Abstract
Objectives:
Pain catastrophizing has been associated with higher pain intensity, increased risk of developing chronic pain and poorer outcomes after treatment. Despite this, the mechanisms by which pain catastrophizing influences pain remain poorly understood. It has been hypothesized that pain catastrophizing may impair descending inhibition of spinal level nociception. The aims of this study were to compare spinal nociceptive processing in people with chronic widespread pain and pain-free controls and examine potential relationships between measures of pain catastrophizing and spinal nociception.
Materials and Methods:
Twenty-six patients with chronic widespread pain and 22 pain-free individuals participated in this study. Spinal nociception was measured using the nociceptive flexion reflex (NFR) threshold and NFR inhibition, measured as the change in NFR area during exposure to a second, painful conditioning stimulus (cold water immersion). Pain catastrophizing was assessed using the Pain Catastrophizing Scale and a situational pain catastrophizing scale.
Results:
Compared with pain-free controls, patients with chronic widespread pain had higher pain catastrophizing scores and lower NFR thresholds. Although NFR area was reduced by a painful conditioning stimulus in controls, this was not apparent in individuals with chronic widespread pain. No significant correlations were observed between measures of pain catastrophizing and spinal nociception.
Discussion:
Despite increased excitability and decreased inhibition of spinal nociception in patients with chronic widespread pain, we could find no evidence of a significant relationship between pain catastrophizing and measures of spinal nociceptive processing.
Key Words: nociceptive flexion reflex, pain catastrophizing, chronic widespread pain
Chronic widespread pain (CWP), defined as pain for at least 3 months affecting the trunk, upper and lower quadrants and both sides of the body,1 has an estimated prevalence of 7% to 13%2 and is considered to be the cardinal symptom of fibromyalgia (FM). There is considerable overlap in the clinical features of fibromyalgia and CWP, such as the presence of fatigue, sleep disturbances, depression, and cognitive impairments,3,4 and it has been suggested that these conditions may fit on a clinical continuum.5
CWP may be associated with abnormal nociceptive system processing characterized by central sensitization and signs of allodynia and hyperalgesia.6,7 In support of this, people with FM have reduced nociceptive flexion reflex (NFR) thresholds,8,9 heightened temporal summation of pain,10 and prolonged after sensations following repetitive painful stimuli.10 Deficient endogenous pain inhibition has also been implicated in chronic widespread pain and FM,11,12 with several studies reporting impaired conditioned pain modulation (CPM) efficacy13–15 and reduced concentrations of key neurotransmitters involved in descending inhibitory pathways, including serotonin, norepinephrine, and dopamine.16,17
Psychological factors are known to play an important role in the pain experience. Pain catastrophizing, which involves magnification, helplessness, and rumination about pain, is associated with higher pain intensity in fibromyalgia18,19 and other chronic pain conditions. The long-term consequences of pain catastrophizing include a greater risk for the development and worsening of chronic pain.20–22 Furthermore, it has been shown that reductions in pain catastrophizing during psychologically based pain treatments may mediate positive outcomes23–25 and that early changes in pain catastrophizing during multidisciplinary pain treatment are linked with later improvements in pain intensity.26,27 More recently, situational measures of catastrophizing have been developed, with these also found to correlate with measures of pain sensitivity and clinical pain intensity.28–30
It is possible that pain catastrophizing has a direct effect on descending inhibitory pathways, as areas of the brain mediating attention and pain affect are part of a network involved in the descending modulation of spinal nociception.31–34 In this regard, a recent study35 showed that a cognitive-behavioral therapy intervention reduced secondary hyperalgesia, a biomarker of central sensitization. Of relevance, the amount that pain catastrophizing reduced with the intervention was associated with the size of the reduction in secondary hyperalgesia. Such a finding may reflect altered descending modulation of spinal nociceptive processing. Indeed, there is evidence in healthy controls that pain catastrophizing is associated with reduced CPM efficacy.36,37 However, in these studies, CPM efficacy was assessed as the modification of pain ratings of a test stimulus in response to a second, painful conditioning stimulus. As the CPM induced change in test pain ratings is thought to reflect both cortico-cortical inhibition31,32 and descending inhibition of spinal nociception,38 it is difficult to determine from these studies whether pain catastrophizing alters nociceptive processing at a spinal and/or supraspinal level. The NFR provides an objective measure of spinal nociceptive processing.39 As such, the NFR can be used to directly examine the effect of descending pathways on spinal nociception. Several studies in healthy40,41 and other chronic pain populations42,43 have thus far failed to show a significant association between measures of pain catastrophizing and NFR threshold, a static measure of excitability in spinal nociceptive pathways. Alternatively, the NFR can be utilized within a CPM paradigm to examine the dynamic modulation of spinal nociceptive processing by endogenous pain inhibitory and/or facilitatory pathways. This is commonly achieved by examining changes in the size of the average NFR electromyography (EMG) response with (conditioned) and without (test) a painful conditioning stimulus such as immersion of the hand in cold water. Using such an approach, studies44,45 have observed impaired descending inhibition of spinal nociceptive processing in chronic pain populations, compared with pain-free controls. Given the consistent relationships observed between pain catastrophizing, pain intensity, and pain-related treatment outcomes, it may be of interest to determine if pain catastrophizing is associated with descending modulation of the NFR, particularly in a population with chronic pain. Thus, the aims of the present study were to compare NFR threshold and descending modulation of NFR area in healthy, pain-free controls and individuals with CWP and to explore the possible relationships between these measures and pain catastrophizing. Our hypotheses were that: (1) NFR threshold would be lower in people with chronic widespread pain compared with controls; (2) NFR area would be significantly reduced by a painful conditioning stimulus in controls, but not in people with chronic widespread pain; and (3) there would be a significant association between measures of pain catastrophizing and descending modulation of the NFR, such that greater catastrophizing would be associated with less inhibition of the NFR area.
