Abstract
Objective
The aim of this systematic review and network meta-analysis was to evaluate the comparative efficacy and safety of antiplatelet agents, vitamin K antagonist (VKA) and non-VKA oral anticoagulants (NOACs) in patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI).
Methods
PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials were searched to identify clinical trials comparing antiplatelet drugs with VKA and NOACs or their combination in AF patients undergoing PCI with a mean/median follow-up of at least 12 months. A network meta-analysis was conducted to directly and indirectly compare the efficacy and safety of competitive antithrombotic regimens with a Bayesian random-effects model. Results were presented as relative risks (RRs) and 95% confidence intervals (CIs).
Results
A total of 15 studies enrolling 13,104 patients were included. Among 5 regimens, rivaroxaban 15 mg daily plus P2Y12 inhibitor treatment demonstrated significant superiority over dual- and triple-antiplatelet therapies (DAPT, TT) in reducing thromboembolic events (0.64 [0.38, 0.95] and 0.68 [0.43, 0.98], respectively) but showed the maximum possibility of major bleeding risk, while VKA plus single antiplatelet therapy (SAPT) seemed the safest. Significantly less risk of major bleeding was seen in DAPT group than that in TT group (0.63 [0.39, 0.99]).
Conclusions
The present study suggests that combination of VKA and SAPT is the best choice for AF patients undergoing PCI considering both efficacy and safety. Rivaroxaban 2.5 mg twice daily plus DAPT treatment owns the highest probability to be the optimal alternative to VKA plus SAPT for these patients.
Introduction
Percutaneous coronary intervention (PCI) with dual-antiplatelet therapy (DAPT, aspirin and P2Y12 inhibitor or thienopyridine) is a standardized treatment for coronary artery disease (CAD) patients with moderate or severe coronary artery stenosis, 5% to 15% of whom are concomitant atrial fibrillation (AF)[1–3]. A previous observational study reported that 12% of 113,283 PCI cases were concomitant AF and significantly older, more likely to have comorbid congestive heart failure, cardiomyopathy, cerebrovascular disease, chronic lung disease and in-hospital complications, including in-hospital mortality (3.2% vs. 1.3%, p < 0.001)[4]. Anticoagulant treatment is of great importance to prevent thrombosis in AF patients while DAPT owns Ia evidence after PCI. As to the antithrombotic therapy for AF patients undergoing elective PCI, 2016 European Society of Cardiology (ESC) guidelines recommend triple therapy (TT) including oral anticoagulant (OAC), aspirin 75–100 mg per day and clopidogrel 75 mg per day for a period of 1 months (evidence level IIaB), followed by dual therapy (OAC with aspirin or clopidogrel, evidence level IIaC) till 6 or 12 months according to high or low bleeding risk and then OAC for lifelong (evidence level IB)[5]. However, the clinical evidence by now has been deficient to support the strategy decision and prescribe individualized antithrombotic regimens for AF patients undergoing PCI.
Notably, administration of VKA requires frequent monitoring of coagulant function and adjustment for proper dosage because of its fluctuated pharmacokinetics and susceptibility to food and drugs. Conversely, non-VKA oral anticoagulant (NOAC) without these deficiencies has dramatically optimized anticoagulation management. Although antithrombotic efficacy and bleeding risk of NOACs for AF patients undergoing PCI in the real world has not been well established yet, several clinical studies have yielded different results of several antiplatelet and anticoagulant regimens for these patients[6–20]. This systematic review and network meta-analysis (NMA) aimed to rank efficacy and safety of different antithrombotic therapies available for AF patients in post-PCI.
Methods
Systematic review
A systematic review protocol was prepared to define all aspects of the review beforehand (https://dx.doi.org/10.17504/protocols.io.iijcccn). All the participants, interventions, comparisons, outcomes, and study designs (PICOS) were recorded in the systematic review process.
Search strategy and data sources
We conducted a systematic literature search in PubMed, Embase and Cochrane Central databases from the earliest possible search date through March 2017. The following search terms were used: (“atrial fibrillation” or “AF”) and (“percutaneous coronary intervention” or “PCI” or “coronary stent implantation”) and (“aspirin” or “P2Y12 antagonist” or “P2Y12 inhibitor” or “clopidogrel” or “ticagrelor” or “warfarin” or “vitamin K antagonist” or “VKA” or “dabigatran” or “rivaroxaban” or “apixaban” or “edoxaban” or “novel oral anticoagulant” or “new oral anticoagulant” or “NOAC”).
Study selection and eligibility criteria
Two reviewers (GXX and TSW) performed the literature selection, data extraction, and quality assessment. Disagreements were resolved by the consensus of a third reviewer (LCJ). The study selection process involved literature screening through titles and/or abstracts and further full-text evaluation.
The inclusion criteria were as follows: (1) clinical trials with at least one compared group; (2) study population of CAD patients with paroxysmal, persistent, or permanent AF undergoing PCI; (3) treatment with antiplatelet or anticoagulant agents mentioned before with a target international normalized ratio (INR) of 2.0–3.0 when warfarin was used to reflect present standard practice; (4) outcomes involving main adverse cardiac and cerebrovascular events (MACCEs) and major bleeding; (5) mean or median follow-up period no less than 12 months. Studies were excluded if (1) they were published only in the form of abstracts or in non-English languages; (2) antithrombotic therapies changed differently in post-PCI; (3) compared treatments were not specific such as VKA vs. non-VAK.
Duplicates were firstly and irrelevant or non-English articles were subsequently removed on the basis of titles and/or abstracts. After assessment of full-text studies, eligible ones were finally enrolled in our NMA.
