Abstract
The blue lotus flower (Nymphea caerulea) is an Egyptian water lily containing apomorphine and nuciferine. Apomorphine has been described as a psychoactive alkaloid and is a non-selective dopamine agonist primarily used to treat Parkinson’s disease as it stimulates dopamine receptors and improves motor function. Nuciferine is an alkaloid associated with dopamine receptor blockade. Today, blue lotus flower is used as a sleep aid and anxiety reliever. The rebuildable dripping atomizer (RDA) is an electronic cigarette that allows direct application of an e-liquid onto the coil in the atomizer for aerosolization, compared to a typical electronic cigarette where the e-liquid is wicked from a storage vessel to the coil. Our laboratory received a dark-brown resin material from a concerned parent. The resin had been confiscated from an adolescent who had a reported history of marijuana use. The resin was later identified as blue lotus flower (N. caerulea). This resin, together with four commercially available blue lotus products, was analyzed for content. Apomorphine was detected in two samples, and nuciferine was detected in all five samples. The confiscated resin was determined to contain no apomorphine and 4300 ng/g of nuciferine. The nuciferine resin was shown to aerosolize using aRDA electric cigarette.
Keywords: Apomorphine, blue lotus flower, electronic cigarette, nuciferine
Nymphaea caerulea, popularly known as the blue lotus flower or the blue Egyptian lotus, is a water lily in the genus Nymphaea. Images of the blue lotus flower can be found on ancient Egyptian papyri and tomb depictions. It has been speculated that the flower was used in ancient Egyptian culture as part of healing and shamanistic rituals dating back to the fourteenth century B.C. (Emboden 1989). Today, blue lotus flower is used as a sleep aid and anxiety reliever, but has also been described as a mild stimulant. Blue lotus flower can be purchased primarily as tea extracts or incense. It is not a controlled substance and it is not approved for human consumption in the United States. The flower’s psychoactive effects are most often attributed to two alkaloids, apomorphine and nuciferine.
Apomorphine is a nonselective dopamine receptor agonist and activates serotonin receptors and α-adrenergic receptors (LeWitt 2004; Millan et al. 2002). It has been used as a sedative-hypnotic since the late 1800s to treat insomnia, depression, or schizophrenia (Ribarič 2012). It has been used in the treatment of erectile dysfunction (Gottlieb 2000) and was sold under the trade name Upriama and Ixense. In 1951, it was reported to successfully treat Parkinson’s disease at a subcutaneous dose of 0.5 to 1.0 mg (Schwab, Amador, and Lettvin 1951). It has also been used in the treatment of alcohol and morphine addiction (Ribarič 2012). In veterinary medicine, it has been used to induce vomiting (Scherkl, Hashem, and Frey 1990). It has also been suggested that apomorphine can play a role in the treatment of Alzheimer’s disease (Okun and Foote 2010; Ribarič 2012)
Nuciferine is an antagonist at 5-HT2A, 5-HT2C, and 5-HT2B, an inverse agonist at 5-HT7, a partial agonist at D2, D5 and 5-HT6, an agonist at 5-HT1A and D4 receptors, and inhibits the dopamine transporter (Farrell et al. 2016). Behavioral effects produced in rats include catalepsy, potentiation of hexobarbitone hypnosis, morphine analgesia, and anticonvulsant action (Bhattacharya et al. 1978). It has been suggested that nuciferine may have potential therapeutic applications as an anti-psychotic drug (Farrell et al. 2016) and on vascular diseases associated with aberrant vasoconstriction (Wang et al. 2015).
Presently, blue lotus flower extracts, resins, dried leaves, oils, powders, and electronic cigarette refill liquids (e-liquids) are readily available via the Internet. These products are sold as natural sedatives and/or aphrodisiacs. They are labeled as natural and are not approved by the Federal Drug Administration (FDA). The suggested means for consumption often include drinking in teas and dissolving in ethanol-containing beverages, since the alkaloids are not water soluble, or smoking or vaping in electronic cigarettes.
Electronic cigarettes, known as “personal vaporizers” or electronic nicotine delivery devices (ENDS), have experienced a significant increase in popularity. They work by either drawing negative pressure through the mouthpiece or depressing a button to activate a battery that heats a coil containing a wick saturated with a liquid refill formulation known as the e-liquid. These e-liquids are made of propylene glycol and/or vegetable glycerin containing active ingredient(s) such as a pharmaceutical agent and/or herbal remedy. When the electronic cigarette is activated, the e-liquid is vaporized, followed by rapid condensation into an aerosol that is inhaled by the user (Breland et al. 2016; Peace et al. 2016a).
