Pneumatosis intestinalis (PI) is a rare condition that affects 0.03% of the population.1 Traditionally, PI has been managed surgically; however, many cases can be successfully managed non-surgically.2 As the diagnosis of PI increases with increased use of diagnostic imaging, family physicians will likely encounter more cases in various settings including outpatient offices, emergency departments, and hospitals.1 As such, recognizing PI and managing it appropriately is becoming increasingly important for primary care clinicians.
Case
A 54-year-old African American man presented to the emergency department with left-sided chest pain and diffuse abdominal pain accompanied by dyspnea, nausea, and loss of appetite. His past medical history was relevant for severe emphysema, ischemic dilated cardiomyopathy after implantable cardioverter defibrillator placement, stage IV systolic heart failure, and adenocarcinoma of the lung. He had started a chemotherapy regimen of carboplatin and pemetrexed 3 days before onset of his symptoms.
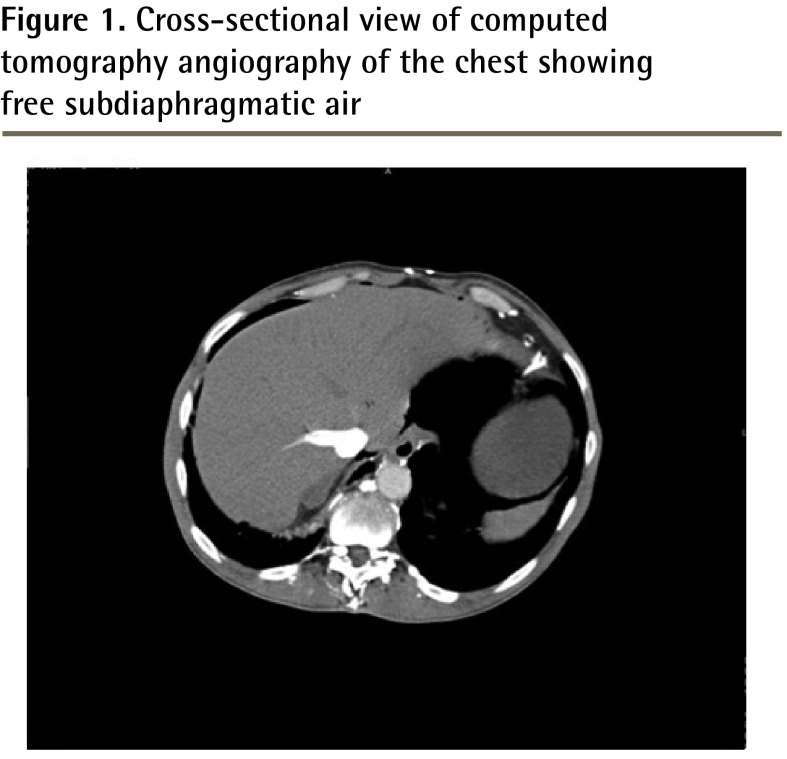
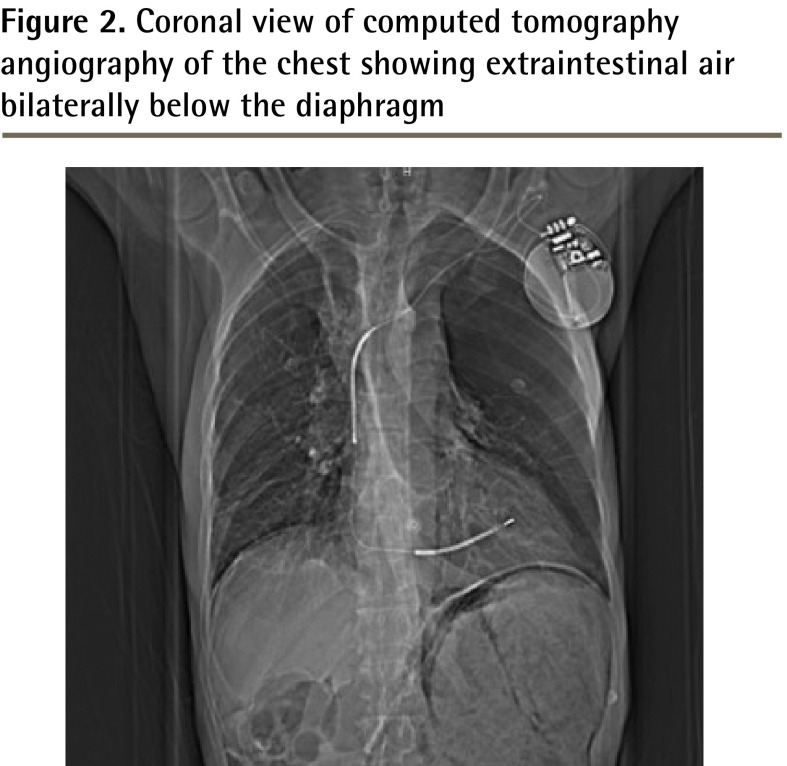
On presentation to the emergency department, his oxygen saturation was 93% on 3 L of oxygen, his heart rate was 70 beats/min, and his blood pressure was 111/66 mm Hg. Findings of an electrocardiogram ruled out ischemia. The patient described his left-sided chest pain as a sharp pain radiating to his epigastric region that became worse with body movement. His physical examination findings were remarkable for generalized abdominal distension, mild epigastric tenderness, and atypical left-sided chest pain, which was reproducible on palpation. He subsequently underwent computed tomography (CT) angiography of the chest to rule out pulmonary embolus; it showed a large apical bulla on the left side with free subdiaphragmatic air consistent with hollow viscus perforation (Figures 1 and 2). A subsequent CT of the abdomen and pelvis showed free extraintestinal air in multiple compartments including the gastric wall; retroperitoneal air; and intrapelvic air in the biliary and portal venous systems and in a left inguinal hernia.