Data extraction and quality assessment
The extracted data mainly included study design, sample size (all and in compared arms), follow-up duration, antithrombotic strategies in post-PCI, efficacy and safety outcomes, baseline characteristics of the patients including mean age, male percentage, mean CHADS2/CHA2DS2-VASc and HAS-BLED scores and most importantly the number and percentage of MACCEs and major bleeding outcomes in every arm.
The qualities of observational studies and randomized controlled trials (RCTs) were assessed by the Newcastle-Ottawa Scale (NOS, evaluation of selection, comparability and outcome)[21] and Jadad Scale (judgment of randomization, double blind, withdrawals and dropouts)[22] respectively. As the Cochrane Collaboration recommended[23], all qualified studies were included in our analysis without considering their qualities.
Outcome measures
The primary endpoint was defined as the occurrence of MACCEs, including all-cause mortality, cardiac death, myocardial infarction (MI), target lesion revascularization (TLR), and stroke. The secondary outcome was major bleeding, defined by TIMI[24] or the Bleeding Academic Research Consortium (BARC)[25] criteria. The endpoints data in around 1-year follow-up available were collected for our analysis.
Statistical analysis
Results for endpoint events were treated as dichotomous data. Risk ratio (RR) and 95% confidence interval (CI) were used to estimate pooled results from studies. A Bayesian NMA was conducted in Addis 1.16.8 software by Markov chain Monte Carlo methods, which provided direct and indirect evidence for any given treatments in one joint analysis and a possible ranking distribution of 2 endpoints for all regimens[26]. If 95% CI of inconsistency factors median contained 0 and p value of node split model was over 0.05, inconsistent model would be considered not significant and then consistent model would be used for further analysis.
Results
Search results and studies characteristics
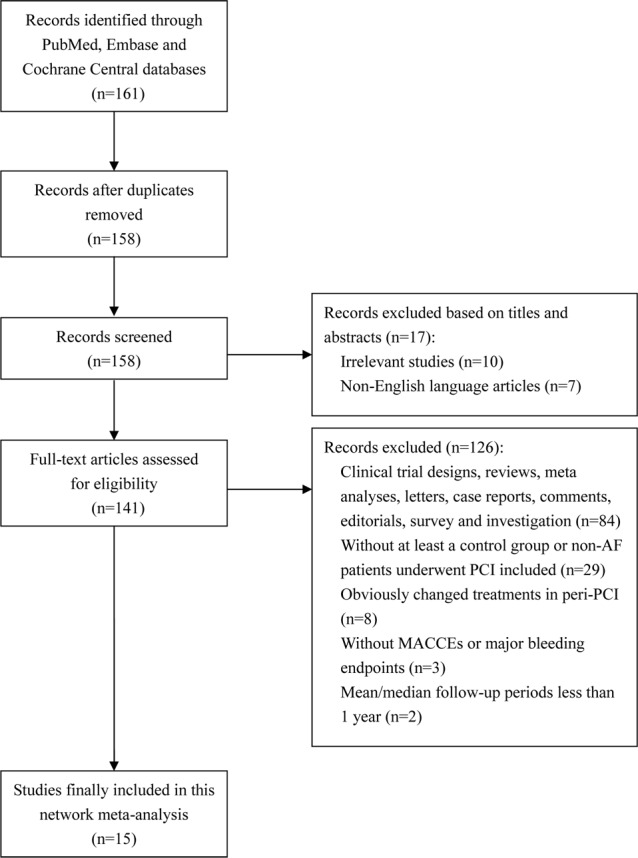
A total of 161 potentially relevant titles were identified by the key words mentioned before. After 146 duplicate analysis (n = 3), non-English articles (n = 7), irrelevant studies (n = 10), non-clinical trials (n = 84), and clinical trials not fulfilling inclusion/exclusion criteria (n = 42) were rejected, 15 studies including 13,104 patients were finally included in this NMA after screening and full-text assessment (Fig 1). According to the principles expressed in the Declaration of Helsinki, all participants provided written consents in every enrolled trial.
Fig 1. Flow diagram of study selection in this net-work meta-analysis.
The studies and patients baseline characteristics are shown in Table 1. We incorporated 3 RCTs and 12 observational cohort studies with a mainly mean or median follow-up of 1 year using 5 regimens: DAPT (n = 6,489), TT (n = 4,721), rivaroxaban 15 mg per day plus P2Y12 inhibitor (n = 757), rivaroxaban 2.5 mg twice daily plus DAPT (n = 706) and VKA plus single antiplatelet therapy (SAPT) (n = 434). INR therapeutic range was 2.0–3.0 when VKA was used. Loading doses of aspirin and ticagrelor or clopidogrel were administered to acute coronary syndrome (ACS) patients and before PCI according to guideline[27]. Among the recruited patients, the reported mean age was similar across trials (68.3–79.6 years) and men were in the majority (45%-82.4%). The mean or median values of 12 CHADS2/CHA2DS2-VASc scores and 7 HAS-BLED scores were mostly over 2 which indicated high risks of both thrombosis and bleeding in enrolled patients. All of the included studies reported thromboembolic events and major bleeding endpoints. The rates of cardiovascular-cause mortality and re-hospitalization were used for MACCEs outcome evaluation of PIONEER AF-PCI trial[19].