The electronic cigarette industry has produced several generations of these systems. The first generation, the “ciga-like,” is a closed system that looks like a real cigarette. It is a simple design with a battery of single, low voltage, a coil, and a tank which is non-refillable. The second generation, a mid-size pen-like electronic cigarette, is more customizable than the ciga-likes. They have re-chargeable variable voltage batteries and can be re-filled with e-liquid. The third generation, called “advanced personal vaporizers” or APVs, has customizable coil and wick configurations and stronger batteries with adjustable settings and refillable e-liquid tanks.
In the last few years, the APVs have led to the development of the rebuildable dripping atomizer (RDA) or “dripper.” The RDA allows an electronic cigarette user to add e-liquid directly onto the atomizer and onto the coil and/or wicking material used to heat and aerosolize the e-liquid. Drippers are frequently used for illicit purposes, allowing the user to drip tinctures or plant materials directly onto the coil after removal of the mouthpiece (Figure 1). This method allows for more efficient vaporization of solid materials dissolved in a small amount of propylene glycol and/or glycerol. Further modification of the dripping technique allows for the placing of a wax or dab directly onto the hot coils and whiffing the aerosol through a straw.
Figure 1.

The plume veil 1.5 clone RDA and how it works.
Our laboratory received a confiscated dark-brown resin material from a concerned parent. The resin had been confiscated from an adolescent male who had a reported prior history of marijuana use. An aliquot of the sample was diluted 1:10 (w:v) with methanol and analyzed by immunoassays (Abbott Diagnostics, Abbott Park, IL) for amphetamines, barbiturates, benzodiazepines, cocaine metabolite, methadone, opiates, phencyclidine, propoxyphene, and tetrahydrocannabinol. The sample screened negative for all immunoassays. The sample was then screened by using Direct Analysis in Real Time-AccuTOF™ Mass Spectrometry (DART-MS) and confirmed by Gas Chromatography Mass Spectrometry (GC-MS) methods, resulting in the identification of nuciferine using a NIST 11.0 library mass spectrometry library. Subsequently, it was identified as blue lotus flower (N. caerulea) resin, used in a relatively new type of electronic cigarette known as a “dripper.”
We obtained, via the Internet, several blue lotus flower products including resins, a powder, and an e-liquid. The confiscated resin and the purchased products were analyzed using DART-MS and GC-MS for active ingredients/drugs. The samples were then quantitated by an UPLC-MS/MS method. An aerosol was then produced by a dripper electronic cigarette using the confiscated resin sample. This aerosol was analyzed by solid phase micro-extraction gas chromatography mass spectrometry (SPME-GC-MS).
Analysis of products
Materials
The four products, labeled “Blue Lotus Herbal E-liquid,” “Blue Lotus Flower Powder,” “Blue Lotus Resin Extract,” and “Space Lotus Resin Extract,” were purchased from www.lotus-extracts.com. The e-liquid was contained in a 10 mL plastic bottle, the resins were in solid blocks, and the powder was in a plastic bag labeled as 10 grams. These products were shipped from Thailand. The e-liquid was labled with the batch number 18–42369 and a best-used-before date of 12/2018. The other products were labeled with batch numbers: Blue Lotus Flower Powder, 16–42356; Blue Lotus Resin Extract, 16–42371; and Space Lotus Resin, 15–42121 (Figure 2). Two flyers were e-mailed along with the receipt for these products. One listed 24 different extract resins for sale and listed advertised effects for these products ranging from sedation to euphoria to stimulation. The second flyer included general herb usage tips and stated that all of the herbs listed are legal in the USA. It also included a section of vaping tips that stated, “Using a vaporizer is probably the most efficient way to the effective component from the herbs. Most people use the leaves powder or resin in vaporizers….”
Figure 2.

The confiscated resin for different blue lotus flower products labeled “Blue Lotus Herbal E-Liquid,” “Blue Lotus Flower Powder,” “Blue Lotus Resin Extract,” and “Space Lotus Resin Extract.”