The patient’s laboratory values included a normal white blood cell count and a normal lactic acid level. His serum urea nitrogen and creatinine levels were mildly elevated, whereas his serum bicarbonate level was mildly decreased. Other serum laboratory values such as complete blood count, cytidine 5′-monophosphate level, and cardiac enzyme levels were measured to rule out other ischemic, infectious, or electrolyte abnormalities in the patient. Despite fairly unremarkable laboratory values (Table 1) and almost benign physical examination findings, a consultation with the surgery department was initiated owing to CT findings that raised suspicion of acute intestinal perforation. Perforation was later ruled out, as were acute chronic obstructive pulmonary disease (COPD) exacerbation and acute myocardial infarction. The patient was diagnosed with PI, likely secondary to his underlying emphysema or chemotherapy agents. He was subsequently admitted to our service for further management.
Over the following 2 days, we managed him conservatively and he underwent serial abdominal examinations. His diet was gradually advanced after 24 hours of nothing by mouth. The patient tolerated his diet and continued to do well and was subsequently discharged home, with outpatient follow-up scheduled with his family physician.
Figure 1.
Cross-sectional view of computed tomography angiography of the chest showing free subdiaphragmatic air
Figure 2.
Coronal view of computed tomography angiography of the chest showing extraintestinal air bilaterally below the diaphragm
Table 1.
Initial laboratory values measured in the emergency department
| INVESTIGATION | VALUE | REFERENCE RANGE |
|---|---|---|
| White blood cell count, × 109/L | 9.1 | 4.0–10.0 |
| Hemoglobin level, g/L | 126 (low) | 140–174 |
| Hematocrit | 0.398 (low) | 0.420–0.520 |
| Platelet count, × 109/L | 355 | 130–400 |
| Sodium level, mmol/L | 138 | 135–145 |
| Potassium level, mmol/L | 5.3 (high) | 3.5–5.0 |
| Chloride level, mmol/L | 103 | 98–106 |
| Bicarbonate level, mmol/L | 18 (low) | 24–30 |
| Serum urea nitrogen level, mmol/L | 18.21 (high) | 2.5–8.0 |
| Creatinine level, µmol/L | 123.76 (high) | 70–120 |
| Glucose level, mmol/L | 4.72 | < 6.5 |
| Calcium level, mmol/L | 2.12 | 2.18–2.58 |
| Phosphorus level, mmol/L | 0.58 (low) | 0.80–1.50 |
| Magnesium level, mmol/L | 1.12614 (high) | 0.75–0.95 |
| Total bilirubin level, µmol/L | 6.84 | < 26 |
| AST level, U/L | 19 | 0–35 |
| ALT level, U/L | 15 | 3–36 |
| ALP level, U/L | 87 | 35–100 |
| Troponin level, µg/L | < 0.01 | < 0.01 |
| Total protein level, g/L | 77 | 60–80 |
| Albumin level, g/L | 44 | 35–50 |
| Prothrombin time, s | 13 | 10–13 |
| International normalized ratio | 1.1 | 0.9–1.2 |
| Lactic acid level, mmol/L | 0.79 | 0.7–1.8 |
ALP—alkaline phosphatase, ALT—alanine aminotransferase, AST—aspartate aminotransferase.