Table 1. Main characteristics of studies and patients included in the network meta-analysis.
| Studies characteristics | Patients baseline characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Trial name/country, author, published year | Study design | Sample size | Follow-up period | Treatment groups | Age (years) | Male (%) | CHADS2/ CHA2DS2-VASc score3 | HAS-BLED score4 |
| Germany, Lars Maegdefessel, et al, 2008[6] | Single-center, prospective cohort trial | 117 | 1.4 years | DAPT, TT | 69.6 | 74.4 | - | - |
| WOEST, Willem J M Dewilde, et al, 2013[7] | Multi-center, randomized, open-label, controlled trial | 573 | 1 year | TT, VKA + clopidgrel1 | 68.7 | 78.2 | ≥2, 49.7% | - |
| Poland, Magdalena Dąbrowska, et al, 2013[8] | Single-center, prospective cohort trial | 104 | 1 year | DAPT, TT | 70.2 | 58.7 | - | - |
| CRUSADE, Emil L. Fosbol, et al, 2013[9] | Multi-center, retrospective cohort trial | 1648 | 1 year | DAPT, TT | 77.7 | 58.3 | 2/4 | - |
| Japan, Hideki Kawai, et al, 2014[10] | Multi-center, retrospective cohort trial | 146 | 37 months | DAPT, TT, VKA + SAPT | 72.0 ± 8.1 | 72.9 | 2.14 | ≥3, 32.9% |
| AFCAS, Andrea Rubboli, et al, 2014[11] | Multi-center, prospective cohort trial | 914 | 1 year | TT, DAPT, VKA + clopidgrel1 | 73 ± 8 | 70 | 2.2 ± 1.2 | 3.0 ± 0.7 |
| Korea, Soon Yong Suh, et al, 2014[12] | Single-center, retrospective cohort trial | 203 | 42.0 ± 29.0 months | DAPT, TT | 68.3 ± 10.1 | 62.6 | 1.92 ± 1.19 | 1.97 ± 0.64 |
| Korea, Dong Oh Kang, et al, 2015[13] | Two-center, retrospective cohort trial | 367 | 2 years | DAPT, TT | 68.1 | 65.1 | 1.82 | - |
| ACTION Registry–GWTG, Connie N. Hess, et al, 2015[14] | Multi-center, prospective cohort trial | 4959 | 2 years | DAPT, TT | 77.5 | 57.5 | 2.54 | - |
| AVIATOR, Marco G. Mennuni, et al, 2015[15] | Multi-center, prospective cohort trial | 859 | 1 year | DAPT, TT | 73 ± 9.6 | 71 | 2.7 ± 1.2 | 2.9 ± 0.7 |
| Span, Antonia Sambola, et al, 2016[16] | Multi-center, prospective cohort trial | 585 | 1 year | DAPT, TT | 73.2 ± 8.2 | 75.2 | ≥2, 73.2% | ≥3, 39.2% |
| Triple Therapy in Elderly Patients, Antonia Sambola, et al, 2016[17] | Multi-center, prospective cohort trial | 289 | 1 year | DAPT, TT | 79.6 ± 3.4 | 67.1 | 4.0 ± 1.4 | ≥3, 84.4 |
| ROCKET AF, Matthew W. Sherwood, et al, 2016[18] | Multi-center, randomized, double-blind, double-dummy noninferiority controlled trial | 153 | 806 days | TT, rivaroxaban2 + P2Y12 inhibitor | 73 (67, 79) | 82.4 | 3.5 ± 1.0 | - |
| PIONEER AF-PCI, Gibson CM, et al, 2016[19] | Multi-center, randomized, open-label, controlled trial | 2124 | 1 year | TT, rivaroxaban 15 mg/d + P2Y12 inhibitor; rivaroxaban 2.5 mg bid + DAPT | 70.1 | 74.4 | 3.77 | - |
| Italy, Renato De Vecchis, et al, 2016[20] | Single-center, retrospective cohort trial | 98 | 378 ± 15.7 days | DAPT, TT, VKA + SAPT | 73 ± 7.5 | 45 | 1.8 ± 1.5/5.3 ± 1.6 | 2.3 ± 0.5 |
1. Clopidogrel 75 mg/d
2. Rivaroxaban 20 mg/d or 15 mg/d in participants with a creatinine clearance of 30–90 ml/min
3. 1 point each for the presence of congestive heart failure, hypertension, age 75 years or older, and diabetes mellitus, and 2 points for history of stroke or transient ischemic attack
4. 1 point each for hypertension, abnormal renal and liver function, stroke, bleeding, labile INRs, age at least 65 years, drugs or alcohol. Results were shown as mean ± standard deviation (SD) or median value with or without interquartile range.
Abbreviations: VKA: vitamin K antagonist; DAPT: dual antiplatelet therapy including aspirin 75–100 mg/d, clopidogrel 75 mg/d; TT: triple therapy including aspirin 75–100 mg/d, clopidogrel 75 mg/d and VKA; SAPT: single antiplatelet therapy, aspirin 75–100 mg/d or clopidogrel 75 mg/d.
Individual studies results
The raw data of this NMA are presented in S1 Table. All of 15 enrolled trials reported TT outcomes and 13 demonstrated DAPT endpoints. The remaining 3 regimens including VKA plus SAPT, rivaroxaban 15 mg per day plus P2Y12 inhibitor and rivaroxaban 2.5 mg twice daily plus DAPT were observed in 4, 2 and 1 trials respectively. MACCEs incidence rates varied from 3.3% in DAPT arm[8] to 37.3% in VKA with SAPT arm[10], and major bleeding rates were observed from 0[6] to 21.4%[10] both in TT group.