The primary reference standards for apomorphine and nuciferine standard were purchased from Cayman Chemical (Ann Arbor, MI) and ALB Technology (Richmond, VA), respectively. Oxycondone-d6 was purchased from Cerilliant Corporation (Round Rock, TX). HPLC-grade methanol was used for all dilutions; stock and working solution preparations were purchased from Pharmco-Aaper (Brookfield, CT). Polyethylene glycol (PEG), with an average molecular mass of 600 Da used for DART-MS calibration, was obtained from ULTRA Inc. (North Kingstown, RI). Certified ACS-grade ammonium acetate, 1,4-butanediol, formic acid, optima grade acetone, ethanol, methanol, n-propanol, and isopropanol were purchased from Fisher Scientific (Hanover Park, IL). Nitrogen and helium gases were purchased from Praxair and Airgas (Richmond, VA). Propylene glycol and glycerin were purchased from Wizard Labs (Altamonte Springs, FL). The electronic cigarette used was a Plume Veil 1.5 Clone dual coil dripper by Tobeco from Peak Vapor (Taylorsville, UT) attached to an IPV Mini 2 70 W battery supply from Wake and Vape (Miami, FL). The coils were 26 American Gauge Wire Kantal A-1 wire purchased from Lightning Vapes (Bradenton, FL) and wrapped in contact configuration with a total resistance of 0.6 Ω. The wicking material used was the Cotton Bacon- Organic Cotton purchased from Lightning Vapes (Bradenton, FL). The flow meter used for the trap system was purchased from Cole Palmer (Vernon Hills, IL). Solid-phase micro-extraction (SPME) fibers, 7 um PDMS, were purchased from Supelco (Bellefont, PA). All tubing, glassware, and the fitted gas dispersion tubes were purchased from Colonial Scientific (Richmond, VA).
Methods
The e-liquid was mixed by hand for 20 seconds before aliquoting. The resins and powder were extracted 1:10 (w:v) with methanol. The initial screening was performed on a JEOL JMS T100LC Accu-TOF™ DART-MS controlled by Mass Center software version 1.3.4 m (JEOL Inc. Tokyo, Japan) using modifications of previously published methods (Peace et al., 2016b; Polkis et al., 2015). In brief, a capillary tube was dipped into an aliquot of the e-liquid or extracts and then introduced into the helium stream of a DART-MS for the analysis. The instrument was run in positive-ion mode with a helium stream temperature of 350°C. The flow rate was 2.0 L/min with a discharge electrode needle voltage at 150 V, and the grid electrode at 250 V. The ion guide peak voltage was 400 V, reflectron voltage was 900 V, orifice 2 was set to 5 V, and the ring lens was set to 3 V with orifice 1 set operating in function switching mode at 20, 30, 60, or 90 V while a single data file was created for all four voltages. The range of masses measured was from 40 to 1100 Da. Each sample was analyzed five separate times to ensure reproducibility of the results. The data were analyzed by the creation of averaged, background subtracted, centroided mass spectra that were calibrated using the PEG 600. The identification of apomorphine and nuciferine was made when the exact mass was detected within 5 mDa of its calculated monoisotopic mass (M + H)+ and the fragmentation pattern in function switching mode matched that of the primary reference materials.
For the direct analysis of the e-liquids, 100 μL aliquots of each e-liquid and methanol extracts, along with primary reference materials consisting of apomorphine and nuciferine at a concentration of 10 μg/mL in methanol, 100 % propylene glycol, and 100 % glycerin, were diluted 1:10 with methanol. These samples, along with methanol blanks injected between the samples, were analyzed by gas chromatography mass spectrometry (GC-MS). For the qualitative analysis of the aerosol, a Plume Veil 1.5 Clone RDA electronic cigarette was used to generate an aerosol. Thirty-five milligrams of the original resin sample was dissolved in 0.5 mL of a 50:50 propylene glycol: glycerin mixture (v:v). A 200 microliter aliquot of these mixtures was dripped onto the heated coils after activating the electronic cigarette for four seconds. The aerosol was introduced into a trap consisting of two Erlenmeyer flasks in tandem attached to a vacuum pump with a flow rate of 2.3 L/min. DI-water was placed in the flasks with a gas dispersion tube through which the aerosol was bubbled into the water. Glass wool was placed between the two traps to contain the aerosol in the first trap. A 7 μm polydimethylsiloxane (PDMS) SPME fiber was inserted through a stopper in the top of the first trap. The fiber was introduced into the trap when the electronic cigarette was activated and the aerosol filled the trap. After five minutes, the fiber was retracted and removed. The fiber was then inserted into the injection port of the GC-MS and allowed to thermally desorb for 15 mins.