MATERIALS AND METHODS
Participants
Twenty-six people with CWP were recruited from a hospital based multidisciplinary pain service, volunteering to participate in the study (Table 1). A sample size of 26 allowed 80% power to detect a clinically relevant association of r=0.48 between pain catastrophizing and NFR inhibition at an alpha level of 0.05. Before volunteering for the study, all participants in the CWP group were assessed and diagnosed by a physician with specialist qualifications in pain medicine. To be eligible, participants had to meet the American College of Rheumatology’s 1990 diagnostic criteria for CWP, requiring axial pain in addition to pain in the upper and lower quadrants and right and left sides of the body for at least 3 months.1 A control group of 22 pain-free participants of a similar age and sex balance was also recruited from flyers on a local university notice board.
TABLE 1.
Participant Demographic and Clinical Characteristics
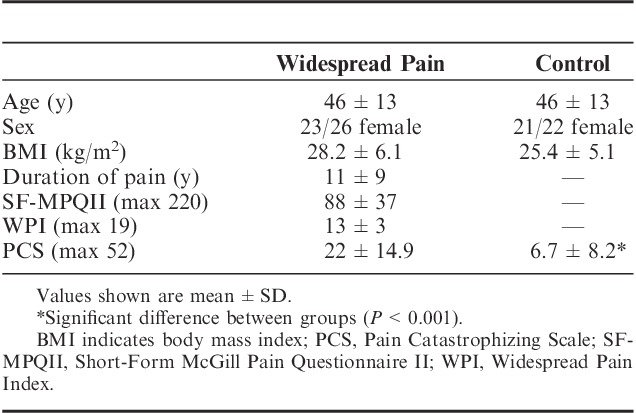
All participants had to be a fluent English speaker and reader. Participants were excluded if they were younger than 18; had a history of major psychiatric illness (eg, schizophrenia, bipolar disorder); had a known medical condition that could account for the widespread nature of their pain (eg, rheumatoid arthritis, cancer); had a documented loss of normal sensory perception or a neurological condition that could interfere with quantitative sensory testing procedures (eg, polyneuropathy); were currently pregnant; or had any condition that may prevent weight-bearing on 1 leg to collect NFR responses.
Before testing, all participants were asked to refrain from taking analgesics for 24 hours and to abstain from caffeine, nicotine, alcohol, and strenuous exercise for at least 4 hours. Female participants were tested between days 17 and 28 of the menstrual cycle to reduce the potential effects of hormonal fluctuations on the NFR.46 All study procedures were approved by the local Ethics Committee and informed consent was gained before initiation of study procedures.