NMA results
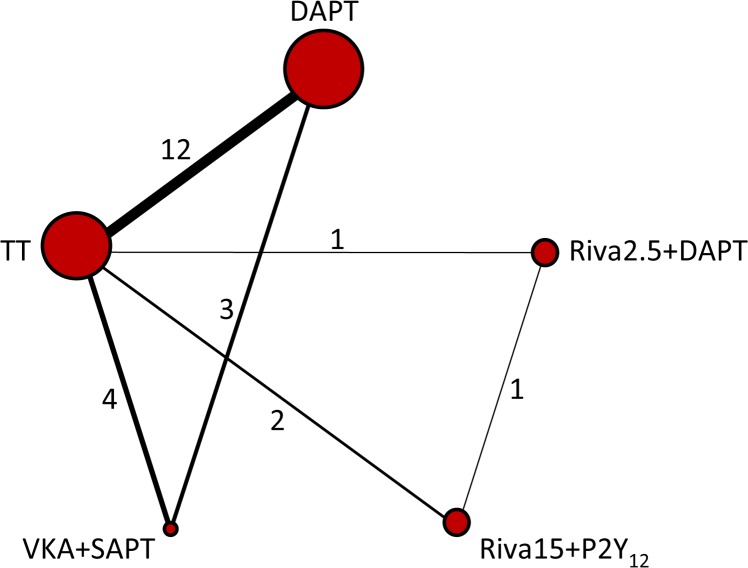
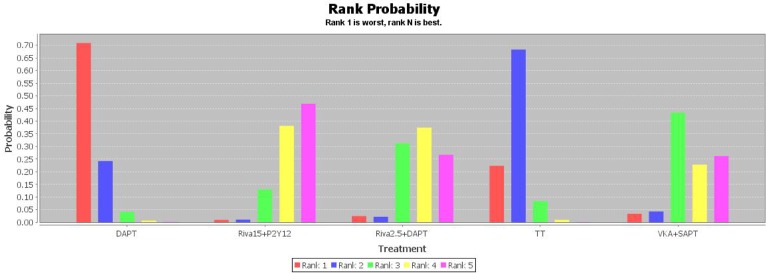
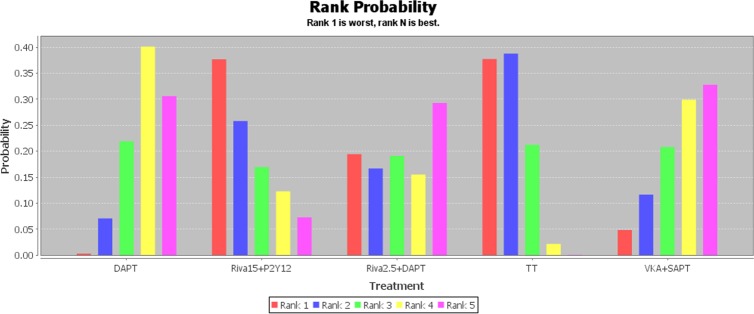
Network of evidence for MACCEs and major bleeding outcomes was displayed in Fig 2. In all, 12 studies made a comparison between TT and DAPT, followed by 4 and 3 trials comparing TT and DAPT with VKA plus SAPT respectively. The distributions of two outcomes probabilities of every treatment being ranked at each of the possible 5 positions were demonstrated in Figs 3 and 4. RRs and 95% CIs for all treatments relative to each other in MACCEs and major bleeding endpoints under the consistency model were presented in Tables 2 and 3.
Fig 2. Network diagram of treatment comparisons for MACCEs and major bleeding of competitive antithrombotic regimens.
MACCEs, main adverse cardiac and cerebrovascular events; DAPT, dual-antiplatelet therapy; TT, triple-antiplatelet therapy; VKA, vitamin K antagonist; SAPT, single antiplatelet therapy; Riva15 + P2Y12, rivaroxaban 15 mg/d plus P2Y12 inhibitor; Riva2.5 + P2Y12, rivaroxaban 2.5 mg bid plus P2Y12 inhibitor. The size of each node is proportional to the overall sample size of the corresponding therapy. Each line represents the direct comparison between two treatments, and the corresponding width is proportional to the number of trials. Number next to the line indicates the specific number of studies.
Fig 3. The distribution of MACCEs probabilities of 5 treatments being ranked at possible positions.
MACCEs, main adverse cardiac and cerebrovascular events; DAPT, dual-antiplatelet therapy; Riva15 + P2Y12, rivaroxaban 15 mg/d plus P2Y12 inhibitor; Riva2.5 + P2Y12, rivaroxaban 2.5 mg bid plus P2Y12 inhibitor; TT, triple-antiplatelet therapy; VKA, vitamin K antagonist; SAPT, single antiplatelet therapy.
Fig 4. The distribution of major bleeding probabilities of 5 treatments being ranked at possible positions.
DAPT, dual-antiplatelet therapy; Riva15 + P2Y12, rivaroxaban 15 mg/d plus P2Y12 inhibitor; Riva2.5 + P2Y12, rivaroxaban 2.5 mg bid plus P2Y12 inhibitor; TT, triple-antiplatelet therapy; VKA, vitamin K antagonist; SAPT, single antiplatelet therapy.
Table 2. NMA results of MACCEs risk for all treatments relative to each other under the consistency model.
| DAPT | 0.64 (0.38, 0.95) | 0.69 (0.41, 1.03) | 0.95 (0.79, 1.13) | 0.75 (0.52, 1.08) |
| 1.56 (1.05, 2.60) | Riva15 + P2Y12 | 1.07 (0.70, 1.68) | 1.47 (1.03, 2.35) | 1.20 (0.70, 2.16) |
| 1.45 (0.97, 2.46) | 0.94 (0.59, 1.42) | Riva2.5 + DAPT | 1.39 (0.94, 2.25) | 1.09 (0.66, 2.01) |
| 1.05 (0.89, 1.26) | 0.68 (0.43, 0.98) | 0.72 (0.44, 1.06) | TT | 0.80 (0.55, 1.11) |
| 1.33 (0.92, 1.94) | 0.83 (0.46, 1.43) | 0.92 (0.50, 1.52) | 1.25 (0.90, 1.83) | VKA + SAPT |
Results are presented as risk ratios (95% confidence intervals). Significant results indicated in bold. MACCEs, main adverse cardiac and cerebrovascular events; DAPT, dual-antiplatelet therapy; Riva15 + P2Y12, rivaroxaban 15 mg/d plus P2Y12 inhibitor; Riva2.5 + P2Y12, rivaroxaban 2.5 mg bid plus P2Y12 inhibitor; TT, triple-antiplatelet therapy; VKA, vitamin K antagonist; SAPT, single antiplatelet therapy.