An Agilent 6890 N Gas Chromatograph with a 5973 Mass Selective Detector (Santa Clara, CA) was used for both the e-liquid and aerosol analyses. The chromatographic separation was performed on a HP-5MS column 30 m × 0.25 mm id × 0.25 μm (Agilent, Santa Clara, CA). The GC-MS was operated in splitless mode. The carrier gas was helium at a linear velocity of 35 cm/s. The oven temperature was programmed from 120°C to 300°C at a rate of 10°C/min and held for 12 min. The total run time was 30 min. The scan range for the mass spectrometer was 40–500 m/z. SPME fibers were thermally cleaned between runs following manufacturer specifications in order to ensure that no carryover occurred between samples. The comparison of the retention time and mass spectrum of the samples versus the primary reference materials was used to identify apomorphine and nuciferine in both the direct analysis of the e-liquids and in the aerosol.
Quantification of apomorphine and nuciferine was performed using a Waters AcQuity Xevo TQD system UPLC–MS/MS (Milford, MA) controlled by MassLynx 4.5 software. Chromatographic separation was performed on a Restek BiPhenyl, 3.0 um, 3.0 × 100 mm (Bellefonte, PA) (Figure 3). The mobile phase consisted of 2 mM ammonium formate in water: acetonitrole 50:50 (v:v). The column flow rate was 0.6 mL/min. The source and desolvation temperatures were 150°C and 200°C, respectively. Desolvation and cone gas flows were 650 and 100 L/hr, respectively. The capillary voltage was 4.0 kV and the cone voltage was 46 V for oxycodone-d6 and 16 V for apomorphine and nuciferine, respectively. The acquisition mode was multiple reaction monitoring (MRM). The following transition ions (m/z) were monitored in MRM mode with their corresponding collision energies (eV) in parentheses: oxycodone-d6: 322 > 304 (18) and 322 > 247 (24); apomorphine: 268 > 191 (31) and 268 > 237 (15); and nuciferine: 296 > 234 (31) and 296 > 250 (21). The total run time for the analytical method was 6.0 min. A seven-point calibration prepared with a range of 1–100 ng/mL of apomorphine and nuciferine along with a blank, double blank control, and controls containing both apomorphine and nuciferine was analyzed. The controls consisted of a limit of quantification control at 1 ng/mL; low control at 3 ng/mL; mid-control at 30 ng/mL; and high control at 75 ng/mL. The internal standard (10 ng/mL oxycodone-d6) was added to each calibrator, blank, control, sample extracts, dilutions of the e-liquid, and dilutions sample extracts. Dilutions of the samples were prepared to assure that all samples were braced within the calibration range. The limit of detection (LOD) and the limit of quantitation (LOQ) were administratively set at 0.25 ng/mL and 1.0 ng/mL, respectively. The coefficient of determination (r2) was < 0.9994 for all calibration curves. The accuracy for all the controls (n = 3) for both apomorphine and nuciferine ranged from 87–114% with coefficient of variations (CVs) < 15%.
Figure 3.
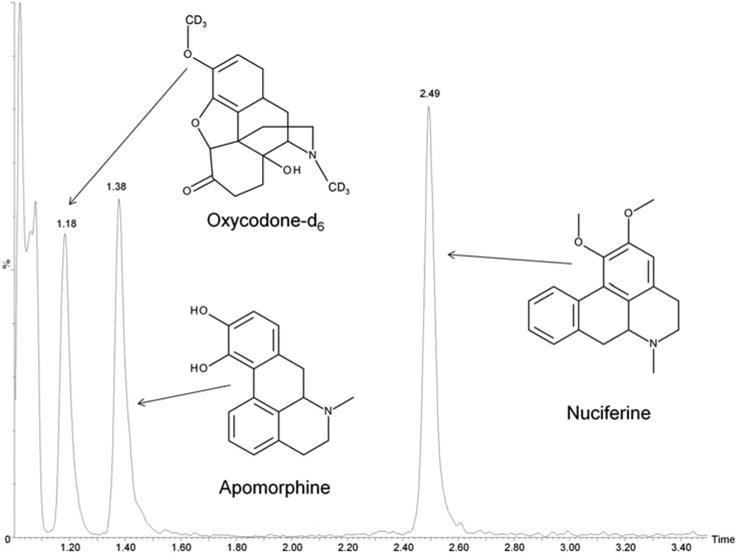
The UPLC-MS/MS total ion chromatograph of oxycodone-d6, apomorphine and nuciferine.