Procedures
All participants attended a single test session lasting 60 to 90 minutes. Participants first completed questionnaire data, including the Short-Form McGill Pain Questionnaire (SF-MPQII), the Widespread Pain Index, and the Pain Catastrophizing Scale (PCS). The SF-MPQII consists of 22 common words used to describe pain and related symptoms and asks participants to rate each of these on a numerical rating scale from 0 (none) to 10 (worst possible) in relation to their own pain experience.47 The Widespread Pain Index is calculated by dividing a body chart into 19 predefined regions and counting how many of these regions are (partially or completely) shaded in when the participant completes a pain drawing.48 The PCS is a 13-item self-report questionnaire which asks participants to rate the extent to which they agree with statements regarding their thoughts and feelings during previous painful experiences.49 Following completion of the questionnaires, NFR responses were collected, as outlined below. Technical difficulties meant that measures of NFR inhibition were only possible in 22 of the 26 participants with CWP. However, NFR threshold was recorded for all 26 participants. All NFR measures were completed in the control participants. Following the NFR measures, situational pain catastrophizing was assessed using the 6-item “in vivo/situational” version of the PCS.28 Participants were asked to answer the questions in relation to their thoughts and feelings during the painful testing procedures they had just experienced.28
NFR Threshold
The NFR was elicited by electrocutaneously stimulating the plantar aspect of the foot of the dominant leg.50 Responses were recorded in the biceps femoris muscle using surface EMG. All EMG recordings were amplified (×1000), filtered (10 to 1000 Hz; AMT-8 amplifier; Bortec Biomedical, Alberta, Canada), and sampled at 2000 Hz (Micro 1401; Cambridge Electronic Design, Cambridge, UK). Following skin preparation, the stimulating electrode (D.O. Weaver & Co., Colorado) was placed 2 cm proximal to the joint line of the first metatarsophalangeal joint, with the anode positioned distally. Each stimulation consisted of a train of 5 rectangular pulses (perceived as a single stimulus) of 1 ms pulse width with a 3 ms interpulse interval (17 ms total duration) delivered using a constant current electrical stimulator (Digitimer DS7A, Hertfordshire, UK). To reduce habituation and/or predictability, a random interval of 8 to 12 seconds separated each stimulus. A bipolar (AgCl) recording electrode (Norotrode) was placed over the biceps femoris, 10 cm proximal to the popliteal crease, with a ground electrode placed on the proximal tibia. All testing was performed with the participant standing on a 26 cm box on the nondominant leg, enabling the dominant leg to hang freely. A handrail was provided for support. Quiescence in the biceps femoris muscle of the dominant leg was ensured by continuous monitoring of the real-time EMG signal on an oscilloscope (Textronix TDS2014B, Beaverton, Oregon).50 This method of eliciting the NFR has been shown to be reliable as well as more comfortable for participants when compared with traditional methods involving sural nerve stimulation in a seated position.50
To allow participants to be familiarized with the procedures, 20 electrocutaneous stimuli of random intensity were first delivered to the foot. The NFR threshold was then determined using an established staircase method and a Z-score of≥10.32 to determine whether a true reflex was present or not, using the following formula: Z-score=(NFR interval peak EMG–baseline EMG mean)/baseline EMG SD.51 The NFR interval was defined as the period 85 to 150 ms after electrocutaneous stimulation, while baseline EMG activity was measured in a period 70 to 5 ms before stimulation. Stimulation intensity was increased in increments of 4 mA until a NFR was detected, then decreased in 2 mA increments until a reflex was no longer present. This procedure was repeated using 1 mA increments until the reflex appeared and disappeared 2 further times. The mean of the final 4 stimulation intensities was then determined and a further 4 stimuli were delivered at this intensity. If 3 of 4 stimuli elicited a NFR, this intensity was considered to be the NFR threshold. If fewer than 3 of 4 stimuli elicited a NFR, the intensity was increased in 1 mA increments until 3 or more NFR responses were elicited, which was then taken as NFR threshold.52
Descending Modulation of the NFR
Descending modulation of the NFR was then evaluated by collecting 2 sets of NFR responses, 10 at baseline (test responses), and 10 during painful cold water immersion of the contralateral hand (conditioned responses). Each of the 10 stimuli was separated by a randomized interstimulus interval of 4 to 8 seconds. A stimulation intensity of 120% to 200% of NFR threshold was used depending on participant tolerance and to ensure a reflex response was consistently elicited. For each participant, the stimulation intensity was kept the same for the test and conditioned responses. After the test responses were collected, a 2-minute break was given, before participants underwent cold water immersion by submerging the contralateral hand up to the wrist crease in circulating 10°C water. After 1-minute of submersion, a set of 10 conditioned responses were collected using identical stimulation parameters. At the conclusion of the conditioned responses, participants were asked to remove their hand from the cold water and rate the maximum pain associated with the cold water immersion on a scale from 0=no pain to 100=maximum tolerable cold pain. If participants could not tolerate the cold water immersion for the full time to complete the 10 stimuli, they were allowed to withdraw their hand and a hand pain rating of 100 was allocated, as this represented their maximum tolerable cold pain. Where this occurred, the second set of (conditioned) NFR responses were still collected and were completed within a maximum of 90 seconds after hand withdrawal. To calculate NFR area, the signals were first rectified and averaged after the removal of any frames that contained background muscle activity before stimulation (<5% of responses). The area of the NFR was measured separately for the test and conditioned responses using the averaged, rectified EMG responses in a period 85 to 150 ms after stimulation.