Table 3. NMA results of major bleeding risk for all treatments relative to each other under the consistency model.
| DAPT | 1.77 (0.49, 7.11) | 1.24 (0.25, 7.19) | 1.83 (1.11, 3.28) | 1.03 (0.40, 2.68) |
| 0.57 (0.14, 2.03) | Riva15 + P2Y12 | 0.70 (0.14, 3.49) | 1.03 (0.30, 3.50) | 0.58 (0.12, 2.59) |
| 0.81 (0.14, 4.02) | 1.43 (0.29, 7.09) | Riva2.5 + DAPT | 1.49 (0.30, 7.03) | 0.83 (0.13, 4.95) |
| 0.55 (0.31, 0.90) | 0.97 (0.29, 3.35) | 0.67 (0.14, 3.32) | TT | 0.56 (0.22, 1.37) |
| 0.97 (0.37, 2.48) | 1.72 (0.39, 8.19) | 1.20 (0.20, 7.72) | 1.78 (0.73, 4.58) | VKA + SAPT |
Results are presented as risk ratios (95% confidence intervals). Significant results indicated in bold. DAPT, dual-antiplatelet therapy; Riva15 + P2Y12, rivaroxaban 15 mg/d plus P2Y12 inhibitor; Riva2.5 + P2Y12, rivaroxaban 2.5 mg bid plus P2Y12 inhibitor; TT, triple-antiplatelet therapy; VKA, vitamin K antagonist; SAPT, single antiplatelet therapy.
Results of NMA for MACCEs
Combination of rivaroxaban 15 mg per day and P2Y12 inhibitor showed a statistically lower risk of MACCEs compared with DAPT and TT regimens for AF patients undergoing PCI (0.64 [0.38, 0.95] and 0.68 [0.43, 0.98], respectively) and owned the first place of efficacy possibility among 5 therapies (Fig 3). Besides, rivaroxaban 2.5 mg twice daily with DAPT, VKA with SAPT treatments ranked in the second and third places of efficacy possibility respectively (Fig 3) were similarly superior to TT and DAPT in efficacy but with no statistical significance (Table 2). DAPT was associated with an obviously higher hazard of MACCEs than others (Fig 3).
Importantly, there were no significant inconsistencies between the direct and indirect evidence of MACCEs in all closed loops (inconsistency factors median -0.22 [-0.90, 0.29]; direct effect model, -0.18 [-0.84, 0.37], indirect effect model, -0.59 [-1.24, -0.01], overall node split models, N/A, p = 0.36).
Results of NMA for major bleeding
Regarding the outcome of major bleeding, the least risk was observed in VKA plus SAPT then DAPT treatment, whereas rivaroxaban 15 mg per day plus P2Y12 inhibitor and TT regimens were ranked in the first and second place of risk possibility respectively (Fig 4). Besides, DAPT was significantly safer than TT in the rate of major bleeding (0.55 [0.31, 0.90]) (Table 3).
Likewise, no significant inconsistencies between the direct and indirect evidence of major bleeding were seen in all closed loops (inconsistency factors median -0.00 [-1.28, 1.22]; direct effect model, -0.03 [-1.24, 1.18], indirect effect model, 0.02 [-1.84, 1.88], overall node split models, 0.03 [-0.91, 0.99], p = 0.96).
Results of quality assessment
As were shown in S2 and S3 Tables, the mean Jadad score of 3 RCTs was 3.67 ± 1.15 and mean NOS score of 12 cohort trials was 7.17 ± 0.58. Although there were some differences between the trials in terms of study design and patient characteristics, all included studies were judged to be of good quality.
Discussion
Our NMA, overcoming the major limitation of routine pairwise meta-analyses, provides evidence-based grading for the efficacy and safety of available oral antiplatelet and anticoagulant therapies for AF patients undergoing PCI. Although no statistical significance was identified, combination of VKA and SAPT treatment showed a trend toward achieving the greatest beneficial effect compared to other regimens, followed by rivaroxaban 2.5 mg twice daily with DAPT. DAPT only showed the weakest antithrombotic efficacy but relative safety among 5 therapies. In addition, rivaroxaban 15 mg per day plus P2Y12 inhibitor revealed the highest risk of major bleeding in spite of its best efficacy. The worst results were observed in TT arm which ranked in the second place of both MACCEs and major bleeding outcomes probabilities.