Results
The screening of the unnamed confiscated resin, Blue Lotus Herbal E-liquid, Blue Lotus Flower Powder, Blue Lotus Resin Extract, and Space Lotus Resin Extract by DART-MS and GC-MS resulted in the detection of apomorphine in only the Space Lotus Resin Extract and detection of nuciferine in the confiscated resin, Blue Lotus Resin Extract, and Space Lotus Resin. The UPLC-MS/MS quantification resulted in the detection of apomorphine in two of the five samples and nuciferine in all five samples. The confiscated resin was determined to contain the greatest concentration of nuciferine, 4300 ng/g, but no apomorphine was detected in this sample. The Blue Lotus Herbal E-liquid was described on the flyer as 4% resin extract and was determined to have <1.0 ng/mL of both apomorphine and nuciferine. The Blue Lotus Flower Powder and Blue Lotus Resin Extract were determined to have <1.0 ng/g and 25 ng/g nuciferine, respectively. Space Lotus Resin Extract was determined to contain 130 ng/g of apomorphine and 2700 ng/g nuciferine (Table 1). The SPME-GC-MS analysis revealed that the nuciferine detected in the original blue lotus flower resin sample was successfully aerosolized using the RDA.
Table 1.
Concentrations of apomorphine and nuciferine.
| Sample (n = 3; mean ± SD) |
Apomorphine (ng/mL or ng/g) |
Nuciferine (ng/mL or ng/g) |
|---|---|---|
| Confiscated resin | ND | 4,300 ± 170 |
| Blue Lotus Herbal E-Liquid | <1.0 | <1.0 |
| Blue Lotus Flower Powder | ND | <1.0 |
| Blue Lotus Resin Extract | ND | 25 ± 3 |
| Space Lotus Resin Extract | 130 ± 10 | 2,700 ± 400 |
| ND = None detected |
Discussion
The confiscated resin and the four purchased products were determined to contain nuciferine and only the e-liquid and Space Lotus Resin Extract were determined to contain apomorphine. The detection of nuciferine as a major alkaloid found in all of the products was coincident with them being products from the blue lotus flower. The products were also consistent with statements on the included flyers that the resin products contained much greater concentrations of nuciferine, and the Space Lotus Resin extract contained more apomorphine than the powder.
Anecdotal information found on various Internet drug forums and blogs state that the dose of dried blue lotus flower needed to produce an effect ranges from 1 to 3 g. Users often recommend that the flower be dissolved in wine (ethanol) prior to consumption. The concentrations of the confiscated sample and the commercial resins and powder evaluated herein were determined to vary. Even so, it is unclear how the determined concentrations of apomorphine and nuciferine correspond to the suggested doses outlined in these drug forums and whether the effects reported were placebo effects, drug effects, or as a result of ethanol solvent.
Given that nuciferine was demonstrated to successfully aerosolize with the RDA electronic cigarette, inhalation may be a more efficient means of delivering the active ingredients of apomorphine and nuciferine in the blue lotus products. Inhalation should allow for better bioavailability depending on the ability of the RDA to produce particles of appropriate sizes for pulmonary delivery. This will allow the compound to directly enter the blood stream, thereby avoiding first-pass metabolism.
An emerging trend in which naturally occurring psychedelic drugs are being successfully used in electronic cigarettes has been demonstrated. The recent commercial availability of modified electronic cigarettes such as the RDA has allowed for vaping of nontraditional e-liquid products, such as blue lotus flower powders and resins described here. The formulation of the confiscated resin was successfully used to aerosolize nuciferine, the active ingredients of the blue lotus flower, in a Plume Veil 1.5 Clone RDA.
Based on the information provided with the confiscated sample, the ease with which blue lotus flower products were obtained, and the flyers provided with those products, it is evident that a variety of commercially available natural products contain psychoactive substances. Further, the development and modifications of the traditional electronic cigarette show that it has been and is being used as a delivery system for these products. However, it remains unclear if these products, in their present form, are potent enough to produce the advertised psychoactive effects.