Statistical Analysis
Shapiro Wilk tests were undertaken to examine the normality of the distribution for all variables of interest, and thus the suitability of parametric analysis. All variables of interest apart from NFR threshold were non-normally distributed. As such, between group comparison of NFR threshold was made using an independent sample t-test while between group comparisons of pain catastrophizing, situational pain catastrophizing, peak pain rating of the conditioning stimulus (cold water immersion) and NFR area stimulus (% threshold) were made using the Mann-Whitney U-test. For each group, differences in NFR area between the test and conditioned responses were examined using the Wilcoxon signed-rank tests. For both the CWP group and the control group, a Spearman rank correlation coefficient was used to examine the relationships between (1) pain catastrophizing and NFR threshold; (2) pain catastrophizing and NFR modulation (percent change in NFR area between test and conditioned responses); (3) situational pain catastrophizing and NFR threshold; and (4) situational pain catastrophizing and NFR modulation (percent change in NFR area between test and conditioned responses). Statistical significance was set at P<0.05. Results are reported as mean±SD. Effect size estimates were calculated using the formula: mean 1−mean 2/pooled SD.53 The strength of the effect can be interpreted as 0.2 to 0.49=small; 0.5 to 0.79=medium; ≥0.8=large).53
RESULTS
Table 1 shows summary demographic and clinical information for the 2 groups. The CWP group demonstrated significantly higher pain catastrophizing on the PCS (Z=4.09; P<0.001; effect size=1.39). No significant between group difference was found for situational pain catastrophizing (Z=1.19; P>0.05; effect size=0.44).
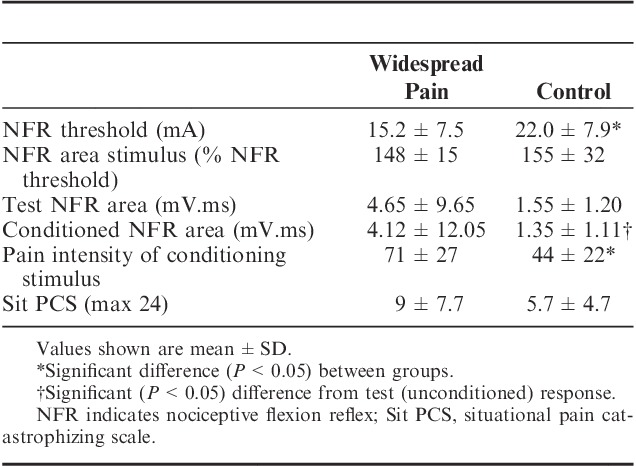
NFR data for the 2 groups are shown in Table 2. The CWP group displayed significantly lower NFR thresholds than the control group (t=−3.06; P<0.01; effect size=0.88). There was no significant difference in the test stimulus intensity (% of NFR threshold) used to elicit NFR area between groups (Z=−0.94; P>0.05). The control group had a significantly smaller NFR area (Z=−3.30; P<0.001; effect size=−0.17) during the conditioned responses compared with the test responses. In contrast, NFR area (Z=−1.64; P>0.05; effect size=−0.05) did not change during the conditioned responses in the CWP group. The peak pain induced by the conditioning stimulus was significantly higher in the CWP group than in the control group (Z=−3.29; P=0.01, effect size=0.83). The conditioning stimulus was unable to be tolerated for the full amount of time in 5% of the control group and 32% of the CWP group.
TABLE 2.
Nociceptive Flexion Reflex and Situational Pain Catastrophizing Summary Data for Chronic Widespread Pain and Control Groups
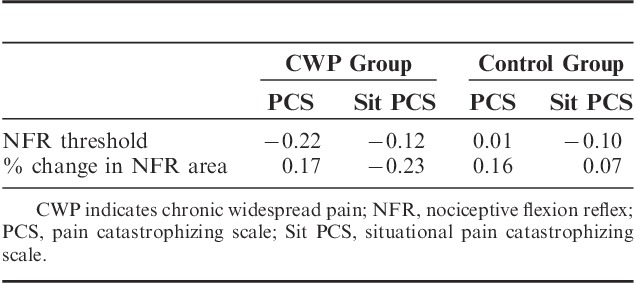
The Spearman rho data for the CWP and control groups are presented separately in Table 3. There were no significant correlations between measures of pain catastrophizing and spinal nociceptive processing in either the CWP group or control group (all P>0.05).
TABLE 3.
Spearman’s Rho Values Between Pain Catastrophizing Scale Scores and Measures of Spinal Nociceptive Processing in Individuals With Chronic Widespread Pain and a Pain-Free Control Group
DISCUSSION
An important finding of this study was that the NFR area was not inhibited by a painful conditioning stimulus in individuals with CWP. In contrast, significant inhibition of the NFR area was observed in the pain-free control group. This supports several other studies that have shown impaired CPM efficacy in CWP and/or FM,13–15,54 thought to at least partly reflect a dysfunction in the normal descending modulation of spinal nociception. In contrast to previous work, the current study used the NFR to directly examine descending modulation of spinal nociception in people with CWP. As such, the lack of NFR inhibition with a painful conditioning stimulus provides direct evidence of impaired descending inhibition and/or enhanced descending facilitation of spinal level nociceptive processing in this population.