So far, no other NMA on antithrombotic therapy for AF patients undergoing PCI was done. Because of the heterogeneous methodology, an early systematic review by Barry AR et al[28] including 10 cohort studies and 1 meta-analysis failed to quantify the efficacy and safety of TT compared with DAPT in patients undergoing PCI with stent implantation and with multiple indications for long-term oral anticoagulation. Evidence from small cohort studies supported the benefit of TT at reducing major adverse cardiac events (MACEs) and all-cause mortality with higher rates of bleeding. However, recruited AF patients accounted for 59% to 100% in the enrolled trials who were not really representative for all AF patients. Another meta-analysis[29] on antithrombotic therapy after PCI in patients requiring OAC treatment yielded the conclusion that TT was more efficacious than dual therapy (DAPT or OAC plus SAPT) in reducing MACE/stroke [0.76 (0.70, 0.83), p < 0.00001 and 0.67 (0.59, 0.75), p < 0.00001, respectively] and significantly increased the risk of major bleeding compared with DAPT [1.36 (1.17, 1.58), p < 0.0001]. In the comparison between TT and OAC plus clopidogrel, there were no differences in MACCEs and major bleeding events. In our NMA, TT was associated with significantly higher risk of major bleeding compared with DAPT [1.83 (1.11, 3.28)] which was consistent with the above two meta-analyses, while VKA with SAPT seemed better than TT in efficacy for AF patients undergoing PCI (Fig 3) despite no statistical significance was reached [0.80 (0.55, 1.11)]. The population heterogeneity may be a reason for that discrepancy. A recent meta-analysis[30] compared the clinical outcomes in 20,456 AF patients undergoing PCI and stenting who received DAPT (13,253 patients) and TT (7,203 patients) with a mean follow-up period of 15 months in 18 studies and found that TT was associated with significantly lower risk of stroke, ST, and all-cause mortality compared with DAPT (odd ratios and 95% CIs were 1.98 [1.03, 3.81], p = 0.04; 1.59 [1.08, 2.34], p = 0.02; and 1.41 [1.03, 1.94], p = 0.03, respectively) and a significantly higher risk of major bleeding (0.62 [0.50, 0.77], p < 0.0001). Only 2 or 3 traditional antithrombotic treatments without NOACs were compared in the above meta-analyses, and thus not enough evidence was available for clinical decision in this condition. However, our NMA provides a rank of 5 antithrombotic therapies including NOACs for AF patients undergoing PCI.
To guarantee the study quality, several studies were discreetly excluded in out NMA for patient heterogeneity, narrow time in therapeutic range (TTR) when warfarin was used, mean/median follow-up period less than 1 year, or unspecific comparison between treatments such as VKA vs. non-VKA. Besides, studies in which antithrombotic therapies changed differently after PCI were also excluded to reflect their true antithrombotic efficacy and bleeding effect. A relative meta-analysis[31] which compared uninterrupted oral anticoagulation (UAC) with VKA vs. interrupted oral anticoagulation (IAC) with or without bridging anticoagulation before coronary procedures found that there was no difference in MACCE or major bleeding between UAC and IAC [0.74 (0.34, 1.64), p = 0.46; 0.62 (0.16, 2.43), p = 0.49, respectively]; but as compared to IAC with bridging, there was a statistically significant MACCE risk reduction and 65% lower risk of major bleeding with UAC [0.52 (0.29, 0.95), p = 0.03; 0.35 (0.13, 0.92), p = 0.03, respectively]. Thus UAC is at least as safe as IAC, and seems to be much safer than IAC with bridging anticoagulation in patients undergoing coronary angiography with or without PCI. Furthermore, only 3 RCTs out of 15 included studies could be identified to satisfy our inclusion and exclusion criteria. Several large-size RCTs for AF patients undergoing PCI with other NOACs treatments such as REDUAL-PCI (dabigatran, NCT02164864), AUGUSTUS (apixaban, NCT02415400), and ENTRUST-AF-PCI (edoxaban, NCT02866175) are on-going or just completed and published outcomes have not been available yet[32]. More results from RCTs are needed to provide evidence of optimal antithrombotic strategies for these patients.
The optimal strategy and duration of combination antithrombotic therapy for AF patients undergoing PCI is not known, but as guidelines mentioned, the continued bleeding risk suggests a short duration[5], which is consistent with the worst results observed in TT arm in this NMA. Given the lack of evidence and the greater risk of major bleeding compared with clopidogrel, the use of prasugrel or ticagrelor as part of TT should be avoided unless there is a clear need for these agents (e.g. stent thrombosis on aspirin plus clopidogrel)[33, 34]. Rivaroxaban, a direct factor Xa inhibitor, has been reported to prevent venous thromboembolism more effectively than enoxaparin in patients undergoing orthopedic surgery[35,36]. As for anticoagulant therapy for AF, ROCKET-AF demonstrated that a daily dose of 20 mg rivaroxaban was noninferior to dose-adjusted warfarin for the prevention of stroke or systemic embolism, while major bleeding from a gastrointestinal site was more common in the rivaroxaban group (3.2% vs. 2.2%, p < 0.001)[37]. This may partly explain why rivaroxaban 15 mg daily with P2Y12 inhibitor treatment ranks the worst in safety endpoint, in spite of its best efficacy in our study. The types of stent and CAD, CHA2DS2-Vasc scores and bleeding risk should be well considered of antithrombotic strategies for these patients. Besides, patients’ compliance is vital for INR management when warfarin is used. Anyhow, NOACs instead of VKA stand for the mainstream of anticoagulant therapy in the future, and proper dosage of NOACs with combined antithrombotic regimen needs further research.
In summary, this NMA suggests that combination of VKA with SAPT is an optimized strategy for AF patients undergoing PCI. Rivaroxaban 2.5 mg twice daily with DAPT may be the best alternative for VKA plus SAPT in the treatment for these patients.
Supporting information
(DOCX)
Abbreviations: MACCEs, main adverse cardiac and cerebrovascular events; DAPT, dual-antiplatelet therapy; Riva15 + P2Y12, rivaroxaban 15 mg/d plus P2Y12 inhibitor; Riva2.5 + P2Y12, rivaroxaban 2.5 mg bid plus P2Y12 inhibitor; TT, triple-antiplatelet therapy; VKA, vitamin K antagonist; SAPT, single antiplatelet therapy.
(DOCX)
Abbreviations: RCT, randomized clinical trial.