Acknowledgments
The authors would like to thank Shelly N. Butler for taking the photographs of the rebuildable dripping atomizer (RDA) and for the use of these images.
Funding
This project was supported by the National Institutes of Health (P30DA033934) and by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice (2016-DN-BX-0150). The opinions, findings, and conclusions or recommendations expressed in this publication/program/exhibition are those of the author(s) and do not necessarily reflect those of the Department of Justice.
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/ujpd.
ORCID
Justin L. Poklis http://orcid.org/0000-0001-5470-5717
References
- Bhattacharya SK, Bose R, Ghosh P, Tripathi VJ, Ray AB, Dasgupta B. Psychopharmacological studies on (–)-nuciferine and its Hofmann degradation product atherosperminine. Psychopharmacology (Berl) 1978;59(1):29–33. doi: 10.1007/BF00428026. [DOI] [PubMed] [Google Scholar]
- Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, Eissenberg T. Electronic cigarettes: What are they, and what do they do? Annals of the New York Academy of Sciences. 2016 doi: 10.1111/nyas.12977. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emboden W. The sacred journey in dynastic Egypt: Shamanistic trance in the context of the narcotic water lily and the mandrake. Journal of Psychoactive Drugs. 1989;21(1):61–75. doi: 10.1080/02791072.1989.10472144. [DOI] [PubMed] [Google Scholar]
- Farrell MS, McCorvy JD, Huang XP, Urban DJ, White KL, Giguere PM, Doak AK, Bernstein AI, Stout KA, Park SM, Rodriguiz RM, Gray BW, Hyatt WS, Norwood AP, Webster KA, Gannon BM, Miller GW, Porter JH, Shoichet BK, Fantegrossi WE, Wetsel WC, Roth BL. In vitro and in vivo characterization of the alkaloid nuciferine. PLOS One. 2016;11(3):e0150602. doi: 10.1371/journal.pone.0150602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S. FDA committee recommends approval for Viagra rival. The BMJ. 2000;320(6):1094. [PMC free article] [PubMed] [Google Scholar]
- LeWitt PA. Subcutaneously administered apomorphine: Pharmacokinetics and metabolism. Neurology. 2004;62(Suppl. 4):S8–S11. doi: 10.1212/WNL.62.6_suppl_4.S8. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. Journal of Pharmacology and Experimental Therapeutics. 2002;303(2):791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Okun MS, Foote KD. Parkinson’s disease DBS: What, when, who and why? The time has come to tailor DBS targets. Expert Review of Neurotherapeutics. 2010;10(12):1847–57. doi: 10.1586/ern.10.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace MR, Baird TR, Smith N, Wolf CE, Poklis JL, Poklis A. Concentration of nicotine and glycols in 27 electronic cigarette formulations. Journal of Analytical Toxicology. 2016a;40(6):403–07. doi: 10.1093/jat/bkw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace MR, Stone JW, Poklis JL, Turner JBM, Poklis A. Analysis of a commercial marijuana e-cigarette formulation. Journal of Analytical Toxicology. 2016b;40(5):374–78. doi: 10.1093/jat/bkw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis JL, Raso SA, Alford KA, Poklis A, Peace MR. Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and other dimethoxyphenyl-N-[(2-methoxyphenyl) methyl] ethanamine derivatives on blotter paper. Journal of Analytical Toxicology. 2015;39(8):617–23. doi: 10.1093/jat/bkv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribarič S. The pharmacological properties and therapeutic use of apomorphine. Molecules. 2012;17(5):5289–309. doi: 10.3390/molecules17055289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherkl R, Hashem A, Frey H-H. Apomorphine-induced emesis in the dog-routes of administration, efficacy and synergism by naloxone. Journal of Veterinary Pharmacology and Therapeutics. 1990;13(2):154–58. doi: 10.1111/j.1365-2885.1990.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Schwab RS, Amador LV, Lettvin JY. Apomorphine in Parkinson’s disease. Transactions of the American Neurological Association. 1951;56:251–53. [PubMed] [Google Scholar]
- Wang X, Cheang WS, Yang H, Xiao L, Lai B, Zhang M, Ni J, Luo Z, Zhang Z, Huang Y, Wang N. Nuciferine relaxes rat mesenteric arteries through endothelium-dependent and -independent mechanisms. British Journal of Pharmacology. 2015;172(23):5609–18. doi: 10.1111/bph.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]


