROVIRAL AGENTS
Although this review focuses on long-acting cART regimens, other long-acting ARVs have been explored for the treatment of HIV infection and are either at a very early stage of development or have been utilized in combination with oral medication, in select populations. Ibalizumab is a humanized monoclonal antibody that binds between the domain 1 and 2 interface of the extracellular portion of CD4, blocking CD4-dependent cell entry of HIV [15]. Ibalizumab has been evaluated through phase II trials as an intravenous infusion every 2 or 4 weeks, in combination with optimized oral cART, in heavily treatment-experienced patients.
The pharmacokinetics of other nanoformulated ARV drugs, such as the protease inhibitors atazanavir and ritonavir, has been investigated in preclinical rodent and mammal studies following the administration of long-acting injectable nanoformulations. However, further research in humans is warranted to fully characterize these formulations for clinical use [16].
ANTIRETROVIRAL LONG-ACTING FORMULATIONS: THE PATIENT'S PERSPECTIVE
Importantly, data on patient acceptability of these treatments are very limited. Interestingly, Williams et al.[17] conducted a survey among 400 PLWH to measure their willingness in being administered ARV therapy parenterally rather than orally. Overall, 73% of individuals responded that they would be definitely or probably interested in trying injectable nanoformulated ARV therapy. Notably, this is in accordance with patients’ opinion in the United Kingdom, as development of injectable ARVs seems to have strong community support as a novel therapeutic option (Collins S, personal communication). Finally, some in the community have stated that their hope is that these new therapies may be able to lead to avoidance of forgotten doses, protection of patient privacy, and improvement in tolerability by reducing gastrointestinal adverse effects (Collins S, personal communication). This anticipation must be balanced by potential risks, some of which may be unique to injectable therapy, including pain, erythema, and/or nodule formation at the site of injection and the potential for the prolongation of adverse effects or the emergence of HIV resistance because of the persistence of drug concentrations following intramuscular dosing. Furthermore, PLWH who are stable on cART and asymptomatic are typically seen only twice yearly by their treating physicians and would need to increase the number of visits to the clinic to receive monthly or bimonthly injections. Long-acting regimens for the treatment of HIV are unlikely to ever replace oral cART regimens but can provide additional treatment options for PLWH and may provide advantages in select populations with challenges adhering to daily oral therapy.
CONCLUSION
Cabotegravir and rilpivirine have been formulated as long-acting injectable nanosuspensions to enable dosing at intervals greater than once monthly. A phase IIb study evaluating the intramuscular coadministration of rilpivirine and cabotegravir to HIV-infected individuals with an undetectable viral load is currently underway and will assess the safety, tolerability, and efficacy of this fully injectable regimen for people living with HIV.
Acknowledgements
The authors thank all study participants for the contribution to the research studies that have made this work possible.
Financial support and sponsorship
No financial support was provided for the drafting of this manuscript.
Conflicts of interest
D.M. is an employee of and owns stock in GlaxoSmithKline. GlaxoSmithKline has a majority shareholding in ViiV Healthcare, which is developing cabotegravir for HIV treatment. M.B. has acted as an advisor and has received speaking fees and travel and research grants from ViiV Healthcare, Janssen, Merck Sharp & Dohme, Bristol-Myers Squibb, and Gilead Sciences.
The authors have no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Coleman CI, Roberts MS, Sobieraj DM, et al. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr Med Res Opin 2012; 28:669–680. [DOI] [PubMed] [Google Scholar]
- 2.Low-Beer S, Yip B, O'Shaughnessy MV, et al. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr 2000; 23:360–361. [DOI] [PubMed] [Google Scholar]
- 3.Nelson MR, Elion RA, Cohen CJ, et al. Rilpivirine versus efavirenz in HIV-1-infected subjects receiving emtricitabine/tenofovir DF: pooled 96-week data from ECHO and THRIVE Studies. HIV Clin Trials 2013; 14:81–91. [DOI] [PubMed] [Google Scholar]
- 4.Baert L, van ’t Klooster G, Dries W, et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm 2009; 72:502–508. [DOI] [PubMed] [Google Scholar]
- 5.van ’t Klooster G, Hoeben E, Borghys H, et al. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother 2010; 54:2042–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verloes R, Deleu S, Niemeijer N, et al. Safety, tolerability and pharmacokinetics of rilpivirine following administration of a long-acting formulation in healthy volunteers. HIV Med 2015; in press. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Jackson AG, Else LJ, Mesquita PM, et al. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for preexposure prophylaxis. Clin Pharmacol Ther 2014; 96:314–323. [DOI] [PubMed] [Google Scholar]; This article describes the pharmacokinetics of long-acting rilpivirine and delineated tissue distribution, relevant to HIV treatment and PrEP.
- 8▪.Spreen W, Min S, Ford SL, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials 2013; 14:192–203. [DOI] [PubMed] [Google Scholar]; This article provides the first description of antiviral activity and supportive oral dosing pharmacokinetics for cabotegravir.
- 9.Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS 2013; 8:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪▪.Spreen W, Ford SL, Chen S, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr 2014; 67:481–486. [DOI] [PubMed] [Google Scholar]; This article characterizes the first administration of single-dose long-acting cabotegravir in humans, supporting monthly or greater dosing in follow-up studies.
- 11▪▪.Spreen W, Williams P, Margolis D, et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr 2014; 67:487–492. [DOI] [PubMed] [Google Scholar]; This article describes the first repeat dosing of long-acting cabotegravir as well as co-administration of injectable cabotegravir and rilpivirine, supporting the dosing strategy for follow-up clinical studies.
- 12▪.Lou Y, Gould E, Chen S, et al. Meta-analysis of safety data from 8 clinical studies with GSK1265744, an HIV integrase inhibitor, dosed orally or as injection of long-acting parenteral nanosuspension (LAP) [Abstract 1752]. 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 10–13 September 2013; Denver, CO. [Google Scholar]; This poster described the safety of cabotegravir, oral and injectable, across eight phase I studies.
- 13▪▪.Margolis DA, Brinson CC, Eron JJ, et al. 744 and rilpivirine as two drug oral maintenance therapy: LAI116482 (LATTE) week 48 results [Abstract 91 LB]. 21st Conference on Retroviruses and Opportunistic Infections; 3–6 March 2014; Boston, MA Provided proof-of-concept for the oral two drug regimen of cabotegravir and rilpivirine for the maintenance of virologic supression following triple drug induction therapy. [Google Scholar]; The proportion of participants achieving HIV-1 RNA less than 50 copies/ml at Week 48 was comparable with standard of care efavirenz plus two nucleoside reverse transcriptase inhibitors.
- 14.Ford SL, Chiu J, Lovern M, et al. Population PK approach to predict cabotegravir (CAB, GSK1265744) long-acting injectable doses for phase 2b [Abstract H-645]. 54th Interscience Conference on Antimicrobial Agents and Chemotherapy; 5–9 September 2014; Washington, DC, USA. [Google Scholar]
- 15.Bruno CJ, Jacobson JM. Ibalizumab: an anti-CD4 monoclonal antibody for the treatment of HIV-1 infection. J Antimicrob Chemother 2010; 65:1839–1841. [DOI] [PubMed] [Google Scholar]
- 16.Gautam N, Roy U, Balkundi S, et al. Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy. Antimicrob Agents Chemother 2013; 57:3110–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams J, Sayles HR, Meza JL, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine 2013; 8:1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]