The NFR threshold was also significantly lower in individuals with CWP compared with the control group, providing further evidence of altered nociceptive processing. This finding supports recent studies which have demonstrated consistently lower NFR thresholds in fibromyalgia and other chronic pain conditions compared with healthy pain-free populations.55,56 As wide dynamic range neurons are thought to play a critical role in mediating the NFR response,57 a reduced NFR threshold in the CWP group may indicate heightened spinal nociceptive sensitivity due to central sensitization of wide dynamic range neurons within the dorsal horn.
No other studies have specifically explored the relationships between pain catastrophizing and measures of spinal nociceptive processing in individuals with CWP. Our findings provide no evidence of an important relationship between measures of pain catastrophizing and measures of spinal nociceptive processing in this population, or in pain-free controls. This was evident using both standard and situational measures of pain catastrophizing. It has been shown that CPM is influenced by expectations38,39 and can be modified by verbal suggestion,58 demonstrating that cognitive factors can alter the efficacy of endogenous pain modulatory pathways. However, findings from studies relating neurophysiological measures of nociceptive system function to psychological factors such as catastrophizing are inconsistent. Our findings support several other studies that have shown no relationship among these factors in people with other chronic pain conditions56,59–63 or in healthy controls.64–66 In contrast, some studies have reported that pain catastrophizing is related to various measures of endogenous pain inhibition or facilitation.44,63,67–69 Typically, these studies have found that greater pain catastrophizing is associated with enhanced facilitation of pain and/or impaired inhibition. It is possible that discrepancies among study protocols may account for some of the different outcomes. Different measures, including those relying solely on pain report, as well as different forms of conditioning and test stimuli (eg, mechanical, electrical, thermal) have been used among the studies, which likely influences the precise component of the nociceptive system under study. The chronic pain population studied may also influence findings. For example, Hassett et al19 found that catastrophizing had a greater influence on pain intensity in women with FM than in women with rheumatoid arthritis, suggesting that the influence of catastrophizing may be different among pain conditions.
The findings of the current study suggest that catastrophizing may primarily affect nociceptive processing at a supraspinal, rather than a spinal level CWP. In support of this, Ruscheweyh et al70 recently examined the effect of catastrophizing statements on nociceptive NFR area and temporal summation of pain in healthy, pain-free individuals. Catastrophizing increased both pain ratings and NFR area but the increase in pain ratings was much greater, leading the authors to suggest that catastrophizing predominantly acted at a supraspinal level to modify nociceptive processing, a finding supported by other observations.64–66 Taken together, these findings suggest that factors other than pain catastrophizing may contribute to increased excitability and impaired descending modulation of spinal nociception. Furthermore, treatments aimed at modifying pain catastrophizing (eg, cognitive-behavioral therapy) are less likely to significantly alter spinal nociceptive processing, as has recently been demonstrated in healthy controls.41 As such, additional interventions (eg, antidepressant medications) may be needed to effectively reduce sensitization of spinal nociceptive pathways and maximize pain relief. To further enhance our understanding of the mechanisms by which catastrophizing affects the pain experience, future research should utilize longitudinal study designs to examine the effects of a clinical intervention aimed at reducing pain catastrophizing on measures of spinal and supraspinal nociceptive processing in chronic pain populations.
A limitation of the current study is that we did not measure participants’ expectation regarding the effects of the conditioning stimulus on NFR area. Previous studies have shown that verbally induced expectations of analgesia or hyperalgesia can modify the effects of the conditioning stimulus, even at a spinal level.38,71 In contrast, when no attempt is made to manipulate participant expectation before applying the conditioning stimulus, other studies72,73 have found no association between a priori expectation and the analgesic effects associated with CPM. Future studies may wish to examine the role of conditioning stimulus expectation and whether this may partially explain the deficient CPM response that is often observed in chronic pain populations.74 Another possible limitation of the current study is that the peak pain associated with the conditioning stimulus was not matched between the 2 groups, with the CWP group reporting higher intensity pain in response to cold water immersion of the hand at 10°C. This is unlikely to have influenced the results markedly as several studies have shown no association between conditioning pain intensity and the extent of CPM.75–77 Moreover, one might expect that the higher cold pain intensity reported in the CWP group may lead to stronger inhibition of the NFR but in fact, we observed the opposite, with no significant inhibition of NFR area in the CWP group and an intact inhibitory response in controls. Finally, the conditioning stimulus was unable to be tolerated for the full amount of time by 5% of the control group and 32% of the CWP group. When this occurred, the second set of NFR responses were still collected and completed within a maximum time of 90 seconds after hand withdrawal. As the inhibitory response is known to persist for several minutes after the conditioning stimulus has ceased,77–79 we do not anticipate that this had an important bearing on our results.
CONCLUSIONS
Compared with pain-free control participants, we observed a lower NFR threshold and reduced descending inhibition of the NFR in people with CWP. However, we could not demonstrate a significant relationship between measures of spinal nociceptive processing and pain catastrophizing in these participants, providing further evidence that pain catastrophizing may largely modify nociceptive processing at a supraspinal rather than a spinal level, even in individuals with chronic pain.