(DOCX)
(DOCX)
Acknowledgments
The authors thank LCJ for his review of the manuscript, Shaowen Tang for his assistance with conducting the statistical analysis and LJJ, ZXW, TXY, and MSR for their provision of materials/analysis tools.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Lamberts M, Olesen JB, Ruwald MH, Hansen CM, Karasoy D, Kristensen SL, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 2012. September 4;126(10):1185–93. doi: 10.1161/CIRCULATIONAHA.112.114967 . [DOI] [PubMed] [Google Scholar]
- 2.Hansen ML, Sørensen R, Clausen MT, Fog-Petersen ML, Raunsø J, Gadsbøll N, et al. Risk of bleeding with single, dual, or triple therapywith warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010. September 13;170(16):1433–41. doi: 10.1001/archinternmed.2010.271 . [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Nodar JM, Marín F, Hurtado JA, Valencia J, Pinar E, Pineda J, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol. 2008. February 26;51(8):818–25. doi: 10.1016/j.jacc.2007.11.035 . [DOI] [PubMed] [Google Scholar]
- 4.Sutton NR, Seth M, Ruwende C, Gurm HS. Outcomes of Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. J Am Coll Cardiol. 2016. August 30;68(9):895–904. doi: 10.1016/j.jacc.2016.05.085 . [DOI] [PubMed] [Google Scholar]
- 5.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016. ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016 November;18(11):1609–1678. doi: 10.1093/europace/euw295 . [DOI] [PubMed] [Google Scholar]
- 6.Maegdefessel L, Schlitt A, Faerber J, Bond SP, Messow CM, Buerke M, et al. Anticoagulant and/or antiplatelet treatment in patients with atrial fibrillation after percutaneous coronary intervention. A single-center experience. Med Klin (Munich). 2008. September 15;103(9):628–32. doi: 10.1007/s00063-008-1101-4 . [DOI] [PubMed] [Google Scholar]
- 7.Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013. March 30;381(9872):1107–15. doi: 10.1016/S0140-6736(12)62177-1 . [DOI] [PubMed] [Google Scholar]
- 8.Dąbrowska M, Ochała A, Cybulski W, Tendera M. Balancing between bleeding and thromboembolism after percutaneous coronary intervention in patients with atrial fibrillation. Could triple anticoagulant therapy be a solution? Postepy Kardiol Interwencyjnej. 2013;9(3):234–40. doi: 10.5114/pwki.2013.37501 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fosbol EL, Wang TY, Li S, Piccini J, Lopes RD, Mills RM, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J. 2013. November;166(5):864–70. doi: 10.1016/j.ahj.2013.08.005 . [DOI] [PubMed] [Google Scholar]
- 10.Kawai H, Watanabe E, Yamamoto M, Harigaya H, Sano K, Takatsu H, et al. Major bleeding complications related to combined antithrombotic therapy in atrial fibrillation patients 12 months after coronary artery stenting. J Cardiol. 2015. March;65(3):197–202. doi: 10.1016/j.jjcc.2014.08.013 . [DOI] [PubMed] [Google Scholar]
- 11.Rubboli A, Schlitt A, Kiviniemi T, Biancari F, Karjalainen PP, Valencia J, et al. One-year outcome of patients with atrial fibrillation undergoing coronary artery stenting: an analysis of the AFCAS registry. Clin Cardiol. 2014. June;37(6):357–64. doi: 10.1002/clc.22254 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh SY, Kang WC, Oh PC, Choi H, Moon CI, Lee K, et al. Efficacy and safety of aspirin, clopidogrel, and warfarin after coronary artery stenting in Korean patients with atrial fibrillation. Heart Vessels. 2014. September;29(5):578–83. doi: 10.1007/s00380-013-0399-x . [DOI] [PubMed] [Google Scholar]
- 13.Kang DO, Yu CW, Kim HD, Cho JY, Joo HJ, Choi RK, et al. Triple antithrombotic therapy versus dual antiplatelet therapy in patients with atrial fibrillation undergoing drug-eluting stent implantation. Coron Artery Dis. 2015. August;26(5):372–80. doi: 10.1097/MCA.0000000000000242 . [DOI] [PubMed] [Google Scholar]
- 14.Hess CN, Peterson ED, Peng SA, de Lemos JA, Fosbol EL, Thomas L, et al. Use and Outcomes of Triple Therapy Among Older Patients With Acute Myocardial Infarction and Atrial Fibrillation. J Am Coll Cardiol. 2015. August 11;66(6):616–27. doi: 10.1016/j.jacc.2015.05.062 . [DOI] [PubMed] [Google Scholar]
- 15.Mennuni MG, Halperin JL, Bansilal S, Schoos MM, Theodoropoulos KN, Meelu OA, et al. Balancing the Risk of Bleeding and Stroke in Patients With Atrial Fibrillation After Percutaneous Coronary Intervention (from the AVIATOR Registry). Am J Cardiol. 2015. July 1;116(1):37–42. doi: 10.1016/j.amjcard.2015.03.033 . [DOI] [PubMed] [Google Scholar]
- 16.Sambola A, Mutuberría M, García Del Blanco B, Alonso A, Barrabés JA, Alfonso F, et al. Effects of Triple Therapy in Patients With Non-Valvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention Regarding Thromboembolic Risk Stratification. Circ J. 2016;80(2):354–62. doi: 10.1253/circj.CJ-15-0923 . [DOI] [PubMed] [Google Scholar]