Discussion
Pneumatosis intestinalis is the presence of a gas cyst in the bowel wall. Gas can be located in the mucosa or submucosa.3 It is often identified on abdominal radiographs or CT imaging, and the causes vary from benign to life-threatening conditions.4 It is important to mention that PI is a radiographic sign but not a disease itself.4 The infantile form of PI has high mortality, as it presents as a manifestation of acute necrotizing enterocolitis.5 Pneumatosis intestinalis in adults is usually benign or presents with limited physical examination findings and is often diagnosed incidentally.5 It is usually characterized into primary and secondary forms. Primary PI is found incidentally in approximately 15% to 20% of cases and patients are typically asymptomatic.4 Secondary PI is found in approximately 80% to 85% of cases and has multiple causes in various gastrointestinal and nongastrointestinal illnesses.6 These causes include but are not limited to bowel ischemia, trauma, bowel infection, COPD, and medication or immunosuppressive therapy.6 Complications can occur in approximately 3% of cases of secondary PI, often requiring emergency surgical intervention.7 Overall mortality from these complications appears to be high, ranging from 50% to 75%, especially in PI patients with bowel ischemia.8 Therefore, the patient’s history, physical examination findings, and laboratory test results should be used to help identify the underlying cause of PI, with particular attention paid to risk factors for intestinal ischemia and obstruction.8
Although the cause of PI appears to be multifactorial, the pathogenesis remains unclear.9 There are several mechanical, bacterial, and pulmonary theories; however, none of them have been validated.9 According to the mechanical theory, a mucosal defect in the gastrointestinal tract transmits the gas, which dissects the tissue layers of the bowel wall. Peristalsis then propagates the gas to distant sites.9 The bacterial theory proposes that the gut’s normal bacteria, such as Escherichia coli and Clostridia, enter the bowel wall and lymphatic system through mucosal defects and subsequently produce gas.9 The pulmonary theory applies to patients with COPD, which hypothesizes that air from ruptured pulmonary blebs enters the mediastinal space and travels into the retroperitoneum, finally tracking along the bowel mesentery until it reaches the bowel. Over time, the air becomes surrounded by the body’s inflammatory cells, creating air cysts within the bowel wall.9
While PI has multiple benign causes, primary care clinicians should be aware that some cases potentially have life-threatening causes.3,6,7 The task of the primary care clinician is to identify and differentiate between benign cases of PI and those requiring surgery. This can be challenging owing to the array of PI presentations.10 Patients with benign physical examination findings and serum laboratory test results should be carefully monitored and observed with serial examinations until asymptomatic.11 Patients with life-threatening physical examination findings or serum test results should undergo surgical procedures.11 Patients who remain symptomatic despite observation and medical therapy or who develop complications from PI should also be referred for surgery.11 Until a validated algorithm is created, primary clinicians should interpret radiographic findings in conjunction with clinical presentations to ensure a correct diagnosis and appropriate management.10
Once a correct diagnosis of benign PI is established, a proposal to discharge asymptomatic patients from the emergency department who do not have any risk factors for intestinal ischemia or obstruction might sound reasonable, especially in an era of providing cost-effective medical care. However, limited conclusions can be drawn from our case study. There is also limited evidence in the existing literature about PI. An extensive PubMed search only yielded a handful of small observational studies. A large multicentre cohort study to explore the outcomes of these asymptomatic patients discharged home from the emergency department should be conducted in the future.
Conclusion
Pneumatosis intestinalis is often benign, but in rare cases it can be caused by life-threatening illness. Primary care physicians should differentiate between benign cases that should be managed conservatively and serious cases that should be managed surgically.
Acknowledgments
I express my sincere gratitude to my previous residency supervisor, Dr David Holmes, for providing unrelenting support, guidance, knowledge, enthusiasm, and motivation.
EDITOR’S KEY POINTS
Pneumatosis intestinalis (PI) is usually managed conservatively, with surgical intervention only if signs and symptoms are present. Patients with benign physical examination findings and serum laboratory test results should be carefully monitored and observed until asymptomatic; patients with life-threatening physical examination findings or serum test results should undergo surgery; and patients who remain symptomatic despite observation and medical therapy or who develop complications from PI should also be referred for surgery.
The patient’s history, physical examination findings, and laboratory test results should be used to help identify the underlying cause of PI. While PI has multiple benign causes, primary care clinicians should be aware that some cases potentially have life-threatening causes.