Footnotes
Supported by Awhina Contestable Research Fund, Waitemata District Health Board, Auckland, New Zealand. The authors declare no conflict of interest.
REFERENCES
- 1.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
- 2.Neumann L, Buskila D. Epidemiology of fibromyalgia. Curr Pain Headache Rep. 2003;7:362–368. [DOI] [PubMed] [Google Scholar]
- 3.Arnold LM, Crofford LJ, Mease PJ, et al. Patient perspectives on the impact of fibromyalgia. Patient Educ Couns. 2008;73:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe F, Clauw DJ, Fitzcharles M-A, et al. The American College of Rheumatology Preliminary Diagnostic Criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610. [DOI] [PubMed] [Google Scholar]
- 5.Toda K. Comparison of symptoms among fibromyalgia syndrome, chronic widespread pain, and an incomplete form of chronic widespread pain. J Musculoskelet Pain. 2011;19:52–55. [Google Scholar]
- 6.Clauw DJ, Arnold LM, McCarberg BH. The science of fibromyalgia. Mayo Clin Proc. 2011;86:907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersel DL, Dror V, Cheung R. Central amplification and fibromyalgia: disorder of pain processing. J Neurosci Res. 2011;89:29–34. [DOI] [PubMed] [Google Scholar]
- 8.Banic B, Petersen-Felix S, Andersen OK, et al. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004;107:7–15. [DOI] [PubMed] [Google Scholar]
- 9.Desmeules JA, Cedraschi C, Rapiti E, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420–1429. [DOI] [PubMed] [Google Scholar]
- 10.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julien N, Goffaux P, Arsenault P, et al. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295–302. [DOI] [PubMed] [Google Scholar]
- 12.Lannersten L, Kosek E. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain. 2010;151:77–86. [DOI] [PubMed] [Google Scholar]
- 13.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. [DOI] [PubMed] [Google Scholar]
- 14.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. [DOI] [PubMed] [Google Scholar]
- 15.Potvin S, Larouche A, Normand E, et al. DRD3 Ser9Gly polymorphism is related to thermal pain perception and modulation in chronic widespread pain patients and healthy controls. J Pain. 2009;10:969–975. [DOI] [PubMed] [Google Scholar]
- 16.Russell IJ, Vaeroy H, Javors M, et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–556. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe F, Russell IJ, Vipraio G, et al. Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J Rheumatol. 1997;24:555–559. [PubMed] [Google Scholar]
- 18.Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. [DOI] [PubMed] [Google Scholar]
- 19.Hassett AL, Cone JD, Patella SJ, et al. The role of catastrophizing in the pain and depression of women with fibromyalgia syndrome. Arthritis Rheum. 2000;43:2493–2500. [DOI] [PubMed] [Google Scholar]
- 20.Haythornthwaite JA, Clark MR, Pappagallo M, et al. Pain coping strategies play a role in the persistence of pain in post-herpetic neuralgia. Pain. 2003;106:453–460. [DOI] [PubMed] [Google Scholar]
- 21.Picavet HS, Vlaeyen JW, Schouten JS. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156:1028–1034. [DOI] [PubMed] [Google Scholar]
- 22.Forsythe ME, Dunbar MJ, Hennigar AW, et al. Prospective relation between catastrophizing and residual pain following knee arthroplasty: two-year follow-up. Pain Res Manag. 2008;13:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moseley GL. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur J Pain. 2004;8:39–45. [DOI] [PubMed] [Google Scholar]
- 24.Smeets RJEM, Vlaeyen JWS, Kester ADM, et al. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. [DOI] [PubMed] [Google Scholar]
- 25.Vowles KE, McCracken LM, Eccleston C. Processes of change in treatment for chronic pain: the contributions of pain, acceptance, and catastrophizing. Eur J Pain. 2007;11:779–787. [DOI] [PubMed] [Google Scholar]
- 26.Burns JW, Glenn B, Bruehl S, et al. Cognitive factors influence outcome following multidisciplinary chronic pain treatment: a replication and extension of a cross-lagged panel analysis. Behav Res Ther. 2003;41:1163–1182. [DOI] [PubMed] [Google Scholar]
- 27.Burns JW, Kubilus A, Bruehl S, et al. Do changes in cognitive factors influence outcome following multidisciplinary treatment for chronic pain? A cross-lagged panel analysis. J Consult Clin Psychol. 2003;71:81–91. [DOI] [PubMed] [Google Scholar]
- 28.Campbell CM, Kronfli T, Buenaver LF, et al. Situational versus dispositional measurement of catastrophizing: associations with pain responses in multiple samples. J Pain. 2010;11:443–453.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards RR, Campbell CM, Fillingim RB. Catastrophizing and experimental pain sensitivity: only in vivo reports of catastrophic cognitions correlate with pain responses. J Pain. 2005;6:338–339. [DOI] [PubMed] [Google Scholar]