- 17.Sambola A, Mutuberría M, García Del Blanco B, Alonso A, Barrabés JA, Bueno H, et al. Impact of Triple Therapy in Elderly Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. PLoS One. 2016. January 25;11(1):e0147245 doi: 10.1371/journal.pone.0147245 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherwood MW, Cyr DD, Jones WS, Becker RC, Berkowitz SD, Washam JB, et al. Use of Dual Antiplatelet Therapy and Patient Outcomes in Those Undergoing Percutaneous Coronary Intervention: The ROCKET AF Trial. JACC Cardiovasc Interv. 2016. August 22;9(16):1694–702. doi: 10.1016/j.jcin.2016.05.039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson CM, Pinto DS, Chi G, Arbetter D, Yee M, Mehran R, et al. Recurrent Hospitalization Among Patients With Atrial Fibrillation Undergoing Intracoronary Stenting Treated With 2 Treatment Strategies of Rivaroxaban or a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy. Circulation. 2017. January 24;135(4):323–333. doi: 10.1161/CIRCULATIONAHA.116.025783 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vecchis R, Cantatrione C, Mazzei D. Clinical Relevance of Anticoagulation and Dual Antiplatelet Therapy to the Outcomes of Patients With Atrial Fibrillation and Recent Percutaneous Coronary Intervention With Stent. J Clin Med Res. 2016. February;8(2):153–61. doi: 10.14740/jocmr2443w . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M, Schneider M, Davey SG. Spurious precision? Meta-analysis of observational studies. BMJ, 1998, 316(7125): 140–144. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010. May;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232 . [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011. October 18;343:d5928 doi: 10.1136/bmj.d5928 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, et al. Thrombolysis in Myocardial Infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987; 76:142–154. . [DOI] [PubMed] [Google Scholar]
- 25.Mehran R, Rao S, Bhatt DL, Gibson MC, Caixeta A, Eikelboom J, et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials A Consensus Report From the Bleeding Academic Research Consortium. Circulation. 2011; 123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 . [DOI] [PubMed] [Google Scholar]
- 26.Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med 2002; 21:2313–2324. doi: 10.1002/sim.1201 . [DOI] [PubMed] [Google Scholar]
- 27.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines.J Thorac Cardiovasc Surg. 2016. November;152(5):1243–1275. doi: 10.1016/j.jtcvs.2016.07.044 . [DOI] [PubMed] [Google Scholar]
- 28.Barry AR, Ackman ML. Triple antithrombotic therapy in patients with atrial fibrillation who have undergone percutaneous coronary intervention with stent implantation. Am J Health Syst Pharm. 2012. September 1;69(17):1485–93. doi: 10.2146/ajhp110556 . [DOI] [PubMed] [Google Scholar]
- 29.Chen CF, Chen B, Zhu J, Xu YZ. Antithrombotic therapy after percutaneous coronary intervention in patients requiring oral anticoagulant treatment. A meta-analysis. Herz. 2015. December;40(8):1070–83. doi: 10.1007/s00059-015-4325-0 . [DOI] [PubMed] [Google Scholar]
- 30.Chaudhary N, Bundhun PK, Yan H. Comparing the clinical outcomes in patients with atrial fibrillation receiving dual antiplatelet therapy and patients receiving an addition of an anticoagulant after coronary stent implantation: A systematic review and meta-analysis of observational studies. Medicine (Baltimore). 2016. December;95(50):e5581 doi: 10.1097/MD.0000000000005581 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalewski M, Suwalski P, Raffa GM, Słomka A, Kowalkowska ME, Szwed KA, et al. Meta-analysis of uninterrupted as compared to interrupted oral anticoagulation with or without bridging in patients undergoing coronary angiography with or without percutaneous coronary intervention. Int J Cardiol. 2016. November 15;223:186–194. doi: 10.1016/j.ijcard.2016.08.089 . [DOI] [PubMed] [Google Scholar]
- 32.Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med. 2016. December 22;375(25):2423–2434. doi: 10.1056/NEJMoa1611594 . [DOI] [PubMed] [Google Scholar]
- 33.Sarafoff N, Martischnig A, Wealer J, Mayer K, Mehilli J, Sibbing D, et al. Triple therapy with aspirin, prasugrel, and vitamin K antagonists in patients with drug-eluting stent implantation and an indication for oral anticoagulation. J Am Coll Cardiol. 2013;61:2060–2066. doi: 10.1016/j.jacc.2013.02.036 . [DOI] [PubMed] [Google Scholar]
- 34.Jackson LR II, Ju C, Zettler M, Messenger JC, Cohen DJ, Stone GW, et al. Outcomes of Patients With Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention Receiving an Oral Anticoagulant and Dual Antiplatelet Therapy: A Comparison of Clopidogrel Versus Prasugrel From the TRANSLATE-ACS Study. JACC Cardiovasc Interv. 2015;8:1880–1889. doi: 10.1016/j.jcin.2015.08.018 . [DOI] [PubMed] [Google Scholar]
- 35.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008. June 26;358(26):2765–75. doi: 10.1056/NEJMoa0800374 . [DOI] [PubMed] [Google Scholar]
- 36.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008. June 26;358(26):2776–86. doi: 10.1056/NEJMoa076016 . [DOI] [PubMed] [Google Scholar]
- 37.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011. September 8;365(10):883–91. doi: 10.1056/NEJMoa1009638 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Abbreviations: MACCEs, main adverse cardiac and cerebrovascular events; DAPT, dual-antiplatelet therapy; Riva15 + P2Y12, rivaroxaban 15 mg/d plus P2Y12 inhibitor; Riva2.5 + P2Y12, rivaroxaban 2.5 mg bid plus P2Y12 inhibitor; TT, triple-antiplatelet therapy; VKA, vitamin K antagonist; SAPT, single antiplatelet therapy.
(DOCX)
Abbreviations: RCT, randomized clinical trial.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.