Discharging asymptomatic patients with benign PI from the emergency department who do not have any risk factors for intestinal ischemia or obstruction might sound reasonable, especially in an era of providing cost-effective medical care. However, limited conclusions can be drawn from this case study, and there is limited evidence in the existing literature.
POINTS DE REPÈRE DU RÉDACTEUR
La pneumatose intestinale est habituellement prise en charge de manière conservatrice, les interventions chirurgicales n’étant réservées qu’en présence de signes et de symptômes. Les patients dont les constatations à l’examen physique et les résultats d’analyses sériques en laboratoire sont bénins peuvent être surveillés et observés de près jusqu’à la disparition des symptômes; les patients dont les constatations à l’examen physique ou les résultats des analyses sériques se révèlent menaçants pour la vie doivent subir une intervention chirurgicale. Les patients qui demeurent symptomatiques en dépit de l’observation et d’un traitement médical ou qui développent des complications de l’affection devraient aussi faire l’objet d’une consultation en chirurgie.
On devrait se servir de l’anamnèse, des constatations à l’examen physique et des résultats des analyses en laboratoire pour aider à cerner la cause sousjacente de la pneumatose intestinale. Même si cette affection a de multiples causes bénignes, les cliniciens en soins primaires devraient être conscients que, dans certains cas, les causes sont potentiellement dangereuses pour la vie.
Il pourrait sembler raisonnable de donner leur congé de l’urgence aux patients asymptomatiques souffrant d’une pneumatose intestinale bénigne qui n’ont pas de facteurs de risque d’ischémie ou d’obstruction intestinale, surtout en cette époque préconisant des soins médicaux rentables. Par ailleurs, des conclusions limitées peuvent être tirées de cette étude de cas et les données probantes sont limitées dans les ouvrages médicaux.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Competing interests
None declared
References
- 1.Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol. 2007;188(6):1604–13. doi: 10.2214/AJR.06.1309. [DOI] [PubMed] [Google Scholar]
- 2.Morris MS, Gee AC, Cho SD, Limbaugh K, Underwood S, Ham B, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195(5):679–82. doi: 10.1016/j.amjsurg.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Cordum NR, Dixon A, Campbell DR. Gastroduodenal pneumatosis: endoscopic and histological findings. Am J Gastroenterol. 1997;92(4):692–5. [PubMed] [Google Scholar]
- 4.Saul T, Palamidessi N. Pneumatosis intestinalis. J Emerg Med. 2011;40(5):545–6. doi: 10.1016/j.jemermed.2008.10.019. Epub 2009 Mar 9. [DOI] [PubMed] [Google Scholar]
- 5.Domino FJ, Baldor RA, Golding J, Grimes JA, editors. The 5-minute clinical consult standard. 23rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2015. Pneumatosis intestinalis. Available from: http://5minuteconsult.com/collectioncontent/1-151977/diseases-and-conditions/pneumatosis-intestinalis. Accessed 2017 Aug 14. [Google Scholar]
- 6.Slesser AA, Patel PH, Das SC, Leahy A, Livingstone J, Riaz AA. A rare case of segmental small bowel pneumatosis intestinalis: a case report. Int J Surg Case Rep. 2011;2(7):185–7. doi: 10.1016/j.ijscr.2011.06.003. Epub 2011 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galandiuk S, Fazio VW. Pneumatosis cystoides intestinalis. A review of the literature. Dis Colon Rectum. 1986;29(5):358–63. doi: 10.1007/BF02554132. [DOI] [PubMed] [Google Scholar]
- 8.Doumit M, Saloojee N, Seppala R. Pneumatosis intestinalis in a patient with chronic bronchiectasis. Can J Gastroenterol. 2008;22(10):847–50. doi: 10.1155/2008/595297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azzaroli F, Turco L, Ceroni L, Galloni SS, Buonfiglioli F, Calvanese C, et al. Pneumatosis cystoides intestinalis. World J Gastroenterol. 2011;17(44):4932–6. doi: 10.3748/wjg.v17.i44.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donovan S, Cernigliaro J, Dawson N. Pneumatosis intestinalis: a case report and approach to management. Case Rep Med. 2011;2011:571387. doi: 10.1155/2011/571387. Epub 2011 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenstein AJ, Nguyen SQ, Berlin A, Corona J, Lee J, Wong E, et al. Pneumatosis intestinalis in adults: management, surgical indications, and risk factors for mortality. J Gastrointest Surg. 2007;11(10):1268–74. doi: 10.1007/s11605-007-0241-9. Epub 2007 Aug 9. [DOI] [PubMed] [Google Scholar]