- 30.Grosen K, Vase L, Pilegaard HK, et al. Conditioned pain modulation and situational pain catastrophizing as preoperative predictors of pain following chest wall surgery: a prospective observational cohort study. PloS One. 2014;9:e90185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moont R, Crispel Y, Lev R, et al. Temporal changes in cortical activation during conditioned pain modulation (CPM), a LORETA study. Pain. 2011;152:1469–1477. [DOI] [PubMed] [Google Scholar]
- 32.Piche M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. J Neurosci. 2009;29:14236–14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprenger C, Bingel U, Büchel C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain. 2011;152:428–439. [DOI] [PubMed] [Google Scholar]
- 34.Wilder-Smith CH, Schindler D, Lovblad K, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salomons TV, Moayedi M, Erpelding N, et al. A brief cognitive-behavioural intervention for pain reduces secondary hyperalgesia. Pain. 2014;155:1446–1452. [DOI] [PubMed] [Google Scholar]
- 36.Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res. 2008;186:79–85. [DOI] [PubMed] [Google Scholar]
- 37.Goodin BR, McGuire L, Allshouse M, et al. Associations between catastrophizing and endogenous pain-inhibitory processes: sex differences. J Pain. 2009;10:180–190. [DOI] [PubMed] [Google Scholar]
- 38.Goffaux P, Redmond WJ, Rainville P, et al. Descending analgesia—when the spine echoes what the brain expects. Pain. 2007;130:137–143. [DOI] [PubMed] [Google Scholar]
- 39.Goffaux P, de Souza JB, Potvin S, et al. Pain relief through expectation supersedes descending inhibitory deficits in fibromyalgia patients. Pain. 2009;145:18–23. [DOI] [PubMed] [Google Scholar]
- 40.France CR, France JL, Absi M, et al. Catastrophizing is related to pain ratings, but not nociceptive flexion reflex threshold. Pain. 2002;99:459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terry EL, Thompson KA, Rhudy JL. Experimental reduction of pain catastrophizing modulates pain report but not spinal nociception as verified by mediation analyses. Pain. 2015;156:1477–1488. [DOI] [PubMed] [Google Scholar]
- 42.Emery CF, Keefe FJ, France CR, et al. Effects of a brief coping skills training intervention on nociceptive flexion reflex threshold in patients having osteoarthritic knee pain: a preliminary laboratory study of sex differences. J Pain Symptom Manage. 2006;31:262–269. [DOI] [PubMed] [Google Scholar]
- 43.France CR, Keefe FJ, Emery CF, et al. Laboratory pain perception and clinical pain in post-menopausal women and age-matched men with osteoarthritis: relationship to pain coping and hormonal status. Pain. 2004;112:274–281. [DOI] [PubMed] [Google Scholar]
- 44.Piche M, Bouin M, Arsenault M, et al. Decreased pain inhibition in irritable bowel syndrome depends on altered descending modulation and higher-order brain processes. Neuroscience. 2011;195:166–175. [DOI] [PubMed] [Google Scholar]
- 45.Sandrini G, Rossi P, Milanov I, et al. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia. 2006;26:782–789. [DOI] [PubMed] [Google Scholar]
- 46.Tousignant-Laflamme Y, Marchand S. Excitatory and inhibitory pain mechanisms during the menstrual cycle in healthy women. Pain. 2009;146:47–55. [DOI] [PubMed] [Google Scholar]
- 47.Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-Form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35–42. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–610. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 50.Lewis GN, Rice DA, Jourdain K, et al. Influence of stimulation location and posture on the reliability and comfort of the nociceptive flexion reflex. Pain Res Manag. 2012;17:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: a comparison of different scoring criteria. Pain. 2007;128:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Micalos PS, Drinkwater EJ, Cannon J, et al. Reliability of the nociceptive flexor reflex (RIII) threshold and association with pain threshold. Eur J Appl Physiol. 2009;105:55–62. [DOI] [PubMed] [Google Scholar]
- 53.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 54.Gormsen L, Bach FW, Rosenberg R, et al. Differential pain modulation in patients with peripheral neuropathic pain and fibromyalgia. Scand J Pain. 2012;3:116–123. [DOI] [PubMed] [Google Scholar]
- 55.Lim ECW, Sterling M, Stone A, et al. Central hyperexcitability as measured with nociceptive flexor reflex threshold in chronic musculoskeletal pain: a systematic review. Pain. 2011;152:1811–1820. [DOI] [PubMed] [Google Scholar]
- 56.Curatolo M, Müller M, Ashraf A, et al. Pain hypersensitivity and spinal nociceptive hypersensitivity in chronic pain: prevalence and associated factors. Pain. 2015;156:2373–2382. [DOI] [PubMed] [Google Scholar]
- 57.You H-J, Morch CD, Chen J, et al. Simultaneous recordings of wind-up of paired spinal dorsal horn nociceptive neuron and nociceptive flexion reflex in rats. Brain Res. 2003;960:235–245. [DOI] [PubMed] [Google Scholar]
- 58.Lewis GN, Leys A, Rice DA, et al. Subconscious manipulation of pain expectation can modulate cortical nociceptive processing. Pain Pract. 2015;15:117–123. [DOI] [PubMed] [Google Scholar]
- 59.Lee YC, Lu B, Edwards RR, et al. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum. 2013;65:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edwards RR, Mensing G, Cahalan C, et al. Alteration in pain modulation in women with persistent pain after lumpectomy: influence of catastrophizing. J Pain Symptom Manage. 2013;46:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johannesson U, de Boussard CN, Brodda Jansen G, et al. Evidence of diffuse noxious inhibitory controls (DNIC) elicited by cold noxious stimulation in patients with provoked vestibulodynia. Pain. 2007;130:31–39. [DOI] [PubMed] [Google Scholar]
- 62.Daenen L, Nijs J, Roussel N, et al. Dysfunctional pain inhibition in patients with chronic whiplash-associated disorders: an experimental study. Clin Rheumatol. 2013;32:23–31. [DOI] [PubMed] [Google Scholar]
- 63.Sterling M, Hodkinson E, Pettiford C, et al. Psychologic factors are related to some sensory pain thresholds but not nociceptive flexion reflex threshold in chronic whiplash. Clin J Pain. 2008;24:124–130. [DOI] [PubMed] [Google Scholar]
- 64.Rhudy JL, France CR, Bartley EJ, et al. Does pain catastrophizing moderate the relationship between spinal nociceptive processes and pain sensitivity? J Pain. 2009;10:860–869. [DOI] [PubMed] [Google Scholar]
- 65.Rhudy JL, Martin SL, Terry EL, et al. Pain catastrophizing is related to temporal summation of pain but not temporal summation of the nociceptive flexion reflex. Pain. 2011;142:794–801. [DOI] [PubMed] [Google Scholar]
- 66.Rhudy JL, Maynard LJ, Russell JL. Does in vivo catastrophizing engage descending modulation of spinal nociception? J Pain. 2007;8:325–333. [DOI] [PubMed] [Google Scholar]
- 67.Goodin BR, Glover TL, Sotolongo A, et al. The association of greater dispositional optimism with less endogenous pain facilitation is indirectly transmitted through lower levels of pain catastrophizing. J Pain. 2013;14:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geisser ME, Casey KL, Brucksch CB, et al. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: association with mood, somatic focus, and catastrophizing. Pain. 2003;102:243–250. [DOI] [PubMed] [Google Scholar]
- 69.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Sex and pain-related psychological variables are associated with thermal pain sensitivity for patients with chronic low back pain. J Pain. 2007;8:2–10. [DOI] [PubMed] [Google Scholar]
- 70.Ruscheweyh R, Albers C, Kreusch A, et al. The effect of catastrophizing self-statements on pain perception and the nociceptive flexor reflex (RIII reflex). Clin J Pain. 2013;29:725–732. [DOI] [PubMed] [Google Scholar]
- 71.Cormier S, Piché M, Rainville P. Expectations modulate heterotopic noxious counter-stimulation analgesia. J Pain. 2013;14:114–125. [DOI] [PubMed] [Google Scholar]
- 72.Grashorn W, Sprenger C, Forkmann K, et al. Age-dependent decline of endogenous pain control: exploring the effect of expectation and depression. PloS One. 2013;8:e75629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lariviere M, Goffaux P, Marchand S, et al. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23:506–510. [DOI] [PubMed] [Google Scholar]
- 74.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13:936–944. [DOI] [PubMed] [Google Scholar]
- 75.Granot M, Weissman-Fogel I, Crispel Y, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2008;136:142–149. [DOI] [PubMed] [Google Scholar]
- 76.Nir R-R, Granovsky Y, Yarnitsky D, et al. A psychophysical study of endogenous analgesia: the role of the conditioning pain in the induction and magnitude of conditioned pain modulation. Eur J Pain. 2011;15:491–497. [DOI] [PubMed] [Google Scholar]
- 77.Lewis GN, Heales L, Rice DA, et al. The reliability of the conditioned pain modulation paradigm to assess endogenous inhibitory pain pathways. Pain Res Manag. 2012;17:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roby-Brami A, Bussel B, Willer J, et al. An electrophysiological investigation into the pain-relieving effects of heterotopic nociceptive stimuli. Brain. 1987;110:1497–1508. [DOI] [PubMed] [Google Scholar]
- 79.France CR, Suchowiecki S. Assessing supraspinal modulation of pain perception in individuals at risk for hypertension. Psychophysiology. 2001;38:107–113. [PubMed] [Google Scholar]


